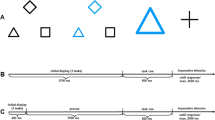
Abstract
In order to flexibly adapt to a permanently changing environment, it is necessary to inhibit previously activated but now irrelevant processing pathways. Empirically, this inhibition manifests itself only indirectly in terms of a cost of reengaging a previously inhibited pathway (n − 2 repetition costs). While imaging studies suggest an involvement of the prefrontal cortex in this type of inhibition, it has recently been argued that the underlying processes are implicated not in triggering inhibition, but in overcoming it. To disentangle these processes on a behavioral level, we investigated the time course of inhibition using a cued task switching paradigm. The response–cue interval (between the response of trial n − 1 and the cue of trial n) was varied in five steps to capture its influence on inhibition in a fine-grained manner. The results suggest that the impact of inhibitory processes increases during the first 200–300 ms after the response of the previous trial, reaches its full extent with about 300 ms, and starts to diminish after that. Therefore, future research on the neural correlates of n − 2 repetition costs should employ techniques with a high temporal resolution that are able to capture this presumed time course of inhibitory processes.


Similar content being viewed by others
References
Aron AR, Monsell S, Sahakian BJ, Robbins TW (2004a) A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain 127:1561–1573
Aron AR, Robbins TW, Poldrack RA (2004b) Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177
Bao M, Li Z-H, Chen X-C, Zhang DR (2006) Backward inhibition in a task of switching attention within verbal working memory. Brain Res Bull 69:214–221
Dreher J-C, Berman KF (2002) Fractionating the neural substrate of cognitive control processes. P Natl Acad Sci USA 99:14595–14600
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G* power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Gade M, Koch I (2005) Linking inhibition to activation in the control of task sequences. Psychon B Rev 12:530–534
Gade M, Koch I (2014) Cue type affects preparatory influences on task inhibition. Acta Psychol 148:12–18
Grange JA, Houghton G (2009) Temporal cue-target overlap is not essential for backward inhibition in task switching. Q J Exp Psychol 62:1068–1079
Grange JA, Houghton G (2011) Task preparation and task inhibition: a comment on Koch, Gade, Schuch, & Philipp (2010). Psychon Bull Rev 18:211–216
Grange JA, Juvina I, Houghton G (2013) On costs and benefits of n − 2 repetitions in task switching: towards a behavioural marker of cognitive inhibition. Psychol Res 77:211–222
Houghton G, Tipper SP (1994) A model of inhibitory mechanisms in selective attention. In: Dagenbach D, Carr TH (eds) Inhibitory processes in attention, memory, and language. Academic Press, San Diego, CA, pp 35–112
Houghton G, Tipper SP (1996) Inhibitory mechanisms of neural and cognitive control: applications to selective attention and sequential action. Brain Cognition 30:20–43
Kiesel A, Steinhauser M, Wendt M, Falkenstein M, Jost K, Philipp AM, Koch I (2010) Control and interference in task switching—a review. Psychol Bull 136:849–874
Koch I, Gade M, Philipp AM (2004) Inhibition of response mode in task switching. Exp Psychol 51:52–58
Koch I, Gade M, Schuch S, Philipp AM (2010) The role of inhibition in task switching: a review. Psychon B Rev 17:1–14
Los SA, Van Den Heuvel EC (2001) Intentional and unintentional contributions to nonspecific preparation during reaction time forperiods. J Exp Psychol Human 27:370–386
Mayr U (2002) Inhibition of action rules. Psychon B Rev 9:93–99
Mayr U, Keele SW (2000) Changing internal constraints on action: the role of backward inhibition. J Exp Psychol Gen 129:4–26
Mayr U, Diedrichsen J, Ivry R, Keele SW (2006) Dissociating task-set selection from task-set inhibition in the prefrontal cortex. J Cogn Neurosci 18:1–8
Philipp AM, Koch I (2006) Task inhibition and task repetition in task switching. Eur J Cogn Psychol 18:624–639
Philipp AM, Gade M, Koch I (2007) Inhibitory processes in language switching? Evidence from switching language-defined response sets. Eur J Cogn Psychol 19:395–416
Scheil J, Kleinsorge T (2014) N − 2 repetition costs depend on preparation in trials n − 1 and n − 2. J Exp Psychol Learn 40:865–872
Schuch S, Koch I (2003) The role of response selection for inhibition of task sets in task shifting. J Exp Psychol Human 29:92–105
Sinai M, Goffaux P, Phillips NA (2007) Cue- versus response-locked processes in backward inhibition: evidence from ERPs. Psychophysiology 44:596–609
Vandierendonck A (2013) On the role of dissipating inhibition in task switching. Z Psychol 221:61–62
Vandierendonck A, Liefooghe B, Verbruggen F (2010) Task switching: interplay of reconfiguration and interference control. Psychol Bull 136:601–626
Whitmer AJ, Banich MT (2012) Brain activity related to the ability to inhibit previous task sets: an fMRI study. Cogn Affect Behav Neurosci 12:661–670
Acknowledgments
We thank Miriam Gade and Jim Grange for their helpful comments on a previous version of this article and Florian Selchow for running the experiments.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The procedures employed in the present study were approved by the ethics committee of the Leibniz Research Centre for Working Environment and Human Factors.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Supplementary analyses
RT and ER data of both experiments were analyzed with the additional factor lag_RCI (i.e., the time interval between the response of trial n − 2 and the cue of trial n − 1). This resulted in a 5 (RCI) × 5 (lag_RCI) × 2 (task sequence) ANOVA. Only results involving the lag_RCI factor are reported.
Experiment I
For RT data, the interaction between RCI and lag_RCI reached statistical significance, F (16, 480) = 3.41, p < .001, \( \eta_{p}^{2} \) = .10. This effect was mainly due to shortest RTs for conditions with an RCI of 550 ms and a lag_RCI of 50 ms (703 ms) as well as highest RTs for the RCI 50 ms/lag_RCI 550 ms condition (781 ms). In addition, the three-way interaction between RCI, lag_RCI, and task sequence was significant, F (16, 480) = 2.82, p < .001, \( \eta_{p}^{2} \) = .09. This interaction mainly resulted from highest n − 2 repetition costs in the conditions with a current RCI of 300 ms and a lag_RCI of either 300 ms (97 ms) or 550 ms (98 ms). An overview of the data is given in Table 3. Note that due to 50 conditions resulting from the present design, net n − 2 repetition costs are presented to reduce the table to 25 cells.
For ER data, neither of the effects reached statistical significance.
Experiment II
For RT data, a significant main effect of lag_RCI occurred, F (4, 80) = 2.74, p < .05, \( \eta_{p}^{2} \) = .12. Duncan’s post hoc test revealed shortest RTs for lag_RCIs of 50 ms (802 ms) that differed significantly (p’s < .05) from RTs for lag_RCIs of length 300 (823 ms), 425 (832 ms), and 550 (826 ms) but not from lag_RCI 175 (817 ms, p > .12). Besides, the interaction between RCI and lag_RCI was significant, F (16, 320) = 2.28, p < .01, \( \eta_{p}^{2} \) = .10. Shortest RTs emerged for conditions with a lag_RCI of 50 ms and an RCI of 175 ms (807 ms). Highest RTs could be observed for conditions with a lag_RCI of 300 ms and a RCI of 50 ms (908 ms) as well as for conditions with a lag_RCI of 550 ms and a RCI of 175 ms (892 ms). Additionally, the three-way interaction of all factors reached statistical significance, F (16, 320) = 2.69, p < .001, \( \eta_{p}^{2} \) = .12. This interaction was mainly caused by highest n − 2 repetition costs for conditions with a current RCI of 300 ms and a lag_RCI of 300 (139 ms), 425 (103 ms), or 550 ms (120 ms). An overview of the data is given in Table 4. Note that, as for Experiment I, net n − 2 repetition costs are presented to enhance readability.
For ER data, there was a significant interaction of RCI and lag_RCI, F (16, 320) = 1.83, p < .05, \( \eta_{p}^{2} \) = .08. This effect was mainly due to lowest ERs when both RCI and lag_RCI had a length of 50 ms (2.6 %) and highest ERs with a RCI of 175 ms and a lag_RCI of 550 ms (6.5 %), with the other conditions lying in between (cf. Table 4).
Please note that the two-way interaction between the current RCI and task sequence was still significant for RT data of both experiments (both p’s < .01), whereas the two-way interaction of lag_RCI and task sequence was far from significance (p > .61 and p > .48, respectively).
Due to the large number of different conditions, the results of the supplementary analyses provide a rather unclear picture for both experiments. To include the current RCI as well as lag_RCI in the analyses in a more transparent way, we used the sum of lag_RCI and RCI as factor in an additional step. This resulted in a new summed RCI variable involving nine different time intervals (100, 225, 350, 475, 600, 725, 850, 957, and 1,100 ms) that was included as within-subjects factor in analyses of variance, conducted for RT and ER net n − 2 repetition costs across the data of both experiments.
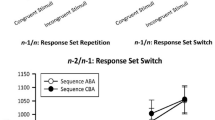
As can be seen from Fig. 3, the sum RCI significantly influenced the size of RT n − 2 repetition costs, F (8, 408) = 4.22, p < .001, \( \eta_{p}^{2} \) = .08. As established by Tukey’s post hoc tests, this effect was due to highest costs for conditions with lag_RCI and RCI summing up to 850 ms that differed significantly from conditions with 225, 475, 975, and 1,100 ms (p’s < .05) and marginally significant (p < .10) from the 350 ms condition.
For ER data, the effect of the summed RCI factor on n − 2 repetition costs was not significant (p > .57).
Discussion
To complete data analyses, additional ANOVAs including the RCI between the response of trial n − 2 and the cue of trial n − 1 were run. For both experiments, an interaction between lag_RCI and RCI occurred. Highest RTs emerged in conditions in which the current RCI was shorter than the lag_RCI, while shortest RTs occurred for RCIs longer than lag_RCI. This data pattern is in line with the trace conditioning account put forward by Los and Van Den Heuvel (2001) that proposes higher RTs when the lag_RCI is longer than the current RCI compared with conditions when the lag_RCI is shorter or equal to the current RCI. However, for the present data, the trace conditioning account holds only for the minimum and maximum RTs, while the other conditions lying in between show no systematic variation that could be explained by the model.
The three-way interaction of lag_RCI, RCI, and task sequence yielded a quite unsystematic picture, although highest n − 2 repetition costs still occurred with a current RCI of 300 ms, as it was the case for the two-way interaction. However, using the sum of both time intervals, the data pattern was much more clear-cut, implying highest n − 2 repetition costs when both RCIs summed up to 850 ms. Considering all time intervals lying between the response of trial n − 2 and the response of the current trial (both RCIs, CSIs, and a mean RT of 775 ms), this leads to the observation of highest costs at about 2,825 ms after the response in trial n − 2 (although noise is added to this estimation due to differences in reaction times).
Rights and permissions
About this article
Cite this article
Scheil, J., Kleinsorge, T. Tracing the time course of n − 2 repetition costs in task switching. Exp Brain Res 232, 3535–3544 (2014). https://doi.org/10.1007/s00221-014-4044-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-4044-4