Published online Dec 18, 2020. doi: 10.5312/wjo.v11.i12.595
Peer-review started: April 11, 2020
First decision: September 24, 2020
Revised: October 9, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 18, 2020
Two-stage revision arthroplasty with an antibiotic-loaded spacer is the treatment of choice in chronically infected total hip arthroplasties. Interval spacers can be functional articulating or prefabricated. Functional results of these spacers have scarcely been reported.
To compare retrospectively the patient reported outcome and infection eradication rate after two-stage revision arthroplasty of the hip with the use of a functional articulating or prefabricated spacer.
All patients with two-stage revision of a hip prosthesis at our hospital between 2003 and 2016 were included in this retrospective cohort study. Patients were divided into two groups; patients treated with a functional articulating spacer or with a prefabricated spacer. Patients completed the Hip Osteoarthritis Outcome Score and the EQ-5D-3L (EQ-5D) and the EQ-5D quality of life thermometer (EQ-VAS) scores. Primary outcomes were patient reported outcome and infection eradication after two-stage revision. The results of both groups were compared to the patient acceptable symptom state for primary arthroplasty of the hip. Secondary outcomes were complications during spacer treatment and at final follow-up. Descriptive statistics, mean and range are used to represent the demographics of the patients. For numerical variables, students’ t-tests were used to assess the level of significance for differences between the groups, with 95% confidence intervals; for binary outcome, we used Fisher’s exact test.
We consecutively treated 55 patients with a prefabricated spacer and 15 patients with a functional articulating spacer of the hip. The infection eradication rates for functional articulating and prefabricated spacers were 93% and 78%, respectively (P > 0.05). With respect to the functional outcome, the Hip Osteoarthritis Outcome Score (HOOS) and its subscores (all P < 0.01), the EQ-5D (P < 0.01) and the EQ-VAS scores (P < 0.05) were all significantly better for patients successfully treated with a functional articulating spacer. More patients in the functional articulating spacer group reached the patient acceptable symptom state for the HOOS pain, HOOS quality of life and EQ-VAS. The number of patients with a spacer dislocation was not significantly different for the functional articulating or prefabricated spacer group (P > 0.05). However, the number of dislocations per patient experiencing a dislocation was significantly higher for patients with a prefabricated spacer (P < 0.01).
Functional articulating spacers lead to improved patient reported functional outcome and less perioperative complications after two-stage revision arthroplasty of an infected total hip prosthesis, while maintaining a similar infection eradication rate compared to prefabricated spacers.
Core Tip: Two-stage revision arthroplasty with an antibiotic-loaded spacer is the treatment of choice in chronically infected total hip arthroplasties. The functional results of these spacers have scarcely been reported. We retrospectively compared all patients treated with two-stage revision arthroplasty of the hip with a functional articulating or prefabricated spacer between 2003 and 2016. We used 15 functional articulating spacers and 55 prefabricated spacers. Patient reported outcome was significantly better for the functional articulating spacer group, while a similar infection eradication rate was achieved.
- Citation: Veltman ES, Moojen DJF, Poolman RW. Improved patient reported outcomes with functional articulating spacers in two-stage revision of the infected hip. World J Orthop 2020; 11(12): 595-605
- URL: https://www.wjgnet.com/2218-5836/full/v11/i12/595.htm
- DOI: https://dx.doi.org/10.5312/wjo.v11.i12.595
When a periprosthetic joint infection (PJI) persists after a debridement, antibiotics and implant retention procedure of an infected prosthesis, or when onset of infection is delayed or late, the PJI is considered chronic[1,2]. Two-stage revision arthroplasty is the standard treatment for chronic PJI of the hip[3]. Antibiotic-loaded interval spacers have proven to be effective in eradicating the infection[3-5]. In contrast to a Girdlestone situation, the antibiotic-loaded hip spacer keeps the soft tissues at length during the interval period[6]. Antibiotic-loaded interval spacers can be either functional articulating, prefabricated or custom-made peroperatively with or without the use of a prefabricated mould[4]. The infection eradication rates for these types of spacers are comparable, while the complication rates of prefabricated spacers are reported to be higher[4,7-9]. Dislocation of prefabricated hip spacers is the most common complication occurring during the spacer interval, which is probably caused by the limited number of options available to adjust the prefabricated spacer to the patients’ anatomy[4,10,11].
Repetitive surgery on a joint causes soft tissue trauma, which can lead to periarticular fibrosis and impaired range of motion[6,12]. Therefore, orthopaedic surgeons have been trying to find a type of antibiotic-loaded spacer with the same efficacy in infection eradication but also facilitating range of motion exercises and ambulation during the spacer period[7,13,14]. Since the functional articulating spacers allow the patient normal activity during the interval period, they may be a good solution for these functional problems and thereby also decrease morbidity and impairments of the patients to a certain extent. Patient related functional assessment of hip function after two-stage revision of the infected total hip arthroplasty with the use of a functional articulating has only scarcely been reported, and these studies did not compare the outcome of the different types of spacers[13,14].
The functional articulating spacers are made of commonly used femoral and acetabular cemented components. During insertion the antibiotic-loaded cement is not pressurized, and care is taken to have no cement distal to the tip of the stem. The type of antibiotics used in the cement can be adjusted to the causative pathogen found in the preoperative cultures. The surgeon has several options to optimise offset and neck length of the femoral component and offset, version and inclination of the acetabular component. The spacer enables patients to practice full range of motion, and patients are allowed to walk bearing 50% to full body weight, irrespective of the extent of bone loss. Prefabricated antibiotic-loaded hip spacers are commercially available with different stem lengths and head sizes. During the spacer interval, the prefabricated spacer allows patients to practice range of motion of the hip. Weight-bearing during the spacer interval is usually limited to less than 25% of body weight.
The aim of this study was to review retrospectively and compare all patients treated with two-stage revision of an infected hip arthroplasty with the use of either a prefabricated or a functional articulating spacer between 2003 and 2016.
The STROBE statement was adhered to while constructing the study and writing the manuscript.
After approval by the local medical ethics committee, the records of all patients who had two-stage revision arthroplasty of the hip between 2003 and 2016 were retrospectively reviewed. All patients with chronic periprosthetic joint infection of the hip that were treated with two-stage revision arthroplasty with the use of an interval spacer and with follow-up of at least 12 mo were included in the study. Exclusion criteria were two-stage revision without the use of a spacer, patients treated with one-stage revision and follow-up of less than 12 mo. Extent of bone loss was not an exclusion criterion for either kind of spacer.
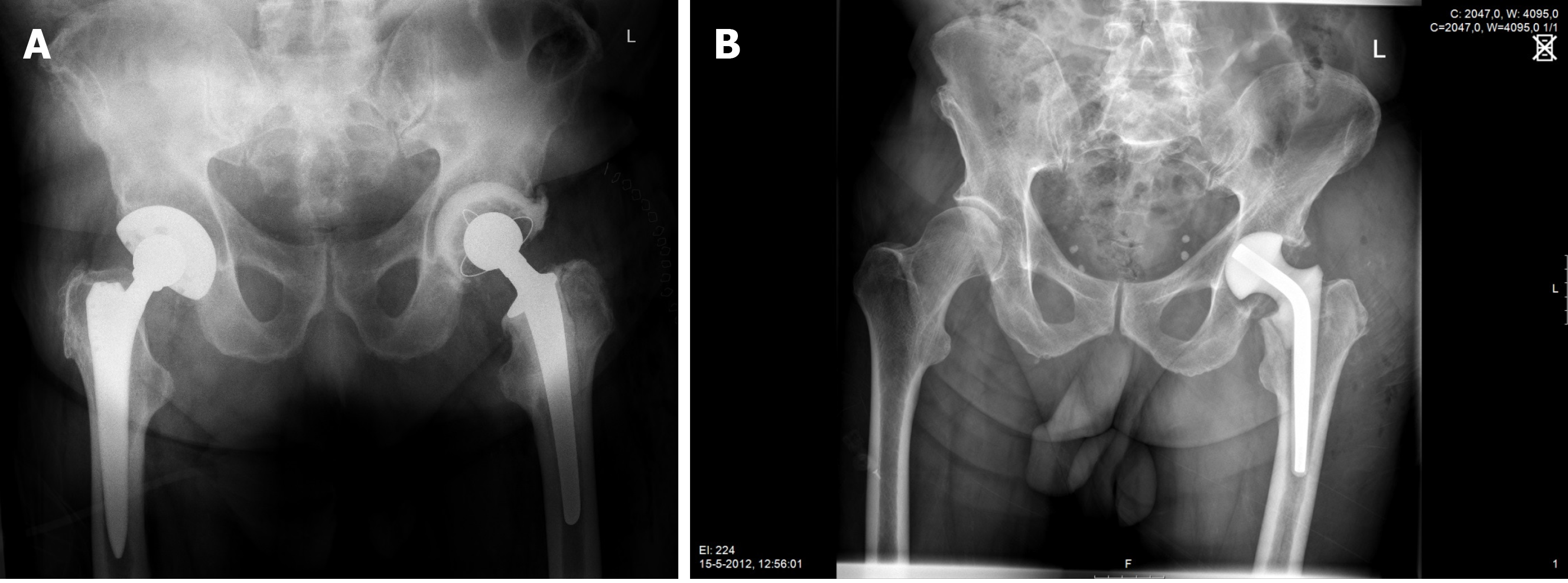
During first-stage surgery the infected prosthesis including bone cement, if present, was removed using a posterolateral approach. After meticulous debridement, a functional articulating-or a prefabricated antibiotic-loaded interval spacer was inserted (Figure 1A and B, respectively). The two groups of patients were treated consecutively, there were no differences in selection criteria for either type of treatment. Initially the prefabricated spacers were used; later the functional articulating spacers. The concentration of antibiotics in the cement were the same in both groups.
All included patients were treated with antibiotics according to the recommendations postulated by Zimmerli et al[2] in 2004. The type of antibiotic treatment was decided in close consultation with a microbiologist and an infection specialist. Two weeks before the second stage procedure, antibiotics were discontinued to achieve a 2-wk antibiotic free interval. During the study period there were no other changes to the treatment practice, except for the implementation of the functional articulating spacers in 2014.
General patient characteristics, complications during treatment and infection status were retrieved from patients’ records. At follow-up, patient reported outcome was measured using the Hip Osteoarthritis Outcome Score (HOOS), EQ-5D-3L (EQ-5D) and the EQ-5D quality of life thermometer (EQ-VAS)[15,16]. The HOOS is a validated score for patients with osteoarthritis of the hip and consists of five domains: symptoms (five questions), pain (10 questions), activities (17 questions), sports (four questions) and quality of life (four questions). Using all answers, a score can be calculated with range of scores between 0-100, with 100 as the optimal score. The EQ-5D is a questionnaire that was developed to describe and value health across a wide range of disease areas. The EQ-5D is comprised of five dimensions: Mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The patient indicates his health state on one of three levels: No problems, some problems or extreme problems, labelled 1-3. The scores can be converted into a value -0.500 to 1.00, with 1.00 as the optimal score. The EQ-5D also contains a visual analogue scale for quality of life (EQ-VAS), where patients can indicate their perceived quality of life on a range of scores 0-100, with 100 as the optimal score.
Primary outcomes were patient related outcome measure scores (PROMs) and infection eradication after second-stage procedure. Secondary outcomes were complications reported during the spacer period and at final follow-up.
The results of the subscores of the HOOS and the result of the EQ-5D were compared to the patient acceptable symptom state (PASS) as described for patients following primary total hip arthroplasty by Paulsen et al[17]. The PASS for the HOOS, EQ-5D and EQ-VAS are 91 (HOOS Pain), 88 (HOOS-PS), 83 [HOOS Quality of life (QoL)], 0.92 (EQ-5D Index) and 85 (EQ-VAS), respectively[17].
Patients were analysed for the type of spacer they were treated with. To be able to compare patient reported outcome after successful treatment and to determine patient reported outcome after failed two-stage revision and subsequent treatment, the PROMs of successfully and unsuccessfully treated patients were analysed separately.
Failure of treatment was defined as persisting infection at final follow-up, removal of the hip prosthesis or use of suppressive antibiotics at follow-up[18]. Descriptive statistics, mean and range are used to represent the demographics of the patients. For numerical variables, we used students’ t-tests to assess the level of significance for differences between the groups, with 95% confidence intervals; for binary outcome, we used Fisher’s exact test. The P values are presented to show the presence or absence of statistical significance. Calculations and statistical analyses were performed using Excel and SPSS software (Armonk, NY, United States).
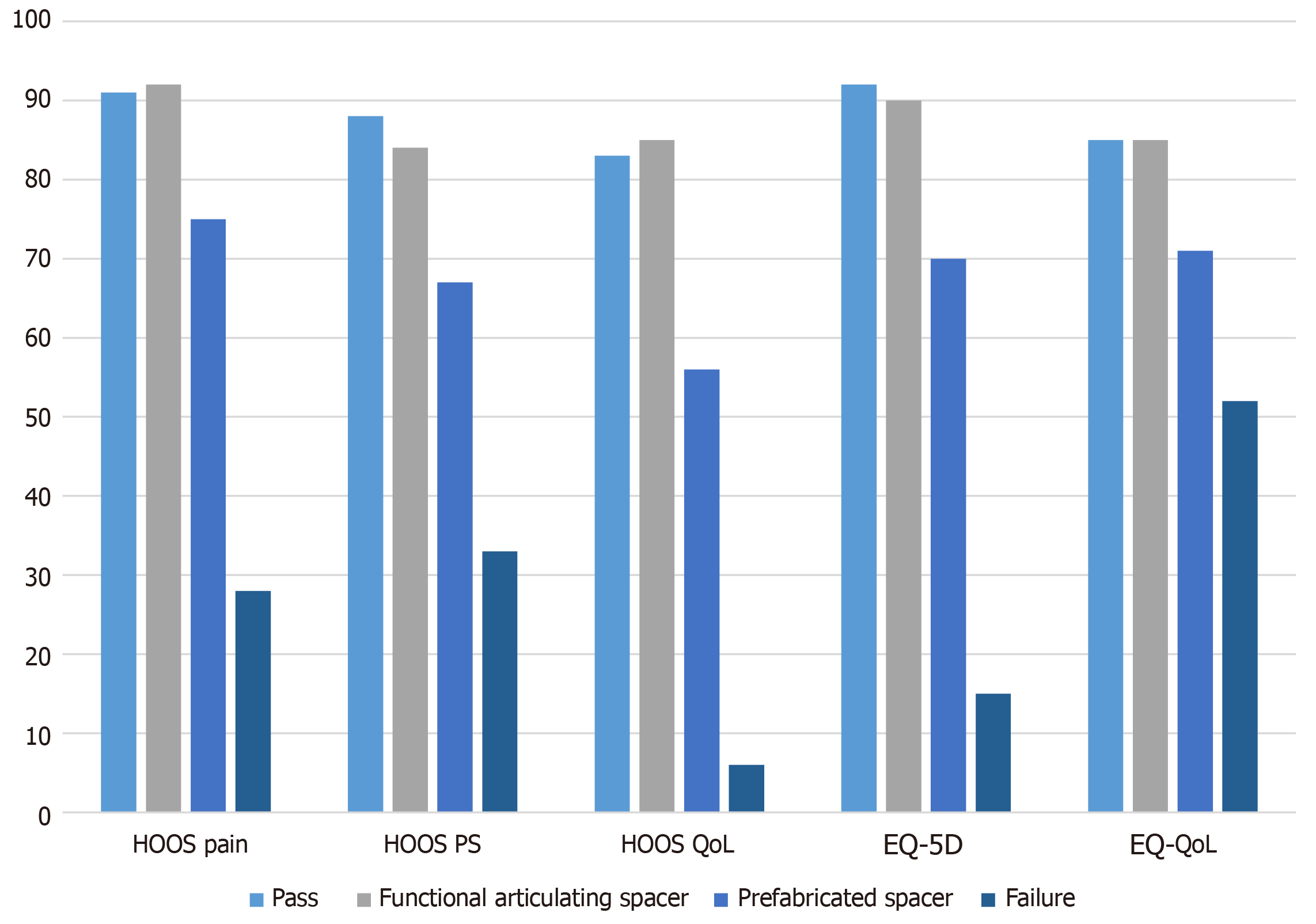
Between 2003 and 2016 we consecutively treated 55 patients with a prefabricated spacer and 15 patients with a functional articulating spacer. General patient characteristics and infection characteristics are listed in Tables 1 and 2. All live patients completed the PROMs. The results of HOOS and EQ-5D scores are displayed in Figure 2.
| Functional articulating spacer group | Prefabricated spacer group | P value | |
| Number of patients | 15 | 55 | |
| Age (range) | 66 (58-76) | 68 (33-88) | NS |
| Gender female | 8 | 25 | NS |
| BMI (range) | 27 (20-35) | 27 (19-41) | NS |
| BMI > 30 | 3 | 13 | NS |
| Diabetes | 4 | 8 | NS |
| ASA 1/2/3 | 1/11/3 | 3/30/22 | NS |
| Post-traumatic (fracture) | 6 | 9 | < 0.05 |
| Months follow-up (range) | 24 (15-85) | 51 (13-129) | < 0.005 |
| Functional articulating spacer group | Prefabricated spacer group | |
| CoNS | 10 | 18 |
| S. aureus | 0 | 9 |
| S. epidermidis | 0 | 1 |
| Propioni acnes | 0 | 5 |
| E. faecalis | 2 | 2 |
| E. coli | 0 | 1 |
| P. aeruginosa | 0 | 1 |
| H. parainfluenzae | 0 | 1 |
| Corynebacterium | 0 | 2 |
| Aerococcus christensenii | 0 | 1 |
| Group B Streptococcus | 1 | 0 |
| Candida albicans | 0 | 2 |
| Culture negative | 0 | 4 |
| Polymicrobial | 2 | 8 |
Fifteen patients were treated with a functional articulating spacer of the hip. At a mean follow-up of 24 mo (range 15-85 mo), one patient had died due to reasons unrelated to treatment.
The mean operating time of the first-stage surgery was 160 min (range 116-290 min). Patients were admitted to the orthopaedic ward for median 13 d (range 5-34 d) after the first stage procedure. Spacer dislocation occurred in two patients. Both patients experienced one dislocation each, which was treated with a closed reduction in both patients. The mean duration of the spacer interval was 8 wk (range 5-12 wk).
The mean operating time of the second stage surgery was 139 min (range 88-188 min). After the second stage procedure patients were admitted for a median 6 d (range 3-12 d) postoperatively. Results of the PROMs are listed in Table 3 and Figure 2; PASS was reached for the mean score of the HOOS pain, HOOS QoL and EQ-VAS.
| Functional articulating spacer group | Prefabricated spacer group | P value | |
| Number of patients | 15 | 55 | |
| HOOS total (SD) | 88 (6) | 67 (14) | < 0.01 |
| HOOS pain (SD), % PASS | 92 (6), 54% | 75 (14), 8% | < 0.01 |
| HOOS PS (SD), % PASS | 85 (6), 15% | 67 (14), 3% | < 0.01 |
| HOOS QoL (SD), % PASS | 85 (12), 46% | 56 (21), 5% | < 0.01 |
| EQ-5D (SD), % PASS | 0.90 (0.17), 46% | 0.69 (0.30), 5% | < 0.01 |
| EQ-VAS (range), % PASS | 85 (65-100), 46% | 71 (45-85), 3% | < 0.05 |
We consider one patient as failure of treatment. Infection persisted after two-stage revision, therefore a Girdlestone situation was created.
Fifty-five patients were treated with a prefabricated spacer of the hip. At a mean follow-up of 51 mo (range 13-129 mo) 10 patients had died; five of these patients had died due to reasons unrelated to treatment.
The mean operating time of the first-stage surgery was 186 min (range 70-360 min). Patients were admitted to the orthopaedic ward for median 31 d (range 5-114 d) after the first stage procedure. Ten patients experienced dislocation of the spacer. In these ten patients a total of 25 dislocations occurred. Revision of the spacer because of multiple dislocations was performed in seven patients. The mean duration of the spacer interval was 8 wk (range 2-28 wk).
The mean operating time of the second stage surgery was 165 min (range 75-326 min). After the second stage procedure patients were admitted for a median 22 d (range 3-63 d) postoperatively. After second-stage procedure dislocation of the hip prosthesis occurred in two patients, both of these patients were treated with a closed reduction. Results of the PROMs are listed in Table 3 and Figure 2; none of the mean outcomes reached the PASS.
We considered 12 patients as failure of treatment. Persistent infection occurred in eight patients, and re-infection with a different bacteria was present in four patients. Two patients were treated with lifelong suppressive antibiotics. Two patients underwent subsequent two-stage revision that was successful in both. Eventually, a Girdlestone situation was created in eight patients. Five of the failure patients had died at time of final follow-up.
With respect to the functional outcome, the HOOS and its subscores (all P < 0.01), the EQ-5D (P < 0.01) and the EQ-VAS scores (P < 0.05) were all significantly better for patients successfully treated with a functional articulating spacer compared to patients successfully treated with a prefabricated spacer. The infection eradication rates were 93% and 78% (P > 0.05) for patients treated with a functional articulating spacer and for patients treated with a prefabricated spacer, respectively. There are differences between the causative pathogens found for both groups (Table 2). The prefabricated group contains more patients with a Staphylococcus aureus, candida and polymicrobial infection.
The mean duration of the first-stage procedure was not statistically different (P = 0.14) and neither was the second-stage procedure (P = 0.13) for the functional articulating and prefabricated groups, respectively. The duration of time patients were admitted to the hospital was significantly shorter for the patients with a functional articulating spacer, both after first-stage surgery (P < 0.01) as well as after the second-stage procedure (P < 0.01).
The number of patients with a spacer dislocation was not significantly different for the functional articulating or prefabricated spacer group (P > 0.05). However, the number of dislocations per patient experiencing a dislocation was significantly higher for patients with a prefabricated spacer (P < 0.01). Revision of the spacer due to recurrent dislocations was performed more often in the prefabricated spacer group, without reaching significance (P = 0.15).
We considered 13 patients as failure of treatment after two-stage revision of the hip. Mean age of these patients was 67 years (range 50-88 years) at first-stage surgery. There were 10 females and three males. Mean body mass index was 32 (range 24-37). Seven patients were American Society of Anesthesiologists score (ASA) 3, the other six were ASA 2. Five patients had died at final follow-up, all of these patients were ASA 3. The eight patients who were alive at follow-up completed the HOOS, EQ-5D and EQ-VAS questionnaires and scored mean 20 (range 5-39), 0.1486 (range -0.128-0.693) and 52 (range 30-80), respectively. None of the patients reached PASS for any of these outcomes. Two of these seven patients received lifelong suppressive antibiotic therapy, the others had a Girdlestone situation.
This study compared patient reported outcome, infection eradication rate and complications for functional articulating spacers and prefabricated spacers used in two-staged revision arthroplasty for PJI of the hip. Infection eradication rate was similar for patients treated with a functional articulating spacer and patients treated with a prefabricated spacer (93% vs 78% respectively, P > 0.05). Both of these infection eradication rates are in concordance with the literature[13,14].
The patients treated with a functional articulating spacer achieved patient reported outcome scores above or close to the PASS, reflecting an acceptable state of functioning from a patient’s perspective as described by Paulsen et al[17], whereas the patients treated with a prefabricated spacer achieve much lower scores[17]. The results of the HOOS, EQ-5D and EQ-QoL show patients treated with a functional articulating spacer achieved significantly higher scores compared to the patients treated with a prefabricated spacer. The difference may be partially explained by heterogeneity of the two patient groups, however correcting for age and comorbidity made no difference. We think these higher scores adequately reflect the better functional recovery of patients with a functional spacer, which has large implications for long-term quality of life. Both Lausmann et al[19] and Zhang et al[9] have found comparable results in their cohorts of patients treated with functional articulating spacers[9,19].
As expected, patients with a Girdlestone situation scored lowest of all groups on the HOOS and the EQ-5D. The impact of permanent explantation of the hip prosthesis on patients’ lives may be reflected even better with the EQ-QoL score, where patients with a Girdlestone situation score only a median 40 of a possible 100. Orthopaedic surgeons should be aware of this very poor functional outcome and decreased quality of life when counselling and preparing their patients for explantation of a hip prosthesis.
Patients treated with a functional articulating spacer had significantly shorter in-hospital stays after both first-stage and second-stage surgery. This effect may be biased by the year of surgery, as patients treated with a functional articulating spacer were treated more recently compared to patients treated with a prefabricated spacer. In recent years there has been increased emphasis on a shorter in-patient period, both after primary and revision arthroplasty[20,21]. However, with a functional articulating spacer, the patients’ mobility is improved and patients can therefore go home more often and sooner and there is less need for discharge to rehabilitation clinics.
Duration of surgery was longer for the prefabricated spacer group during first stage surgery as well as during second stage surgery, without reaching significance. One could expect that spacer removal would be more difficult and time-consuming in patients with a functional articulating spacer, as these stems have been cemented in contrast to the prefabricated spacers. However, by maintaining normal motion with the functional articulating spacer, these patients may suffer less arthrofibrosis of the hip joint due to improved mobilization during the spacer interval, possibly resulting in an overall easier reimplantation procedure. Zhang et al[9] found no significant difference in the mean duration of first- and second stage surgery in their retrospective cohort study including 13 patients with a functional articulating spacer[9].
Spacer dislocation occurred in two out of 15 patients with a functional articulating spacer and in ten out of fifty-five patients with a prefabricated spacer. Both patients with a functional articulating spacer had a single dislocation that was treated with a closed reduction. In patients treated with a prefabricated spacer, dislocation reoccurred 25 times in ten patients. Spacer revision because of repetitive dislocations was performed in seven patients with a prefabricated spacer. The higher dislocation rate in patients with a prefabricated spacer can be explained by the limited number of modifications that can be made to prefabricated spacers, possibly resulting in less soft-tissue balance around the spacer and thus a higher risk of dislocation. Gil Gonzalez et al[22] tried to prevent dislocation by proximal cementation of the prefabricated spacer, but this did not result in significantly less dislocations in their patient series[22]. Yang et al[11] found an incidence as high as 45% for their cohort of patients with prefabricated spacers[11]. Jones and colleagues report that the incidence of complications is significantly associated with spacer design and loss of femoral offset[10].
This study has several limitations that impede drawing definite conclusions. A weak point of this study is reflected by the retrospective design. There were no baseline PROMs available to compare to the PROMs at follow-up, therefore we cannot exclude that the groups had different baseline scores. The number of patients included in this study is low, which is caused by the relative scarcity of PJI requiring two-stage revision. Due to the long period of time in which patients were treated, differences in outcome may partially rely on other smaller changes in treatment that may have occurred over that interval of time. The heterogeneity of the two groups can cause bias in favour of the functional articulating spacer group, as patients in this group are slightly younger, less patients have an ASA classification > 2, there is a difference in causative pathogens between the groups and follow-up is shorter compared to patients in the prefabricated spacer group (Tables 1 and 2). These differences were not caused by patient selection, since initially all patients were treated with a prefabricated spacer and later all patients with a functional articulating one. Duration of in-hospital stay may also be influenced by the year patients were treated, as in recent years the emphasis on short term in-hospital stay has become stronger. Longer follow-up should determine whether the improved outcome of the functional articulating spacer group lasts.
Two-stage revision arthroplasty is a physically and psychologically demanding procedure to endure, especially for frail elderly patients[23]. Although this was not investigated in our cohort, in cases where the spacer is well-fixed, the use of a functional articulating spacer may even facilitate withholding a second stage procedure in high-risk and low-demand patients. Several studies have described patients refusing further procedures because they were satisfied with the function of the spacer[4,24,25]. Long-term results of retained functional articulating spacers have yet to be studied.
This was the first study to compare patient reported outcomes between groups of patients treated with two-stage revision arthroplasty for infection of the hip with a functional articulating or prefabricated spacer. Functional articulating spacers lead to significantly improved patient reported functional outcome, reaching a functional status that is acceptable to patients; comparable infection eradication rate and less perioperative complications, after two-stage revision arthroplasty of an infected total hip prosthesis, compared to prefabricated antibiotic-loaded spacers. The authors believe that, if technically possible, all two-stage revision procedures of the hip should be performed with the use of a functional articulating spacer, as this study shows clear advantages for this type of spacer. There is a need for a prospective randomised controlled trial studying the infection eradication rate and functional outcome of patients during the spacer interval and at long-term follow-up. As randomised trials are difficult to organise due to the low percentage of infections, performing this study as a cluster randomised controlled trial should be executable.
Failure of two-stage revision and subsequent explantation of the prosthesis leads to very poor quality of life. Whenever possible, patients should be counselled about this outcome.
Infection is a tremendous complication of atheroplasty surgery. In case of chronic infection, a two-stage revision procedure is indicated. For the interval period, several types of antibiotic-loaded spacers are available. Prefabricated spacers have a high complication rate, with instability as its main problem. In recent years, we have implemented the use of a functional articulating antibiotic loaded interval spacer.
The current literature lacks reports on the efficacy and safety of different types of spacers used in two-stage revision of an infected total hip arthroplasty. Physicians are still performing two-stage revision with an interval Girdlestone situation or with a prefabricated spacer, even though the patients' mobility is severely compromised and prefabricated spacers are known to have a high dislocation rate.
This study aims to compare the efficacy and safety of the functional articulating spacer to the previously used prefabricated spacer. We compared the groups on infection eradication rate, complications and functional and patient reported outcome.
We retrospectively reviewed all patients treated with two-stage revision of an infected total hip arthroplasty between 2003 and 2016.
We treated 55 patient with a prefabricated spacer and 15 patients with a functional articulating spacer. The patient reported outcomes for the hip osteoarthritis outcome score and EQ-5D-3L were significantly better for the functional articulating spacer group (both P < 0.01). The infection eradication rate was comparable between the groups (93% and 78%, P > 0.05). The risk of dislocation was comparable, but the number of dislocations was significantly higher for the prefabricated spacer group (P < 0.05).
Functional articulating spacers lead to comparable infection eradication rate, improved patient reported outcome and less complications compared to prefabricated spacers used for two-stage revision of the infected hip.
Future studies should evaluate whether our findings can be affirmed in a prospective study with a larger number of patients. Also, it should be evaluated whether it is safe to have a patient retain the spacer when he is satisfied with its function.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Serban ED, Ueda H S-Editor: Zhang H L-Editor: Filipodia P-Editor: Xing YX
| 1. | Amanatullah D, Dennis D, Oltra EG, Marcelino Gomes LS, Goodman SB, Hamlin B, Hansen E, Hashemi-Nejad A, Holst DC, Komnos G, Koutalos A, Malizos K, Martinez Pastor JC, McPherson E, Meermans G, Mooney JA, Mortazavi J, Parsa A, Pécora JR, Pereira GA, Martos MS, Shohat N, Shope AJ, Zullo SS. Hip and Knee Section, Diagnosis, Definitions: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S329-S337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-1654. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2193] [Cited by in F6Publishing: 2026] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 3. | Aalirezaie A, Abolghasemian M, Busato T, Dennis D, Ghazavi M, Holst DC, Kelly M, Kissin YD, Kuijpers M, Lange J, Lichstein P, Moojen DJ, Poolman R, Schreurs BW, Velázquez Moreno JD, Veltman ES. Hip and Knee Section, Treatment, Two-Stage Exchange: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S439-S443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Veltman ES, Moojen DJ, Glehr M, Poolman RW. Similar rate of infection eradication for functional articulating, prefabricated and custom-made spacers in 2-stage revision of the infected total hip: a literature review. Hip Int. 2016;26:319-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Fink B, Vogt S, Reinsch M, Büchner H. Sufficient release of antibiotic by a spacer 6 wk after implantation in two-stage revision of infected hip prostheses. Clin Orthop Relat Res. 2011;469:3141-3147. [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Hsieh PH, Shih CH, Chang YH, Lee MS, Shih HN, Yang WE. Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Joint Surg Am. 2004;86:1989-1997. [PubMed] [Cited in This Article: ] |
| 7. | Faschingbauer M, Reichel H, Bieger R, Kappe T. Mechanical complications with one hundred and thirty eight (antibiotic-laden) cement spacers in the treatment of periprosthetic infection after total hip arthroplasty. Int Orthop. 2015;39:989-994. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Zhang W, Fang X, Shi T, Cai Y, Huang Z, Zhang C, Lin J, Li W. Cemented prosthesis as spacer for two-stage revision of infected hip prostheses: a similar infection remission rate and a lower complication rate. Bone Joint Res. 2020;9:484-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Jones CW, Selemon N, Nocon A, Bostrom M, Westrich G, Sculco PK. The Influence of Spacer Design on the Rate of Complications in Two-Stage Revision Hip Arthroplasty. J Arthroplasty. 2019;34:1201-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Yang FS, Lu YD, Wu CT, Blevins K, Lee MS, Kuo FC. Mechanical failure of articulating polymethylmethacrylate (PMMA) spacers in two-stage revision hip arthroplasty: the risk factors and the impact on interim function. BMC Musculoskelet Disord. 2019;20:372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Kuzyk PR, Dhotar HS, Sternheim A, Gross AE, Safir O, Backstein D. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: techniques, controversies, and outcomes. J Am Acad Orthop Surg. 2014;22:153-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 13. | Chalmers BP, Mabry TM, Abdel MP, Berry DJ, Hanssen AD, Perry KI. Two-Stage Revision Total Hip Arthroplasty With a Specific Articulating Antibiotic Spacer Design: Reliable Periprosthetic Joint Infection Eradication and Functional Improvement. J Arthroplasty. 2018;33:3746-3753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Tsung JD, Rohrsheim JA, Whitehouse SL, Wilson MJ, Howell JR. Management of periprosthetic joint infection after total hip arthroplasty using a custom made articulating spacer (CUMARS); the Exeter experience. J Arthroplasty. 2014;29:1813-1818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095-1108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3241] [Cited by in F6Publishing: 3399] [Article Influence: 125.9] [Reference Citation Analysis (0)] |
| 16. | Nilsdotter AK, Lohmander LS, Klässbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 620] [Cited by in F6Publishing: 682] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 17. | Paulsen A, Roos EM, Pedersen AB, Overgaard S. Minimal clinically important improvement (MCII) and patient-acceptable symptom state (PASS) in total hip arthroplasty (THA) patients 1 year postoperatively. Acta Orthop. 2014;85:39-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 18. | Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res. 2013;471:2374-2382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 19. | Lausmann C, Citak M, Hessling U, Wolff M, Gehrke T, Suero EM, Zahar A. Preliminary results of a novel spacer technique in the management of septic revision hip arthroplasty. Arch Orthop Trauma Surg. 2018;138:1617-1622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Yanik JM, Bedard NA, Hanley JM, Otero JE, Callaghan JJ, Marsh JL. Rapid Recovery Total Joint Arthroplasty is Safe, Efficient, and Cost-Effective in the Veterans Administration Setting. J Arthroplasty. 2018;33:3138-3142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Berg U, BüLow E, Sundberg M, Rolfson O. No increase in readmissions or adverse events after implementation of fast-track program in total hip and knee replacement at 8 Swedish hospitals: An observational before-and-after study of 14,148 total joint replacements 2011-2015. Acta Orthop. 2018;89:522-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Gil Gonzalez S, Marqués López F, Rigol Ramon P, Mestre Cortadellas C, Cáceres Palou E, León García A. Two-stage revision of hip prosthesis infection using a hip spacer with stabilising proximal cementation. Hip Int. 2010;20 Suppl 7:S128-S134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Palmer CK, Gooberman-Hill R, Blom AW, Whitehouse MR, Moore AJ. Post-surgery and recovery experiences following one- and two-stage revision for prosthetic joint infection-A qualitative study of patients' experiences. PLoS One. 2020;15:e0237047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Lee KJ, Min BW, Bae KC, Cho CH, Son ES, Lee SW, Lee SJ, Kang MK. Unintended Retention of Temporary Articulating Spacers in the Treatment of Periprosthetic Hip Joint Infection. Orthopedics. 2020;43:e251-e257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Quayle J, Barakat A, Klasan A, Mittal A, Stott P. External validation study of hip peri-prosthetic joint infection with cemented custom-made articulating spacer (CUMARS). Hip Int. 2020;1120700020960669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |