
Abstract
Aims: We aimed to evaluate the long-term safety and efficacy of the STENTYS self-apposing paclitaxel-eluting stent (STENTYS-PES) in bifurcation lesions in routine clinical practice.
Methods and results: The primary endpoint of the study was the composite major adverse cardiac events (MACE: cardiac death, myocardial infarction, clinically driven target lesion revascularisation, or emergent bypass surgery) assessed at six months after enrolment. This was reported in 21 patients (10.1%), mainly due to clinically driven target lesion revascularisation (TLR). At 12 months, 27 patients experienced MACE (13.0%).
Conclusions: The long-term results of OPEN II show that the STENTYS-PES is safe and effective in the treatment of all-comers with coronary bifurcation lesions.
Introduction
The use of drug-eluting stents (DES) in the setting of percutaneous treatment for coronary bifurcation lesions has improved outcomes and decreased the rate of main branch (MB) restenosis significantly, whilst the rate of side branch (SB) restenosis at follow-up has remained high at approximately 20%1.
In this context, the first-in-man experience with the STENTYS disconnectable self-apposing stent (STENTYS S.A., Paris, France) showed encouraging results in terms of efficacy and safety in a limited patient population2. In the present study, we investigated the long-term efficacy and safety of the STENTYS paclitaxel-eluting stent (STENTYS-PES) in a prospectively multicentre-derived patient cohort with bifurcation lesions.
Methods
STUDY DESIGN, DEVICE AND ENDPOINT DEFINITIONS
The STENTYS Coronary Bifurcation Stent System fOr the PErcutaNeous treatment of de novo lesions in native bifurcated coronary arteries (OPEN II) study prospectively enrolled 207 patients in 22 European centres. Inclusion criteria were: 1) evidence of ischaemia by stress test and/or angina symptoms; 2) lesion at bifurcation with a relevant stenosis in the MB (according to the operator’s judgement); 3) MB diameter between 3.0 and 4.5 mm; 4) SB diameter ≥2.0 mm; 5) SB angle between 30° and 70°; 6) lesion length <25 mm; 7) patient giving written informed consent. Exclusion criteria were: 1) Medina class 0,0,1; 2) chronic total occlusion; 3) expected patient survival <1 year; 4) left main coronary artery disease; 5) cardiogenic shock; 6) impossibility to cross the lesion at first attempt.
The device has been described elsewhere2,3. The stent disconnection was left to the discretion of the operator.
The study’s primary endpoint was the composite of major adverse cardiovascular events (MACE; defined as the composite of cardiac death, myocardial infarction [MI], emergent bypass surgery or clinically driven target lesion revascularisation [TLR] by percutaneous or surgical methods) at six months. MI was defined as new abnormal Q-waves in accordance with the Minnesota Code for pathologic Q-waves, or if the creatinine kinase (CK) was twice the upper limit of normal and there was a rise in the level of MB isoenzyme of CK (CK-MB).
STATISTICAL ANALYSIS
The Student’s t-test or Mann-Whitney test was used to compare continuous variables, as appropriate. Comparisons between categorical variables were evaluated using the two-tailed Fisher’s exact test or Pearson’s χ2 test, as appropriate. Analyses were carried out with SPSS software version 17.0 (SPSS, Chicago, IL, USA).
Results
Ten patients were excluded according to the initially considered exclusion criteria, resulting in a final population of 207 patients. Among the excluded patients, four had left main disease (one of them eventually died), one patient had a Medina 0,0,1 lesion, and the remaining five were excluded due to the final decision of the interventional cardiologist not to place a STENTYS-PES in case the latter could not cross the lesion.
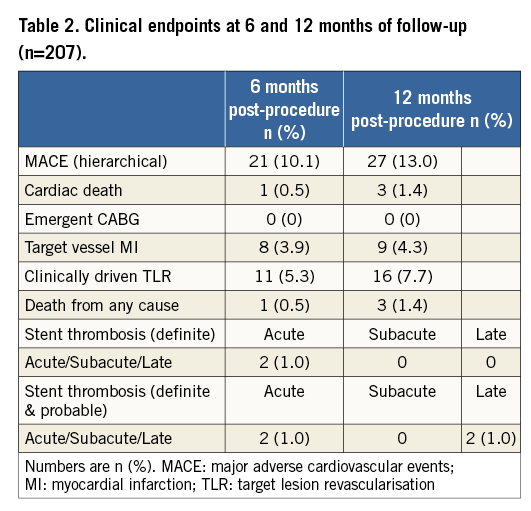
Demographic and clinical characteristics are shown in Online Table 1. Lesion and procedural characteristics are shown in Table 1 and Figure 1. Clinical outcomes are shown in Table 2. The primary endpoint of the study (hierarchical MACE at six months of follow-up) was met in 21 patients. At six and 12 months, the MACE were predominantly driven by target lesion revascularisations.
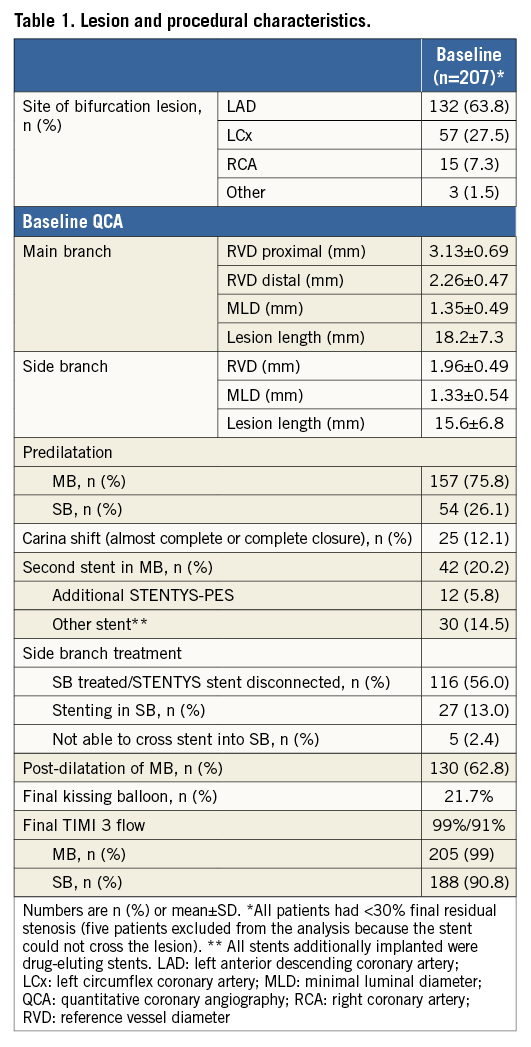
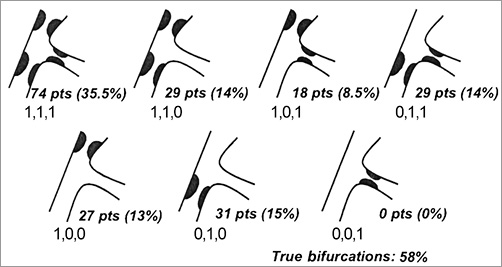
Figure 1. Distribution of bifurcation lesions according to the Medina classification.
Discussion
The present study shows satisfactory midterm and long-term MACE rates with the STENTYS-PES self-expandable stent system in real-world clinical application.
The rate of STENTYS strut disconnection was 56%, somewhat higher than the rate of SB disease observed at baseline angiographic evaluation. Indeed, strut disconnection was performed not only in cases of SB disease at baseline but also for carina shift after STENTYS implantation, although the final decision was left to the operator’s discretion.
In all five cases excluded due to the impossibility to cross the lesion with the delivery system, an attempt to deploy the stent was not performed, based on the operator’s preference to retrieve the delivery system. It is not excluded that a more aggressive lesion preparation with balloon predilatation may have allowed delivery.
No significant differences in MACE rates were found between patients where FKBI was performed and those where it was not (Online Figure 1). However, this latter finding does not itself suggest that FKBI is not helpful. Accordingly, adequately powered patient populations are warranted to address this question as a specific endpoint.
The sirolimus-eluting version of the STENTYS stent has received CE mark approval, and, given that the MACE rate in this study was primarily driven by TLR, potentially this could further reduce adverse events.
STUDY LIMITATIONS
This was an observational single-arm study and, as such, operator-driven selection bias could have influenced the decision to stent the SB provisionally, so the conclusion reached should be seen in that perspective. Approximately 44% of the lesions did not involve significant SB disease. The proximal MB, the distal MB, or both were alternatively involved. Since the reference vessel diameter (RVD) of the SB at baseline was somewhat smaller with respect to previously published experiences, the clinical relevance of SB disease might be questionable in the present patient cohort. Accordingly, a significant impact on clinical outcome, particularly on clinically driven TLR, cannot be excluded. However, strut disconnection towards the SB was performed in the majority of cases (56%), meaning that the operator considered that access to the SB was clinically relevant.
Conclusions
The long-term results of OPEN II show that the STENTYS-PES is safe and effective in the treatment of all-comers with coronary bifurcation lesions.
| Impact on daily practice The OPEN II trial demonstrates safety and efficacy of the STENTYS self-apposing PES at long-term follow-up, with a MACE rate contained to 13%, mainly driven by TLR. Strut disconnection, a unique feature of this dedicated bifurcation system, allowed facilitated side branch access in 56% of the cases. Our findings propose the STENTYS self-apposing PES as a valid alternative to the current standard of care (i.e., provisional stenting) for the treatment of complex bifurcation lesions. |
Conflict of interest statement
D. Bouchez is an employee of STENTYS S.A. The other authors have no conflicts of interest to declare.
Supplementary data
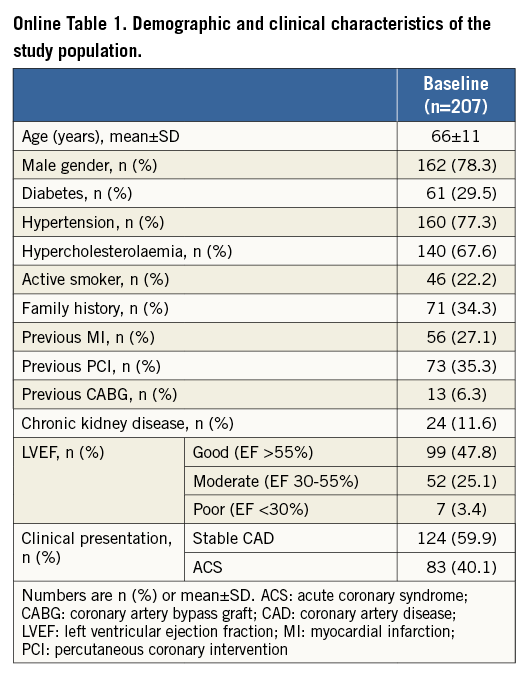
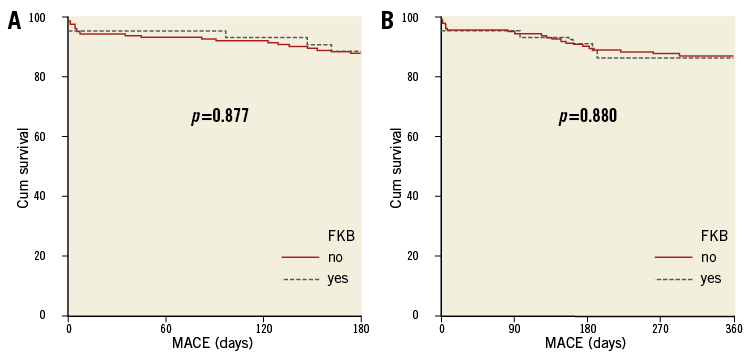
Online Figure 1. Comparison between free-of-MACE survival, as observed between patients who had final kissing balloon inflation (FKBI) and those without FKBI during the index procedure. A) Six-month free-of-MACE survival. B) 12-month free-of-MACE survival.




