
Abstract
Aims: Our aim was to determine (1) periprocedural and 30-day clinical safety and efficacy of the CGuard MicroNet-covered embolic prevention carotid stent system (MN-EPS) in routine use for unselected carotid stenosis (CS) patients undergoing CAS, as well as (2) feasibility of MN-EPS post-dilatation optimisation to minimise residual stenosis after CAS.
Methods and results: This was a non-industry-funded, prospective academic study in all-referrals-tracked symptomatic and asymptomatic CS. In asymptomatic lesions, intervention was mandated only in case of increased stroke risk CS features. There was independent neurologist evaluation before CAS, at 48 hours and 30 days. There was external source data verification, angiographic core lab, and statistical analysis. Over 11 months, 108 referrals were recommended by the NeuroVascular Team for revascularisation: 101 (51-86 years, 55 symptomatic, evolving stroke in nine) underwent 106 (100% MN-EPS use) neuroprotection device-assisted (46% proximal, 54% distal) CAS; CEA was performed in seven. MN-EPS device success was 99.1%. Angiographic diameter stenosis was reduced from 83±9% to 6.7±5% (p<0.001). No MN-EPS foreshortening/elongation occurred (30 mm long was 29.82±0.68 mm; 40 mm long was 39.89±0.59 mm). The periprocedural death/major stroke/MI rate was 0%. One event, with no change in NIHSS or modified Rankin Scale and no clinical sequel, was adjudicated by the clinical events committee as minor stroke (0.9%). By 30 days there were no new events (0%).
Conclusions: These increased risk consecutive patient data (1) indicate safety and efficacy of routine MN-EPS use in achieving endovascular reconstruction across all-comer CS lesion subsets, and (2) are consistent with MN-EPS protection against cerebral events extending throughout the stent healing period.
Introduction
Plaque protrusion through stent struts occurs in up to 65.5% of conventional carotid stents in relation to plaque morphology/symptomatic status and stent type1,2, providing a mechanism for post-carotid artery stenting (CAS) cerebral embolisation, either directly or via additional thrombus formation. Using diffusion-weighted magnetic resonance imaging (DW-MRI), the risk of periprocedural cerebral embolisation in transfemoral CAS was shown to be ≈twofold higher with open-cell vs. closed-cell stents3, suggesting a causal relationship with the ≈twofold relative increase in stroke or death with use of open-cell rather than closed-cell stents demonstrated recently in two major clinical trials4,5. Sequential post-procedural DW-MRI cerebral imaging confirmed that, with conventional carotid stents, CAS-related embolisation also occurs after CAS6. Indeed, large-scale clinical data showed that adverse neurological events in the post-procedural period account for 40-70% of all CAS-related neurological events up to 30 days5,7. The risk of post-procedural adverse cerebral events has been related to the size of the carotid stent-free cell area, particularly in the symptomatic patients3,7, indicating an even more important impact of the carotid stent design than temporary neuroprotection device (NPD) use on CAS outcome4. These data indicated that maximised plaque containment stent design might translate not only into improved periprocedural clinical outcomes of CAS but also into minimised risk of neurological events occurring between the CAS procedure and 30-day follow-up.
We evaluated feasibility, safety and 30-day clinical efficacy of routine use of MN-EPS in otherwise standard clinical practice that included 101 consecutive, unselected carotid stenosis patients undergoing CAS. We also assessed the feasibility of MN-EPS routine post-dilatation optimisation to minimise residual stenosis in order to achieve a carotid endarterectomy (CEA)-like effect of CAS.
Methods
STUDY POPULATION AND MEDICAL THERAPY
This prospective study involved all-comer symptomatic and asymptomatic patients with carotid artery stenosis referred to the Jagiellonian University Department of Cardiac and Vascular Diseases for carotid revascularisation. The definition of symptomatic and asymptomatic carotid artery stenosis and intervention eligibility were according to the CREST study8. In addition, asymptomatic patients had to meet at least one increased stroke risk criterion to be eligible for intervention.
No formal power calculations were performed to determine sample size. Rather, the target study population of 101 consecutive subjects undergoing CAS was determined according to an average yearly volume of the centre. Medical therapy was according to the current standard of care and involved aspirin (75-150 mg daily), clopidogrel (pre-procedural loading dose followed by 75 mg daily for three months) and high-dose statin aimed at reaching the target LDL-cholesterol level9. Other common drugs included beta-blockers and ACE inhibitors/angiotensin receptor inhibitors (withheld on the morning of the day of CAS) and, if required for control of hypertension or heart failure, diuretics9.
STUDY DEVICE
The CGuard™ MN-EPS (InspireMD, Boston, MA, USA) (Figure 1) is an open-cell nitinol carotid stent wrapped with MicroNet mesh and mounted on a self-expanding rapid-exchange delivery system. The strut thickness is 0.24 mm and the nitinol frame open-cell area is 21.66 mm2 (i.e., the largest among open-cell carotid stents7). The nitinol frame is covered, on the outside, with a proprietary closed-cell MicroNet mesh. MicroNet is made of single-knitted PET fibre of 20 µm in thickness, forming mesh cells of only 0.023-0.032 mm2, the most dense closed-cell area among contemporary stents. The outer diameter of the stent delivery system is 6 Fr (2.032 mm). The CGuard MN-EPS is available in a typical range of lengths (20 to 60 mm) and diameters (6 to 10 mm).
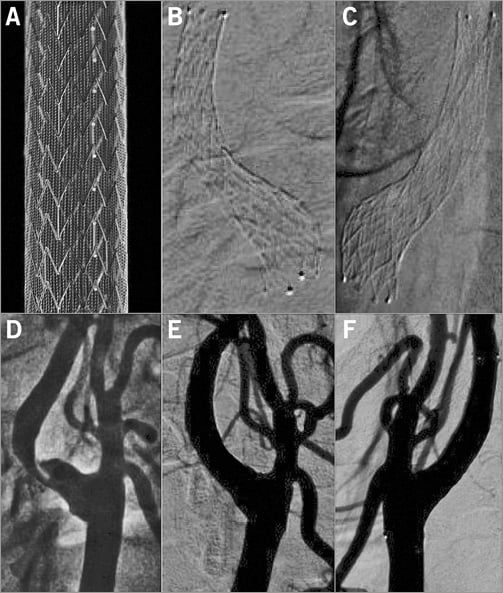
Figure 1. The MicroNet-covered carotid embolic prevention stent system CGuard MN-EPS. The CGuard MN-EPS ex vivo (A). Radiograms in the top panel (B & C) and angiograms in the bottom panel (D, E & F) demonstrate the natural adaptation of the stent to the carotid bifurcation anatomy (self-tapering) while the nitinol frame radial force enables the achievement of no residual stenosis with CAS (“endovascular reconstruction”, CEA-like effect of CAS).
STUDY QUESTIONS
The PARADIGM study was undertaken to evaluate: (1) the feasibility of routinely using the CGuard MN-EPS in an all-comer carotid stenosis population with NeuroVascular Team (NVT) recommendation for revascularisation (all carotid stenosis referral-tracking and all revascularisation-tracking); (2) the device/procedure acute success rate; (3) safety and 30-day clinical efficacy of routine use of the CGuard MN-EPS in unselected carotid stenosis patients undergoing CAS; (4) the proportion of all-comer carotid stenosis patients with an NVT indication for carotid revascularisation that can be safely treated through the endovascular route (CAS) using the study device; and (5) feasibility of MN-EPS post-dilatation optimisation with large balloon/high-pressure inflations performed to minimise residual stenosis after CAS to an average single-digit level by % diameter stenosis (%DS) on an independent angiographic core lab analysis.
The study was registered with a local ethics committee and all subjects gave informed written consent for participation.
CLINICAL DECISION-MAKING PROCESS
Carotid artery stenosis treatment decisions were made by an NVT committee which involved a neurologist, interventional angiologist (interventional vascular specialist), a vascular surgeon and a cardiologist (Figure 2A). Upon a clinical need, the NVT incorporates other medical specialties (anaesthetist, cardiac surgeon, oncologist). Minimum non-invasive imaging preceding carotid revascularisation decision making included cerebral (CT or MRI) scan and carotid duplex ultrasound scan (DUS) in a certified, in-house duplex imaging lab. In case of a “not for carotid revascularisation” decision (Figure 2A), a majority of patients in the cohort also had CT or MRI carotid angiography which, if inconclusive in the context of clinical presentation, was supplemented by conventional angiography.
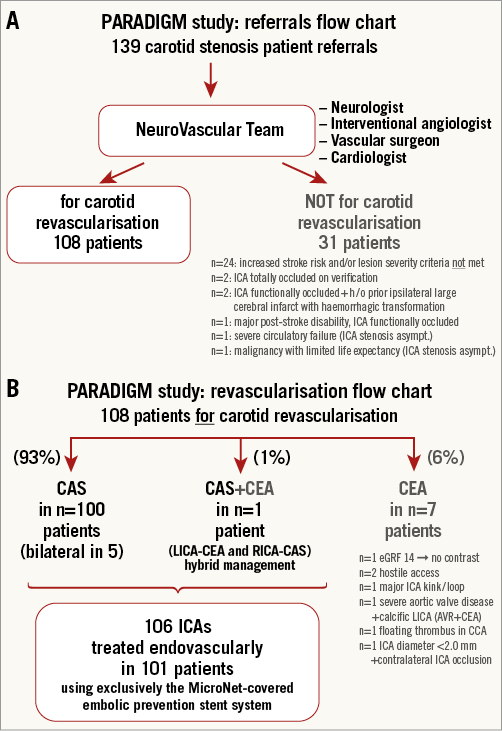
Figure 2. PARADIGM study. A) Referrals flow chart. B) Carotid revascularisation mode flow chart. h/o: history of
It is well known that, with baroreflex-activated bradycardia and hypotension, CAS may be associated with periprocedural MI5,8, particularly when performed in the absence of knowledge of the coexisting significant coronary artery disease (CAD)10 that is also a fundamental determinant of long-term outcome after CAS10. Therefore, coronary angiography was routinely performed during the CAS session consistent with the “Tailored CAS” algorithm10,11 that reflects our routine practice. Our all-comer CAS population included a high proportion of neurologically symptomatic and diabetic patients10,11 who have a poor CAD status discrimination if based on the clinical picture. In case of significant CAD coexisting with carotid stenosis requiring revascularisation, the decision on a one-stage vs. a two-stage procedure (and the order/mode of revascularisation) was made by the combined NVT and Heart Team (HT).
CAS PROCEDURE
The CAS procedure and periprocedural patient management followed our routine “Tailored CAS” algorithm10,11 except for the routine use of the CGuard MN-EPS in consecutive patients as required by the PARADIGM protocol. The CGuard MN-EPS length was to exceed the lesion length by at least 7-10 mm, whereas the diameter was to exceed by 1 mm the diameter of the common carotid artery determined by on-site quantitative angiography (QA). In case of selective internal carotid artery (ICA) stenting, the ICA proximal diameter was used for stent diameter determination. The final decision on the stent length/diameter choice was at the discretion of the operator.
Prior to the point when MN-EPS prevention against embolisation can be established, several steps of the CAS procedure are known to be associated with a substantial risk of cerebral embolisation through plaque/thrombus mobilisation12. The embolisation-prone CAS procedural steps prior to MN-EPS prevention of embolisation include lesion wiring, predilatation, stent deployment and stent post-dilatation12. For this reason, temporary NPD use was recommended as per protocol and was routine in PARADIGM. The choice of NPD type (proximal – temporary flow reversal, or distal – filters) took into consideration lesion morphology, symptoms, target lesion access anatomy and route(s) of cerebral collateral supply consistent with the “Tailored CAS” algorithm10,11. Predilatation (vs. no predilatation) was performed per operator decision using 2.0-4.0 mm coronary balloons. With the DW-MRI evidence for an effective minimisation of cerebral embolisation with MN-EPS13, in PARADIGM large balloon/high-pressure post-dilatations were routinely performed using 4.5-6.0 mm semi-compliant balloons to achieve, whenever feasible, lack of residual in-stent stenosis on visual assessment.
Unfractionated heparin was used according to the activated clotting time (ACT) to ensure an ACT ≥250 sec throughout the procedure. A vascular access closure device14 was used according to availability and anatomic feasibility. Post-procedural arterial blood pressure was tightly controlled to maintain, in general, the systolic range of 100-140 mmHg10,11.
INTERVENTIONAL MANAGEMENT THRESHOLD IN ASYMPTOMATIC CAROTID STENOSIS
Interventional management of asymptomatic carotid stenosis is subject to intense debate, with the approaches ranging from routine intervention (by CEA or CAS) to medical-only management15,16. Our clinical practice of carotid stenosis management has experienced contemporary cases of symptomatic transformation of asymptomatic atherosclerotic carotid stenoses that had been labelled for “watchful waiting” on full medical therapy, and this has included disabling strokes17. We developed an NVT decision-making algorithm which, in addition to the lesion severity requirement as per the CREST criteria11, takes into consideration the following increased stroke risk criteria (with at least one required for intervention in asymptomatic carotid stenosis): (1) documented stenosis progression18-20 by at least one velocity category on DUS assessment or by ≥10% on CT or MRI angiography, (2) thrombus-containing stenosis21, (3) irregular or ulcerated plaque22,23, (4) contralateral transient ischaemic attack (TIA), stroke or carotid occlusion20,24,25, and (5) ipsilateral clinically “silent” brain infarct on cerebral imaging26.
ENDPOINTS AND DEFINITIONS
Stroke and TIA definitions were according to the current American Stroke Association guidelines27. Minor stroke was defined as stroke with a National Institutes of Health Stroke Scale (NIHSS) score ≤328 (more strict than in CREST11 where strokes of NIHSS ≤9 were considered minor). The myocardial infarction (MI) definition was according to the CREST study8.
The primary study endpoint was a composite of death, stroke (major/minor) and MI in the periprocedural period (defined as the period from CAS admission to 48 hours after CAS or to CAS-related discharge, whichever was longer) and at 30 days.
Secondary study endpoints were: (1) acute study device success, defined as the ability to treat the index carotid lesion using the study device (CGuard MN-EPS) successfully delivered and deployed at the lesion site, obtaining residual diameter stenosis <30% by QA, and (2) procedural success, defined as device success in the absence of any vascular complication (e.g., access-site pseudoaneurysm) that would require interventional management.
Since this all-comer study had no exclusion criteria other than lack of an NVT-established indication to carotid revascularisation, there was no per-protocol exclusion of patients with long or tandem lesions.
An independent, external clinical research organisation performed source data verification according to a risk-based monitoring plan.
POST-PROCEDURAL FOLLOW-UP
Patients were evaluated at 48±6 hours and 30±5 days by (i) a neurologist and (ii) an angiologist or cardiologist not involved in the CAS procedure. The evaluator performed clinical examination and reviewed the patient data. If clinically indicated, an additional pre-discharge evaluation was performed; otherwise, the evaluator communicated with the clinical team managing the patient and reviewed patient data and charts. Thirty-day follow-up included DUS of the index artery and, if indicated, of the contralateral artery. Further per-protocol clinical and DUS follow-up is scheduled every 12±1 months up to five years.
ADJUDICATION OF CLINICAL EVENTS
Clinical events were adjudicated by the clinical events committee (CEC) which included an external neurologist, non-invasive cardiologist and vascular specialist (angiologist).
DUPLEX ULTRASOUND AND ANGIOGRAPHIC DATA ANALYSIS
Baseline and 30-day DUS data were evaluated by an independent analyst. Angiographic data analysis was performed by an external angiographic core lab (KCRI Angiographic Core Laboratory, Krakow, Poland) using CAAS Quantitative Vascular Analysis software 5.10.2 (Pie Medical Imaging BV, Maastricht, The Netherlands).
DATA PRESENTATION AND STATISTICAL ANALYSIS
Patient clinical data are provided per patient, with the symptomatic status in relation to the index carotid lesion. In case of NVT recommendation for bilateral carotid stenosis treatment, the lesions were addressed at two separate sessions, with the symptomatic lesion treated, in most cases, first. Procedural data are thus provided on a per lesion basis. Continuous data distribution was evaluated with the Shapiro-Wilk test and, if needed, logarithmic transformation was performed. For between-group comparisons a t-test was used for continuous variables and a chi-square test for categorical ones. Calculations were performed using STATISTICA data analysis software, version 10.0 (StatSoft, Inc., Tulsa, OK, USA).
Results
STUDY POPULATION CHARACTERISTICS
Between October 2014 and September 2015, there were 139 carotid artery stenosis patient referrals (Figure 2A). NVT clinical review of the referred patients taken together with review of patient medical documentation and imaging studies led to an NVT decision on indication to revascularisation in 108 patients, whereas in 31 patients interventional management was deferred (Figure 2A). The main reason for deferral was lack of lesion severity and/or increased stroke risk criteria (indication criteria not met, Figure 2A). Among the 108 subjects with NVT recommendation for carotid revascularisation, seven patients (6%) had contraindication(s) to endovascular management and were thus referred for surgery (CEA, Figure 2B), while one patient underwent hybrid carotid revascularisation (LICA-CEA followed by RICA-CAS, Figure 2B). Five out of 101 endovascular-managed patients were NVT-recommended for bilateral carotid revascularisation, leading to CAS treatment of 106 arteries in the study population of 101 (target) all-comer patients (age 51-86 years, 70% men, Figure 2B, Table 1).
Fifty-five percent of study patients had symptomatic carotid artery disease, including recent (≤14 days) symptoms in 12/55 (i.e., ≈1 in every five symptomatic subjects). Emergent CAS for stroke in evolution was performed in 9/55 patients (16% CAS in the symptomatic cohort). There was an almost equal distribution of LICA and RICA disease (Table 1). The clinical profile of the CAS cohort included 63% coronary artery disease (CAD; previous MI in 32%, history of coronary revascularisation by coronary artery bypass grafting [CABG] or percutaneous coronary intervention [PCI] in 40%), and 41% diabetes. Nine study subjects had a history of atrial fibrillation (AF) or chronic AF (9%), and six had a history of neck/chest radiotherapy (6%). Significant left ventricular dysfunction (LVEF <45%) was present in 13 patients (13%).
There were no significant clinical profile differences between the symptomatic and asymptomatic CAS patients except for a more frequent history of CABG or PCI in the past in the asymptomatic group (26/51 vs. 16/55, p=0.021) and a trend towards an older age (70±8 vs. 67±7 years, p=0.114) and a higher prevalence of diabetes in the symptomatic carotid stenosis patients (27/55 vs. 18/51, p=0.151).
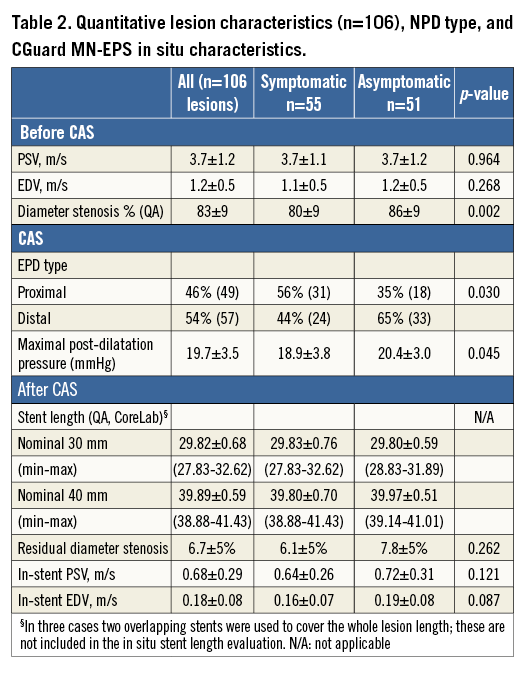
DUS showed index ICA peak systolic velocity (PSV) of 3.7±1.2 m/s and end-diastolic velocity (EDV) of 1.2±0.5 m/s (Table 2). Sixteen (15%) lesions were highly calcific. In the asymptomatic lesion cohort (n=51), one increased stroke risk criterion was present in 14 (27%), two in 23 (45%), three in 12 (24%), while four increased stroke risk criteria were present in two cases (4%).
PROCEDURAL DETAILS
CAS was routinely performed using the transfemoral route. Four patients with symptomatic carotid stenosis and angiographically severe CAD underwent one-stage coronary PCI and CAS (Table 1). Mean angiographic stenosis severity was 83±9% (Table 1). Fifty-seven (54%) procedures were performed under distal NPD, including Spider FX™ (Covidien/Medtronic, Plymouth, MN, USA) in 31 (example in Figure 3), EPI FilterWire EZ™ (Boston Scientific, Marlborough, MA, USA) in 15, and Emboshield® NAV (Abbott Vascular, Santa Clara, CA, USA) in 11. Proximal brain protection by temporary flow reversal was employed in 49 CAS procedures (46%) using either the Gore Flow Reversal system (W.L. Gore and Associates Inc., Flagstaff, AZ, USA) (n=6; example in Figure 4) or Mo.Ma® system (Medtronic, Minneapolis, MN, USA; example in Figure 5) employed routinely to reverse rather than block index ICA flow (n=43). Proximal vs. distal NPD use was more frequent for symptomatic lesion treatment (31/55 vs. 18/51, p=0.03) (Table 2).
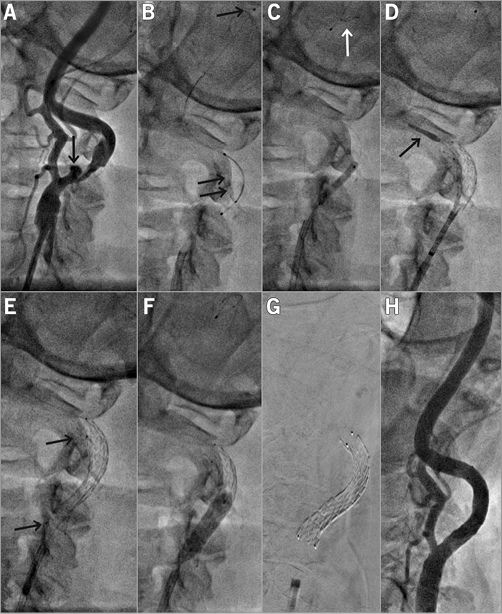
Figure 3. Distal-protected CAS in a 61-year-old male patient with symptomatic LICA stenosis (minor left hemispheric stroke 28 days before) coexisting with asymptomatic RICA occlusion. A) A tight, ulcerated (arrow) lesion. B) Delivery of a Spider FX 6 mm distal embolic protection device (see panel F for the deployed filter image). The lesion was gradually predilated, first with a 2.0×20 mm coronary balloon at 10 atm (C) and then with a 3.5×20 mm coronary balloon at 10 atm. D) Deployment of a 9×30 mm CGuard MN-EPS. E) Stent position after deployment. The stent was post-dilated with a 5.5×20 mm semi-compliant balloon at 18 atm (F). G) A radiograph of the stent (note markers at stent edges). H) Final result of the procedure with no residual stenosis. There were small particles in the filter. The femoral access site was closed with an Angio-Seal™ 8 Fr device (St. Jude Medical, St. Paul, MN, USA).
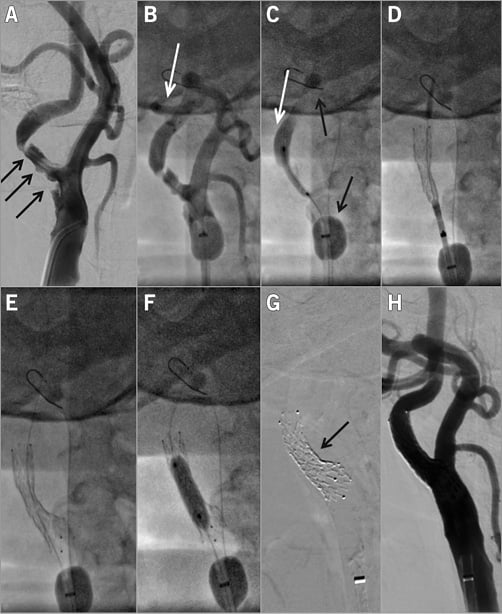
Figure 4. Proximal-protected CAS in a 58-year-old male patient with an asymptomatic but increased stroke risk RICA stenosis. A) Tight, highly ulcerated lesion (black arrows). The lesion was also documented to be progressive. Consistent with the “Tailored CAS” algorithm10,11, the CAS procedure was performed under proximal neuroprotection (Gore Flow Reversal system [G-FR]33). White arrows in panels B and C indicate reversed flow in RICA (“back” pressure was 54/46 mmHg), whereas black arrows in panel C show the G-FR ECA balloon which, in this case, required distal placement for effective ECA occlusion due to the high RECA diameter. The lesion was crossed with a BMW coronary wire (B) and it was gradually predilated, first with a 2.5×20 mm coronary balloon at 12 atm (C) and then with a 3.5×20 mm coronary balloon at 10 atm. Panel D shows positioning and release of the 9×30 mm CGuard MN-EPS while panel E displays the released stent. The stent was post-dilated with a semi-compliant 5.5×20 mm balloon that was inflated up to 20 atm (F). CGuard MN-EPS self-tapering is demonstrated in panel G while panel H shows the final angiographic result of the procedure (endovascular reconstruction; CEA-like effect of MN-EPS–CAS). Flow reversal duration was 7 min 30 sec and it was well tolerated. There was macroscopic debris in the G-FR filter, consistent with the need for temporary embolic protection with MN-EPS–CAS to cover the CAS procedural steps12 prior to MN-EPS-established prevention of plaque/thrombus embolisation.
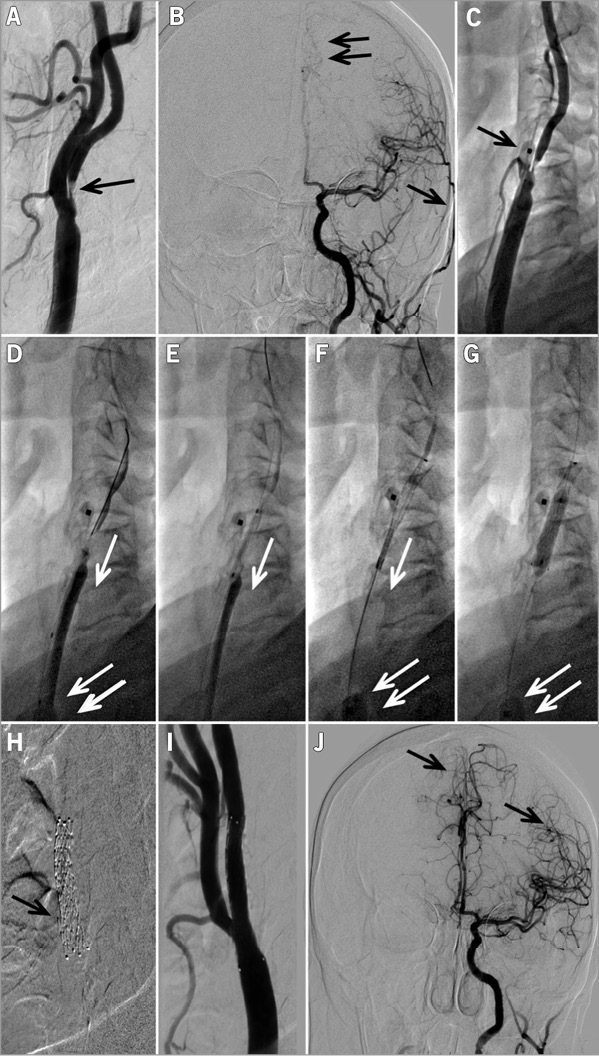
Figure 5. Emergent CAS procedure in an evolving left hemispheric stroke in a 62-year-old female patient. A) A tight thrombus-containing lesion (arrow) in the proximal LICA that is associated with impaired supply from LICA to the left hemispheric vessels (B, double arrow), while the left external carotid artery (LECA) branch is already well filled with the contrast agent (B, single arrow, compare with J). Panel C shows positioning of a Mo.Ma system ECA balloon (arrow) which, in this case, required more proximal repositioning to exclude the left superior thyroid artery that was feasible to perform. Panel D shows wiring of the lesion with a Whisper MS 0.014J coronary wire (Abbott Vascular, Santa Clara, CA, USA) under flow reversal (arrow) with “back” pressure of 65/52 mmHg; the inflated common carotid artery balloon of the Mo.Ma system is indicated with a double arrow in panels D, F and G. Under flow reversal, the lesion was predilated with a 2.0×20 mm coronary balloon (E), and then with a 3.0×20 mm coronary balloon. Panel F shows release of an 8.0×30 mm CGuard MN-EPS under continued flow reversal (single arrow). Stent post-dilatation (4.5×20 mm semi-compliant balloon at 18 atm) is shown in panel G. Panel H is the MN-EPS stent radiogram that illustrates the opening of a stent strut to the ECA (arrow). Panel I is the final result of this procedure, showing minimal residual stenosis (no further stent optimisation using a larger balloon was performed in this highly symptomatic lesion context) while panel J shows a greatly improved supply from LICA to the left hemispheric vessel (arrows) with, now, overshooting to the right hemisphere. The total flow reversal time was 6 min 40 sec, and no intolerance occurred. The arterial access site in the right femoral artery was occluded with an 8 Fr Angio-Seal device.
The study device was used in all 106 procedures (100%; no other carotid stents were used during the study period). MN-EPS of nominal 7.0 to 9.0 mm diameter were employed. Eighty-one lesions were treated with a 30 mm long MN-EPS and 22 with a 40 mm long MN-EPS. In three other cases (2.8%), two 30 mm long MN-EPS were used to cover the whole diseased segment/tandem lesion length. Primary (“direct”) stenting was performed in 9/106 lesions (8.5%) whereas 91.5% of lesions were predilated with a 2.0-4.0 mm semi-compliant coronary balloon prior to MN-EPS insertion. Atropine (1.0-2.0 mg iv.) was given routinely prior to lesion predilatation or MN-EPS placement and post-dilatation.
Consistent with the study protocol, large balloon/high-pressure post-dilatations were routinely performed to minimise residual stenosis. The maximal post-dilatation balloon diameter was 4.5 mm (nine lesions), 5.0 mm (53 lesions), 5.5 mm (37 lesions) and 6.0 mm (seven lesions). Peak post-dilatation pressure ranged from 10 to 24 atm in the symptomatic lesions and 13 to 24 atm in the asymptomatic ones (Table 2). In proximal-protected CAS, mean flow reversal time was 6 min 35 sec (from 3 min 51 sec to 11 min 2 sec).
Macroscopic evidence of debris in the NPD filter (or, in the case of the Mo.Ma system, basket) was present in 19 (17.9%) procedures consistent with the rationale to employ NPDs routinely for cerebral protection prior to the MN-EPS embolic prevention effect. In 16 CAS (15.1%), transient dopamine infusion was used to control post-procedural hypotonia. A vascular closure device was used in 57/106 procedures (54%).
DEVICE/PROCEDURE SUCCESS
In the all-comer CAS cohort, we found 100% feasibility of MN-EPS use. The protocol definition of device and procedure success was met in 105/106 (99.1%) arteries. In one case (0.9%, patient #80) the MN-EPS was successfully delivered and deployed to cover the whole lesion length but it was not optimised (no large balloon/high-pressure inflation performed) due to profound bradycardia/asystole, leaving residual in-stent stenosis of ≈40% DS by visual assessment and 46% by subsequent core lab measurement.
ANGIOGRAPHIC CORE LAB ANALYSIS
The ICA reference diameter was 4.94±0.38 mm (from 3.83 to 6.02 mm) and lesion length was 19.7±5.9 mm (from 4.81 to 33.61 mm). Average in situ length of the nominal 30 mm long MN-EPS was 29.82±0.68 mm (from 27.83 to 32.62 mm), whereas that of the nominal 40 mm in length was 39.89±0.59 mm (from 38.88 to 41.43 mm), consistent with the lack of foreshortening or elongation when placed in situ despite an optimal adaptation to the vascular diameter (self-adaptation) (Figure 1, Figure 3-Figure 5).
In-stent residual DS ranged from 0% to 19% in the 107 procedures with MN-EPS post-dilatation, and from 0% to 46% including patient #80 in whom no MN-EPS post-dilatation optimisation was performed due to profound bradycardia/asystole (patient #80 was included in the mean data analysis). Mean residual diameter stenosis was 6.7±5% (6.1±5% in the symptomatic and 7.8±5% in asymptomatic lesions, p=0.262).
PERIPROCEDURAL CLINICAL OUTCOME
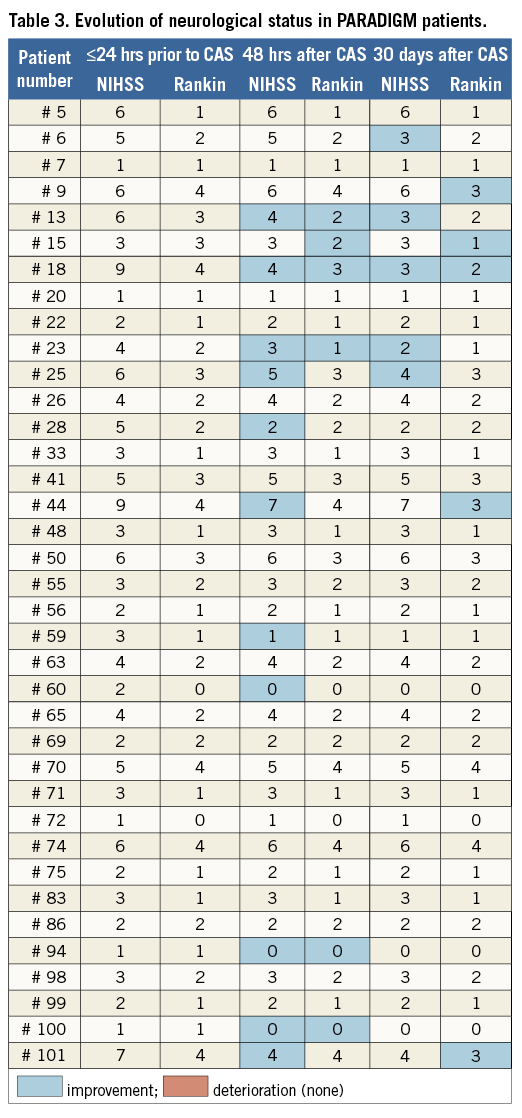
No death/major stroke or MI (0%) occurred at 48 hours and prior to discharge. One patient, with symptomatic RICA stenosis (minor right-hemispheric stroke two months prior to CAS), had hypotonia and transient, fluctuating cognitive dysfunction at 24-48 hours after CAS. The patient had additional neurologic evaluation on discharge (day 7) that showed no change in NIHSS and modified Rankin Scale against 48-hr (and baseline) evaluation (Table 3). CT scan on day 2 showed no new cerebral lesions but day 6 CT revealed an extension of the prior lesion in the right hemisphere. This event was CEC-adjudicated as minor stroke in relation to CAS. Thus, the periprocedural minor stroke rate was 0.9%. No asymptomatic or symptomatic patient at baseline showed deterioration by NIHSS or modified Rankin Scale score at 48 hours or 30 days (Table 3, which also displays neurological status evolution in the study subjects with NIHSS or modified Rankin Scale score ≥1 at any study point).
30-DAY CLINICAL OUTCOME
Thirty-day neurological, duplex, and cardiologic follow-up occurred in 100% of patients (101) and arteries (106). No (0%) new neurological events and no MI occurred between discharge and 30-day follow-up.
Thus, the 30-day death/major stroke/MI rate was 0% and the minor stroke rate was 0.9%. The total 30-day death/any stroke/MI rate was 0.9%.
ECA PATENCY DATA
Prior to CAS, 6/106 (5.6%) ECAs were occluded on the target lesion side, whereas only 3/100 (3.0%; all three with severe ECA stenosis prior to CAS) occluded at CAS. By DUS, no ECA occlusion occurred between CAS and 30 days. The MN-EPS ECA occlusion rate was not different from that in our series using prior-generation carotid stents10.
Discussion
The principal findings from this study, in relation to routine use of the novel MicroNet mesh-covered carotid stent system (CGuard MN-EPS) in a high-volume clinical practice of carotid revascularisation in symptomatic (>50%) and increased stroke risk asymptomatic carotid stenosis based on NVT decision making, are as follows:
1. With the use of MN-EPS, endovascular treatment is feasible in the predominant majority (>90%) of unselected contemporary carotid stenosis patients with NVT-determined indication for revascularisation.
2. MN-EPS can be employed in 100% of CAS procedures and is compatible with all types of temporary neuroprotection systems.
3. MN-EPS allows angiographic optimisation of the CAS result to achieve endovascular reconstruction of the diseased ICA/CCA segment with minimal residual stenosis (CEA-like acute effect of percutaneous revascularisation).
4. Routine MN-EPS use in an increased risk population that includes emergent CAS is safe and is clinically effective across all-comer lesion subsets including thrombus-containing lesions and evolving strokes.
5. Lack of adverse clinical events between discharge and 30 days is consistent with MN-EPS protection extending throughout the stent healing period.
The main strengths of this work are all-comer patient inclusion, NVT decision making on carotid revascularisation with no exclusion criteria other than lack of NVT agreement on the indication for CS revascularisation, independent neurological assessment and CEC adjudication of clinical events, external monitoring and independent core lab angiographic analysis.
CAS is associated with stroke risk due mainly to dislodgement of debris from the target lesion during the procedure and during the stent healing period7,9,12,29. It has been anticipated that the future of carotid stenosis endovascular management would largely depend, in particular in case of symptomatic and increased stroke risk lesions29, on maximised temporary brain protection strategies combined with progress in carotid stent design leading to an effective clinical introduction of novel membrane-covered stents to prevent distal embolisation with CAS29.
The CGuard MN-EPS (Figure 1) was designed with a mesh micro-cell size of only 0.023-0.032 mm2 (i.e., at the level of typical NPD filter pores) for effective prevention of plaque protrusion through stent struts13. The clinical introduction of MN-EPS demonstrates an important, highly anticipated29, technological advancement that may shape the future landscape of carotid revascularisation.
The first MN-EPS clinical study (CARENET)9 had the unique strength of per-protocol evaluation of periprocedural and 30-day cerebral embolisation using the highly sensitive DW-MRI technique with external core lab evaluation. In 30 CARENET patients (33.3% symptomatic), CAS using CGuard MN-EPS was associated with (1) a low incidence (37%) and an extremely low volume (0.039±0.08 mL) of new ipsilateral lesions on DW-MRI at 48 hours, and (2) complete resolution of all but one periprocedural lesion at 30 days and only one new minor (0.116 mL) lesion in relation to the 48-hr scan9. While CARENET demonstrated minimal periprocedural embolisation with MN-EPS and near elimination of embolisation during the stent healing period9, it was not sufficiently powered for device-related and clinical endpoints.
A key finding from the present PARADIGM study is a <1% periprocedural stroke/death/MI rate in an all-comer population with >50% symptomatic carotid stenosis volume (including several strokes in evolution, Table 1) and asymptomatic subjects with an average of two increased stroke risk criteria to warrant interventional management. Another key finding from PARADIGM is the lack of new events by 30 days (100% follow-up). These outcomes, with a reservation for a moderate sample size and single-centre design, compare favourably with 30-day clinical results of two recent major clinical trials in moderate risk patients using conventional (non-covered) carotid stents with free-cell area of 2.74 mm2 and 11.48 mm2, respectively, which reported 2.3-2.8% strokes by 30 days, with a significant proportion of stroke occurring between the procedure and 30 days11,17.
Recent real-life data from a cohort of 794 patients with asymptomatic carotid stenosis in outpatient care at a reputable vascular institution30 confirmed a substantial five-year risk of symptomatic transformation (with stroke as the first manifestation of symptoms in 58% patients) that is not significantly affected by optimal medical therapy (14.1% vs. 10.4%, p=0.32). This finding is not surprising because a number of reasons including comorbidities (e.g., diabetes, history of vascular disease) may lead to an increased-risk referral selection bias. Thus, the population of “asymptomatic” carotid stenosis patients referred to a vascular institution is likely to be of higher risk than patients with asymptomatic carotid stenosis in the general population. Neurological event-prone patients would benefit from low-risk carotid revascularisation, and the ability to offer this with a 30-day complication rate of <1% might have an important effect on patient and physician decision making.
It is widely recognised that the skill and experience of the physician are important in minimising CAS complications4,15,31. The magnitude of the potential role of this important variable in the periprocedural outcome of PARADIGM remains not directly determined. Also, our use of proximal NPD in nearly half of the procedures (Table 2) might have contributed to the low periprocedural32-36 adverse neurological event rate in this study (although there was maximised cerebral protection during CAS, this would have no effect on post-procedural events up to 30 days when the stent design plays a crucial role4). Nevertheless, the complication rate in the present study was lower than results from our group using prior-generation (non-covered) carotid stents and a similar proportion of proximal (vs. distal) neuroprotection in all-comer patients10, suggesting a clinically relevant role of MN-EPS not only in eliminating post-procedural neurological events during stent healing but also in minimising periprocedural events.
With conventional carotid stents, the typical rate of macroscopic evidence of plaque debris is typically found in ≈50-60% of procedures (range 53-100%)35-38. The low proportion of macroscopic debris evidence in the present study (17.9%) is consistent with MN-EPS effective entrapment of plaque and prevention of its intraluminal migration and the low DW-MRI average lesion volume reported by the CARENET study13 with a >10-fold reduction in average lesion volume against conventional carotid stents32 and near elimination of new DW-MRI cerebral lesions at 30 days13. However, the (expected) lack of total elimination of debris when using the new stent type is consistent with a maintained need to use temporary embolic protection (NPD) to cover the emboli-generating CAS phases that cannot be protected by the MN-EPS MicroNet as they occur prior to MN-EPS insertion.
Previous work13 indicated that post-dilatation large balloon/high-pressure inflations are not needed to obtain an angiographic result with MN-EPS as typically seen with conventional carotid stents (16.9±6.5% residual stenosis in CARENET13). However, the MN-EPS effective mesh protection against cerebral embolisation demonstrated in CARENET gave us confidence, allowing the further optimisation of the angiographic result of CAS to the average level of single-digit residual % DS that is not normally depicted by visual estimation (Figure 1, Figure 3, Table 2). This is in contrast to CAS using conventional stents where routine stent optimisation to achieve minimal residual stenosis has not been performed because of substantial risk of adverse cerebral events in relation to plaque protrusion through the stent struts10,31 (thus, in the post-CREST era some CAS operators would routinely refrain from post-dilating a conventional carotid stent, leaving a suboptimal angiographic result). Nevertheless, evidence indicates that carotid residual in-stent stenosis may represent CAS failure in the treatment of carotid stenosis as it may (through favouring exuberant intimal healing with neointimal hyperplasia and/or plaque re-growth31) constitute an important factor for severe vessel reobstruction (in-stent restenosis)39-41 that may pose major management challenges42. In addition, proper apposition of the stent to the arterial wall with minimal residual narrowing has been recognised as an important factor in reducing carotid stent thrombosis8. Recently, 10-year follow-up in the CREST study8 using a conventional carotid stent without angiographic optimisation of the CAS result demonstrated restenosis or repeated revascularisation in 12.2% of patients in the CAS arm.
PARADIGM evaluated the feasibility and safety of minimising residual stenosis after CAS to achieve a CEA-like effect (Figure 1, Figure 3-Figure 5, Table 2). The protocol definition of device success in PARADIGM (30% residual diameter stenosis) was therefore stricter than the one with a conventional carotid stent in CREST or ACT I where <50% diameter stenosis was considered device success8,15. This resulted in 99.1% (rather than 100%) device success in the present study, while the single angiographic result of 46% residual DS would actually have been consistent with device success in CREST or ACT I11,17.
Our strategy routinely to perform coronary angiography preceding CAS (one-stage procedure) may be considered controversial10,11. With the multi-level burden of atherosclerosis and significant coronary involvement in a sizeable proportion of patients with carotid artery disease9,43, MI is a well-known complication of carotid revascularisation, and our team has treated urgent referrals of coronary-complicated CAS performed in the absence of knowledge of the coronary status. Indeed, evidence indicates that coronary and carotid plaque activation (and the risk of their symptomatic transformation) may be cross-linked through the vulnerable blood mechanisms9. Our clinical practice demonstrated that, in the all-comer CAS population with a high proportion of neurologically symptomatic (including post-stroke disability) and diabetic patients10,11, a typical symptom-driven approach may not be sufficient to identify patients at risk of a coronary event at the point of carotid revascularisation. Thus, routine coronary angiography allowed the identification of a proportion of patients at high risk for per-CAS MI and modification of the interventional plan as necessary (starting with PCI of a critical proximal coronary stenosis at the CAS procedure, 4% of PARADIGM patients) or performce of a two-staged procedure (PCI as an elective bridge preceding CAS, 14% of PARADIGM subjects). Whether this strategy can be applied in everyday life in other centres is not certain for logistic and financial reasons. Nevertheless, lack of insurer reimbursement mechanisms should not disable definitive coronary diagnosis and revascularisation as a bridge to CAS in CAS institutions if the latter is considered indicated. A complex coronary intervention may by itself lead to periprocedural MI, and a critical coronary stenosis might not be the sole reason for periprocedural MIs (plaque rupture may occur in non-critical stenosis, vasospasm can provide another pathophysiological mechanism, etc.); thus, the approach we developed may not uniformly protect against MI as a complication of CAS. Therefore, we believe, multi-level revascularisation and its timing as well as sequence decisions need to be adjusted to a particular patient (patient-specific approach) and, at best, they should addressed by a combined NVT and HT recommendation.
Limitations
PARADIGM was a controlled single-arm study rather than a two-arm randomised controlled trial (RCT); thus, by design, it cannot deliver the direct (“level 1”) evidence for the MN-EPS clinical outcome superiority over conventional (non-covered) carotid stents. Initially, we drafted an RCT protocol but, given the totality of data on the impact of the carotid stent cell size on cerebral embolisation and clinical outcome3,4,6,7 taken together with the recent CGuard MN-EPS low lesion prevalence/minimal lesion size DW-MRI evidence13, an RCT design was (1) judged unethical in our increased-risk population due to an unacceptably high likelihood of harm in subjects randomised to treatment with a conventional open-cell carotid stent, through high enhanced cerebral embolisation and adverse neurologic event risks and, on the other hand, (2) likely to be inconclusive/non-contributory to routine clinical practice if performed in a selected low-risk population.
Our decision-making algorithm in asymptomatic carotid stenosis takes into consideration only some of the postulated criteria of an increased stroke risk in asymptomatic carotid stenosis (and these, like other criteria identified to date, may suffer from the absence of prospective large-scale validation in carotid stenosis populations on contemporary “maximal” [or “best”] medical therapy21,44). While any prospective validation of the increased stroke risk criteria in asymptomatic carotid stenosis is beyond the scope of this work, we have been unable to include consistently in our decision-making process some proposed increased risk criteria other than the ones we have used21,44 for the reasons of poor in-house or between-study reproducibility, logistics, or funding. An important increased stroke risk criterion is diabetes20 which, however, we did not believe had sufficient weight to stand as an isolated criterion warranting intervention in asymptomatic carotid stenosis.
In the context of the relationship between post-CAS residual carotid stenosis and the risk of in-stent restenosis39-41 that may lead to vessel occlusion (which can be symptomatic and/or pose significant treatment challenges41,42) and favourable MN-EPS DW-MRI data that suggest the safety of stent post-dilatation13, we routinely performed liberal MN-EPS post-dilatations to minimise residual stenosis.
Large balloon/high-pressure MN-EPS post-dilatations were neurologically and angiographically safe in the present study, and we obtained optimal acute angiographic results (Table 2, Figure 1, Figure 3-Figure 5). Nevertheless, large balloon/high-pressure stent post-dilatations may represent a double-edged sword. Although the coronary artery data on liberal stent post-dilatations are ambivalent45 (and may not be applicable to the carotid territory), it has been hypothesised that large balloon/high-pressure stent optimisation may potentially stimulate, in a subset of lesions, neointimal hyperplasia. Thus, the particular strategy of large balloon/high-pressure post-dilatation in the PARADIGM study may require longer follow-up data before any universal adoption.
Finally, the results from this study require further confirmation in a multicentric setting and patient volume similar to that in CREST8 or ACT I15. In addition, it would be particularly interesting to evaluate MN-EPS in combination with the highly effective transcervical dynamic flow reversal neuroprotection system46,47 both in terms of DW-MRI embolisation incidence and volume and the clinical outcomes periprocedurally and at 30 days.
Conclusions
In conclusion, PARADIGM data indicate that routine use of MN-EPS, a novel carotid stent type covered with a MicroNet mesh to prevent cerebral embolisation by inhibiting plaque protrusion through stent struts (1) may expand the feasibility of endovascular management of carotid stenosis across all-comer symptomatic (including evolving strokes) and increased-risk asymptomatic patients, (2) allows angiographic optimisation of the CAS result to achieve minimal residual stenosis, and (3) is safe and clinically effective in 30-day observation.
The PARADIGM study provides accumulating evidence for a novel carotid revascularisation decision-making model using the CGuard MicroNet-covered embolic prevention stent system as a significant technological advancement translating into maximised patient safety in routine clinical practice.
| Impact on daily practice The long-term effect of CAS is at least equivalent to CEA (ACT I, CREST, EVA-3S, ICSS) but CAS, using conventional stents, has been associated with plaque protrusion through the stent structure, cerebral embolisation and a relative excess of early strokes. This study provides, for the first time, routine clinical evidence that percutaneous carotid stenosis revascularisation using a novel dual-layer embolic prevention stent system (open-cell high radial force nitinol frame covered with ultra closed-cell MicroNet) is safe and effective with no residual stenosis (“CEA-like effect”), and with no post-procedural neurological events during stent healing (“sustained embolic prevention”), in all-comer symptomatic and increased stroke risk asymptomatic patients. Thus, the endovascular route is viable and, with this new technology, is safe and effective in the everyday clinical practice of carotid revascularisation. |
Acknowledgements
We are grateful to the NeuroVascular Team physicians for their determination in shaping the optimal decision on revascularisation in individual patients combining the clinical need with local expertise and the MN-EPS technological advancement. We are also grateful to Justyna Stefaniak, MSc, of Data Management and Statistical Analysis (DMSA, Krakow, Poland) for performing statistical analysis. We would like to acknowledge support from Artur Kozanecki, MD, Piotr Wikolek, MD, PhD, Andrzej Kadzielski, RN, MSc, Miroslawa Merena, RN, MSc, and the patient management physician and nursing teams.
Conflict of interest statement
P. Musialek participated on the InspireMD advisory board and was co-principal investigator in the CGuard MN-EPS CARENET study. The other authors have no conflicts of interest to declare.
Appendix. Neurological status by NIHSS and Rankin Scale in study patients with NIHSS ≥1 or Rankin score ≥1 at any assessment point
Improvement by NIHSS or modified Rankin Scale against the prior neurological assessment point is highlighted in blue, deterioration in red.
There were 55 symptomatic patients in the study. Patients with cortical transient ischaemic attack or amaurosis fugax as CAS-indication symptoms (#11, #19, #21, #52, #53, #61, #62, #66, #68, #81, #84, #87, #92) are not shown in the table as NIHSS and Rankin on admission for CAS were 0, with no change at 48 hours or 30 days. Five patients with minor stroke ≤6 months prior to CAS (#37, #39, #47, #67, #97) are not displayed in Table 3 as their NIHSS and modified Rankin score on CAS admission were 0 with no change at 48 hours or 30 days.
Patient #71 is the index-symptomatic subject (minor ipsilateral, right-hemispheric stroke two months prior to CAS) who had hypotonia and transient, fluctuating cognitive dysfunction at 24-48 hours after CAS; this patient had additional neurologic evaluation on discharge (day 7) that showed no change in NIHSS and Rankin against baseline or 48 hours. Computed tomography (CT) on day 2 showed no new cerebral lesions but day 6 CT revealed an extension of the prior lesion in the right hemisphere; the event (in the absence of right hemispheric symptoms) was CEC-adjudicated as minor stroke in relation to CAS.
No asymptomatic patient at baseline showed deterioration by NIHSS or modified Rankin score at 48 hours or 30 days; thus there are no asymptomatic PARADIGM patients displayed in the table.




