Abstract
Aims: The aim of APPOSITION III was to evaluate the feasibility and performance of the STENTYS Self-Apposing® stent (STENTYS S.A., Paris, France) in the setting of primary percutaneous coronary intervention (PCI).
Methods and results: APPOSITION III was an international, prospective, multicentre registry. The study population consisted of 965 patients. The rate of the primary endpoint major adverse cardiac events (MACE), defined as the composite of cardiac death, recurrent target vessel myocardial infarction (TV-MI), and clinically driven target lesion revascularisation (CD-TLR), at one year was 9.3%. One-year cardiac death rate was 2.0%, TV-MI rate was 1.3%, CD-TLR rate was 7.4% and definite/probable stent thrombosis (ST) rate was 3.5% (definite ST 2.8%). An interim safety analysis of in-hospital outcomes in the first 400 patients showed higher event rates if post-dilation was not performed, and post-dilations became highly recommended in the remaining cohort. Patients undergoing post-dilation eventually showed a numerically lower one-year MACE rate (8.4% vs. 11.3%, p=0.137). One-year TV-MI (0.8% vs. 2.5%, p=0.027) and definite ST (1.9% vs. 5.0%, p=0.010) rates were significantly lower if post-dilation was performed, with the divergence occurring at <30 days.
Conclusions: The use of the STENTYS Self-Apposing® stent in the setting of primary PCI was feasible and associated with acceptable cardiovascular event rates which improved when post-dilation was performed.
Introduction
A recent human autopsy study has revealed acute incomplete stent apposition to be one of the important factors in the induction of stent thrombosis (ST) within 30 days after percutaneous coronary intervention (PCI) in acute coronary syndrome patients1. Other studies have shown that late incomplete stent apposition is associated with the occurrence of late and very late ST2,3. Although the exact pathophysiological mechanism is not completely understood, several factors such as induced shear stress and delayed tissue coverage of these incompletely apposed stent struts are likely to play a role4,5. Sequential intravascular imaging studies have shown distinctive phenotypes of malapposition: acute post-procedural, persistent and late acquired. Stent malapposition occurs more frequently after primary percutaneous coronary intervention for the treatment of patients presenting with ST-segment elevation myocardial infarction (STEMI) when compared with elective PCI for the treatment of stable coronary artery disease6. Acute stent malapposition in STEMI is caused by the presence of thrombus7, which may lead to inadequate judgement of true coronary vessel size by the operator, leading to stent undersizing. On the other hand, (late) acquired stent malapposition after primary PCI may hypothetically be explained by: 1) thrombus dissolution behind the stent struts, and 2) vessel wall relaxation after vasoconstriction in the acute phase, in the days following the acute index event, although these mechanisms remain to be proven8. These problems might be addressed by the STENTYS Self-Apposing® stent (STENTYS S.A., Paris, France). Due to its self-expanding features, it adapts itself to the vessel wall. A previous randomised study has shown the superiority of the STENTYS BMS stent compared with conventional balloon-expandable stents in post-procedural stent strut apposition, as assessed with optical coherence tomography9. Furthermore, it has been shown that, when the STENTYS stent was used, there was a decreased rate of malapposed struts and an increased minimal lumen area and stent area three days post procedure compared with directly post procedure8,9. These findings suggest that the STENTYS stent dynamically adapts to changes in the coronary anatomy during the days after the acute index event. However, these studies were limited due to a relatively small sample size and a small number of participating centres and operators. Larger trials including more patients are therefore needed to evaluate whether superior strut apposition, as previously shown8,9, would indeed lead to improvement in clinical outcomes in a contemporary STEMI patient population. Furthermore, by including a large number of sites across different European countries including a large number of different operators, such a trial could also provide information on how the device is best used in daily clinical practice. Therefore, the multicentre observational APPOSITION III study was performed including 1,000 STEMI patients in 51 sites across 14 European countries to evaluate the feasibility and performance of the use of the STENTYS BMS and DES stents in the setting of primary PCI.
Methods
STUDY OVERVIEW
The APPOSITION III study (A Post-Market registry to assess the STENTYS self-exPanding COronary Stent In AcuTe MyocardIal InfarctiON) was a prospective, multicentre, international registry. The inclusion period started in April 2010 and ended after enrolling 1,000 patients, in January 2012. The study was conducted in 51 sites across 14 European countries (Online Appendix 1). The study objective was to evaluate the safety and performance of the STENTYS Self-Apposing® stent during primary PCI for the treatment of patients presenting with STEMI. The use of the STENTYS stent (BMS or DES) was according to the preference of the operator. To warrant safety and the correct use of the device, an interim analysis of in-hospital outcomes was conducted after the inclusion of the first 400 patients. The conduct of the study was in full conformity with the Declaration of Helsinki, and the study was approved by the local medical ethics committees of participating sites. Written informed consent for inclusion in the registry was obtained from every patient included.
DEVICE
The STENTYS Self-Apposing® stent is self-expanding, made from a nickel-titanium alloy called nitinol. STENTYS is compatible with 6 Fr guide catheters, and is delivered using a rapid-exchange delivery system over a conventional 0.014” guidewire. The stent is deployed by withdrawing a retractable sheath using a thumb slider on the handle. The stent is positioned using two markers on the delivery system, indicating the proximal and distal ends of the stent, and using a third marker on the distal end of the outer sheath so that its retraction can be visualised under fluoroscopy. After deployment, the stent expands until it reaches the vessel wall. During the study period, the stent was available in a bare metal version and a drug-eluting version eluting paclitaxel. The paclitaxel elutes (0.8 microgram/mm2 of stent) from a durable matrix of polysulfone (PSU) and a soluble polyvinylpyrrolidone polymer (PVP) which acts as an excipient. The stent was available in two lengths (22 and 27 mm), and in three sizes: a small-sized STENTYS, indicated for placement in vessels with diameters ranging from 2.5-3.0 mm (BMS version only, available from August 2010), a medium-sized STENTYS for placement in vessels with diameters of 3.0-3.5 mm, and a large-sized STENTYS for diameters of 3.5-4.5 mm. The stent conforms to the shape of the artery and can expand further than the above-mentioned diameters: the small size can gradually grow up to 4.0 mm, the medium size up to 5.0 mm, and the large size up to 6.0 mm, if unconstrained (in the case of ectatic/aneurysmal vessels or positive remodelling, for example). The stent has a nominal strut width of 68 microns (0.0027”) and a strut thickness of 102 microns (0.0040”; small-sized STENTYS) or 133 microns (0.0052”; medium- and large-sized STENTYS). At the time of the study, the STENTYS stent was CE marked and available for clinical use in participating centres.
PATIENT POPULATION
Patients presenting with STEMI undergoing routine primary PCI in which the STENTYS stent was used were eligible for inclusion in the registry. Only patients treated for a de novo lesion in a native coronary artery with a reference vessel diameter of ≥2.5 mm and ≤4.5 mm (visual estimate) were included in the registry, as pre-specified by the protocol. Patients with one of the following criteria were excluded: culprit lesion in the unprotected left main; presentation in cardiogenic shock; any vasculature or lesion characteristic preventing the introduction or placement of the study stent; known allergies or contraindications to antiplatelet medication or stent components; or female patients with child-bearing potential. Patients included in the final study population were those who met all of the above-mentioned criteria and in whom a STENTYS stent had been placed successfully (Figure 1).
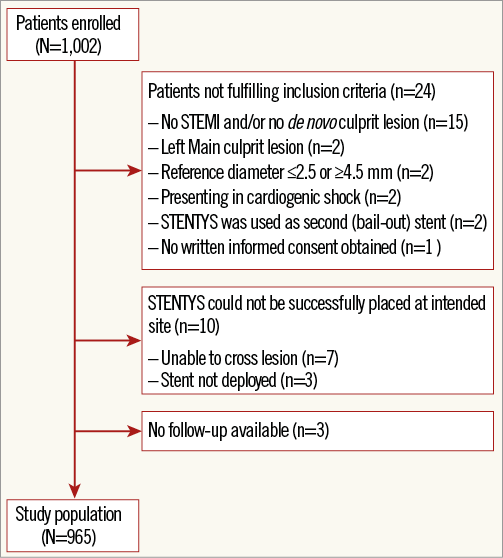
Figure 1. Study population flow chart.
STUDY PROCEDURE AND MEDICATION
Medication administered pre procedure, during PCI and post procedure were in accordance with local standards of care. The choice to use the STENTYS stent was according to the preference of the operator. Lesion preparation, including thrombus aspiration and predilation, and the choice between a bare metal or a paclitaxel-eluting STENTYS stent, if available, was at the discretion of the operator. Preparation and deployment of the stent were according to the instructions for use. During the first phase of the study, post-dilation was left to the discretion of the operator, and was recommended only if the residual stenosis was more than 30%. After the interim analysis of 400 patients was performed, the Steering Committee recommended that the stent should be post-dilated, regardless of the severity of the residual lesion. It was recommended to use normal-sized (i.e., balloon-to-reference vessel ratio <1.1:1) non-compliant balloons at relatively low pressures, starting at 8 atm. This recommendation was based on a higher rate of events observed in patients in whom post-dilation was not performed.
DATA COLLECTION AND MANAGEMENT
All baseline, angiographic (visual estimates), and follow-up data were prospectively collected and independently monitored, and all potential endpoints were adjudicated by an independent clinical events committee (full details on data collection and study management are contained in Online Appendix 2).
STUDY ENDPOINTS
The primary endpoint of the APPOSITION III study was the occurrence of major adverse cardiac events (MACE) at one-year follow-up. MACE was defined as the composite of cardiac death, recurrent target vessel myocardial infarction (TV-MI), and clinically driven target lesion revascularisation (TLR). Secondary endpoints included 30-day and one-year death from any cause, cardiac death, TV-MI, any myocardial infarction (MI), clinically driven TLR, any target vessel revascularisation (TVR), definite ST, probable ST, and the combined endpoints of cardiac death/TV-MI, death from any cause/any MI, death from any cause/any MI/any TVR, and definite/probable ST, as well as 30-day MACE.
All endpoint definitions were according to the Academic Research Consortium (ARC) consensus and are described in detail in Online Appendix 310.
STATISTICAL METHODS
Continuous variables were presented as mean (± standard deviation) or median (interquartile range), where appropriate. Categorical variables were presented as frequencies (percentage). Cumulative event rates were estimated with the Kaplan-Meier method. Follow-up was censored at one year or at last known date of follow-up, whichever came first. Cumulative event rates in different subgroups were compared using the log-rank test. Independent predictors for 30-day MACE were identified by univariate and multivariate Cox proportional hazard models. All hazard ratios (HR) with a p-value <0.10 in the univariate analysis were entered into a multivariate model. HR with a p<0.05 in the multivariate analysis were considered significant. Furthermore, to appreciate differences in early (<30 days) and late (from 30 days up to one year) occurrence of events, we used landmark analyses with the landmark set at 30 days. Statistical analyses were performed at the Academic Medical Center (Amsterdam, The Netherlands) using SPSS software package, version 19.0 (IBM Corp., Armonk, NY, USA).
Results
STUDY POPULATION
A total of 1,002 patients who underwent primary PCI between April 2010 and January 2012 were entered into the electronic database. Twenty-four patients did not meet the pre-specified criteria for inclusion in the registry, and in another 10 patients the STENTYS stent could not be placed. Three patients were lost to follow-up. These patients were excluded from the analysis, resulting in a final study population consisting of 965 patients (Figure 1).
BASELINE CLINICAL, ANGIOGRAPHIC AND PROCEDURAL CHARACTERISTICS
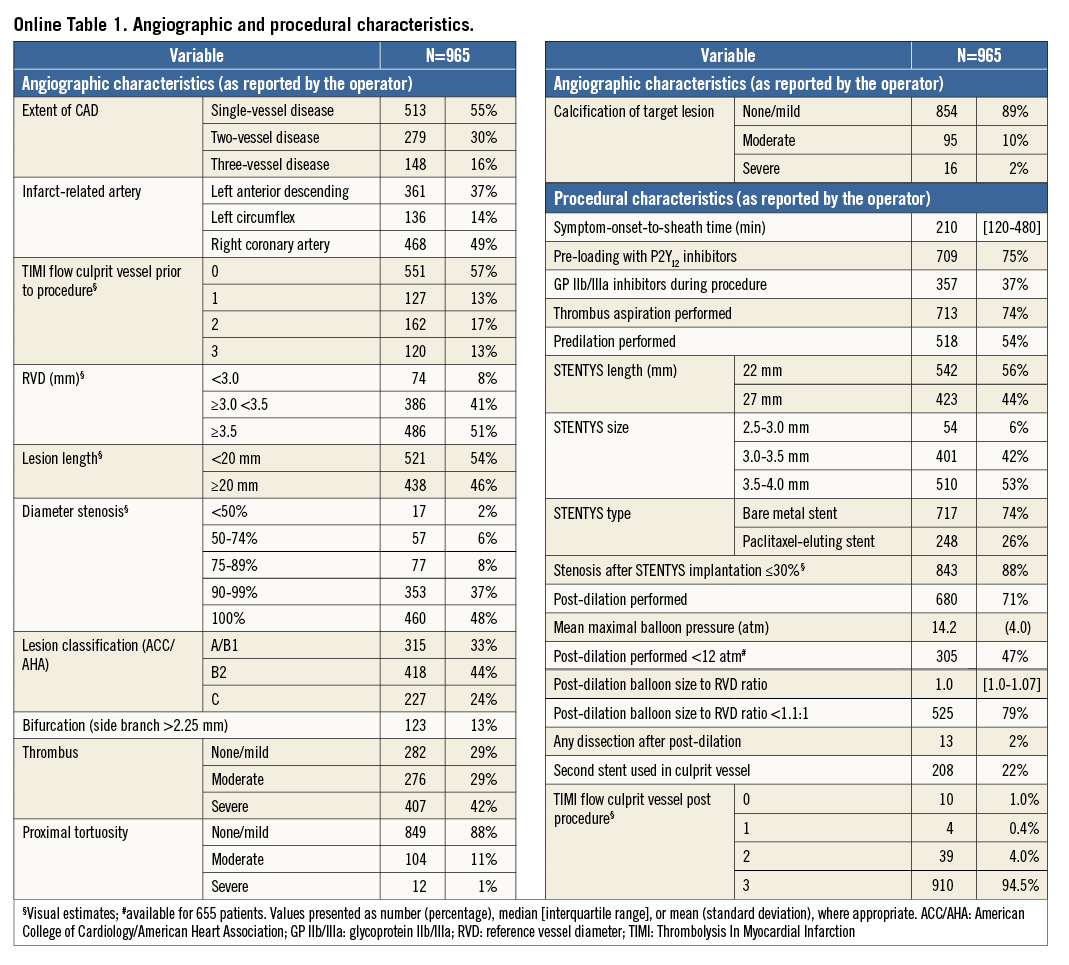
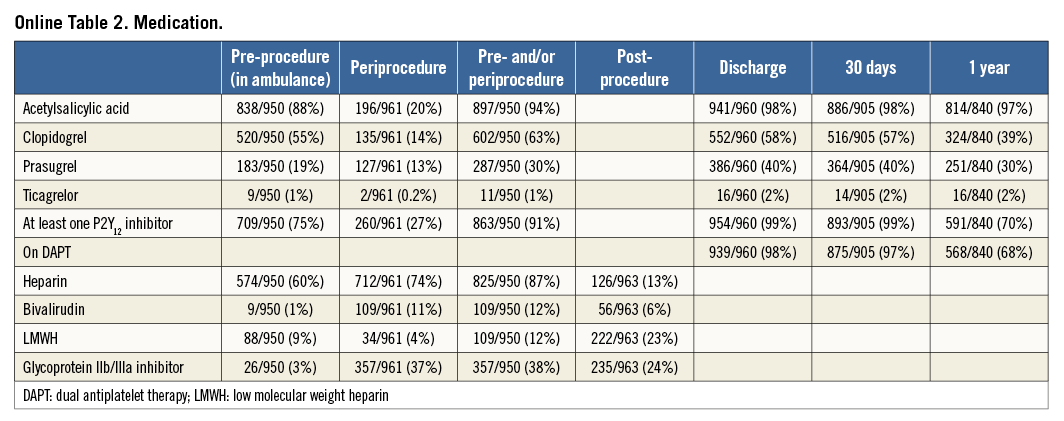
Baseline clinical characteristics are shown in Table 1. Mean age was 60 (±12) years, with the majority being male (77%). Angiographic and procedural characteristics are shown in Online Table 1. Median symptom-onset-to-sheath time was 210 minutes. The vast majority (75% of patients) were pre-loaded with a P2Y12 inhibitor. Pre-procedural Thrombolysis In Myocardial Infarction (TIMI) flow 0/1 was seen in 70% of patients. Thrombus aspiration was performed in 74% of patients and predilatation in 54%. Most (74%) STENTYS stents used were bare metal. Post-dilation was performed in 71% of patients. Ninety-five percent of the patients had TIMI 3 flow on their final angiogram. Pre- and periprocedural medication, and medication at discharge, 30-day and one-year follow-up are outlined in Online Table 2.
CLINICAL OUTCOMES
The thirty-day and one-year clinical outcomes are shown in Table 2. MACE occurred in 86 patients (one-year MACE rate of 9.3%). Twenty-nine patients died, 19 from a cardiac cause, resulting in a one-year cardiac death rate of 2.0%. TV-MI was observed in 12 patients (one-year TV-MI rate of 1.3%) (Figure 2). Clinically indicated TLR was performed in 68 patients (7.4%). Definite/probable ST occurred in 33 patients (27 definite), resulting in a one-year definite/probable ST rate of 3.5% (definite ST rate 2.8%).
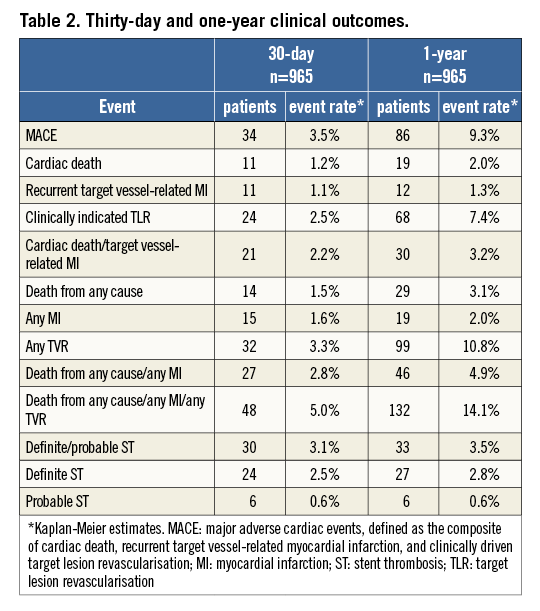
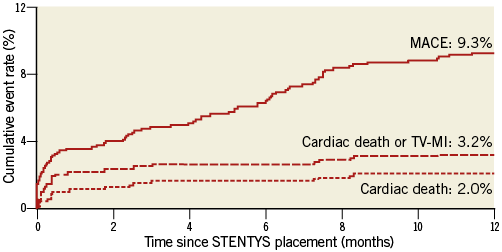
Figure 2. One-year Kaplan-Meier event curves. One-year Kaplan-Meier event curves for major adverse cardiac events (MACE), cardiac death and cardiac death/target vessel myocardial infarction (TV-MI).
0Subgroup analyses
POST-DILATION
The post-dilation rate was 60% in the first 400 patients and 77% in the subsequent 565 patients, after the recommendation of the Steering Committee to perform post-dilation.
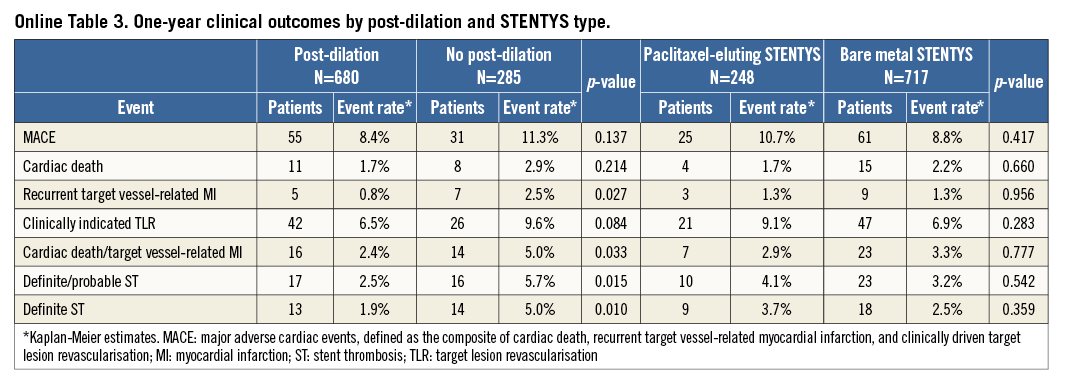
Patients in whom post-dilation was performed showed similar final TIMI 3 flow rates when compared with patients in whom post-dilation was not performed (94% vs. 95%, p=0.836). The one-year MACE rate was numerically lower in patients in whom post-dilation was performed compared with those in whom post-dilation was not performed, although this was not statistically significant (8.4% vs. 11.3%, p=0.14). One-year cardiac death/TV-MI (2.4% vs. 5.0%, respectively, p=0.033) and definite ST (1.9% vs. 5.0%, respectively, p=0.010) rates were lower in patients in whom post-dilation was performed compared with those in whom post-dilation was not performed (Online Table 3).
Landmark analyses showed that these differences in clinical outcomes occurred early, with significantly lower MACE rates at 30-day follow-up in patients in whom post-dilation was performed compared with those in whom post-dilation was not performed (2.7% vs. 5.6%, p=0.02) (Figure 3). The unadjusted hazard ratio (HR) of post-dilation for MACE was 0.47 (95% CI: 0.24-0.92, p=0.027). After adjustment for variables associated with MACE in the univariate analyses (not a current smoker, GP IIb/IIIa inhibitor use, LCx and LAD location), the HR was unaltered (HR 0.49, 95% CI: 0.25-0.96, p=0.03). The same trend was also observed for the individual components of MACE at 30-day follow-up: cardiac death (0.9% vs. 1.8%, p=0.245), TV-MI (0.6% vs. 2.5%, p=0.013), and clinically driven TLR (1.6% vs. 4.6%, p=0.008). Similarly, the occurrence of definite/probable stent thrombosis (2.2% vs. 5.3%, p=0.013) and definite stent thrombosis (1.6% vs. 4.6%, p=0.008) was also significantly reduced in the post-dilation group. In contrast, beyond the 30-day landmark, there were no differences in clinical outcomes observed between the two groups up to one year, with the event curves coinciding (Figure 3).
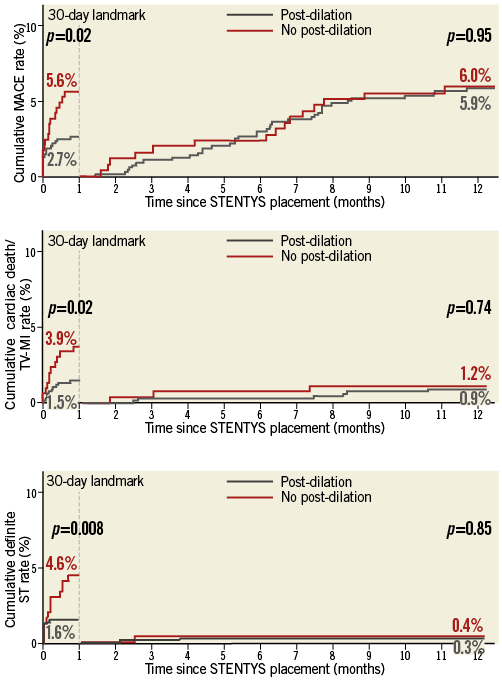
Figure 3. One-year Kaplan-Meier curves with 30-day landmark analysis. One-year Kaplan-Meier event curves for major adverse cardiac events (MACE; upper panel), cardiac death/target vessel myocardial infarction (TV-MI; middle panel), and definite stent thrombosis (lower panel).
DES VERSUS BMS
Compared to the STENTYS BMS patient group, the patients treated with the STENTYS DES were older (62±12 vs. 59±12 years, p=0.016) and there were more diabetic patients (26% vs. 11%, p=<0.00001), more patients with hypertension (55% vs. 45%, p=0.003), more patients with hypercholesterolaemia (51% vs. 39%, p=0.0004), more patients with a previous MI (7.5% vs. 4.0%, p=0.029), more patients with a previous PCI (8.5% vs. 4.6%, p=0.022), and more patients with a bifurcation lesion (17% vs. 11%, p=0.022).
One-year MACE rates were equivalent between the STENTYS DES and STENTYS BMS groups (10.7% vs. 8.8%, respectively, p=0.42) (Figure 4, Online Table 3). This also applied to the other endpoints, including clinically indicated TLR (9.1% vs. 6.9%, respectively, p=0.28), the combined endpoint of cardiac death/TV-MI (2.9% vs. 3.3%, respectively, p=0.78), and definite ST (3.7% vs. 2.5%, respectively, p=0.36) (Online Table 3).
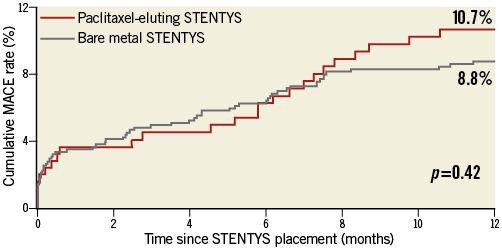
Figure 4. One-year major adverse cardiac event (MACE) rates for bare metal and paclitaxel-eluting STENTYS stent subgroups.
Discussion
This study evaluated one-year clinical outcomes of patients presenting with STEMI who were treated with primary PCI using the STENTYS Self-Apposing stent. The primary endpoint of MACE at one year occurred in 9.3% of the patients. When post-dilation was performed, clinical outcomes were significantly improved, with one-year cardiac death/TV-MI and definite ST rates as low as 2.4% and 1.9%, respectively. With a landmark set at thirty days, we demonstrated that the benefit of post-dilation was achieved early (i.e., <30 days). On the contrary, beyond 30 days there were no differences in clinical outcomes between patients in whom post-dilation was performed and those in whom post-dilation was not performed. This demonstrates that the early benefit of post-dilation did not incur a trade-off in relation to late events.
COMPARISON WITH HISTORICAL STEMI STUDIES
Meta-analyses comparing first-generation DES with BMS in the setting of primary PCI in STEMI patients have shown one-year death rates of ~4.2%, one-year cardiac death rates of ~3.2%, one-year recurrent MI rates of 3.2-3.6%, and one-year ST rates of 1.9-2.7%11-13.
Results from more contemporary randomised trials on second- and third-generation DES in STEMI patients have demonstrated lower event rates. In the XAMI, COMFORTABLE-AMI, and EXAMINATION trials, one-year death rates were around 2.0%-4.1%, cardiac death rates around 1.5%-3.5%, one-year recurrent MI rates 0.5%-3.7%, and one-year definite/probable ST rates around 0.9%-3.7% (of which the definite ST rate was around 0.5%-0.9% in the DES arms, and 1.9%-2.1% in the BMS arms)14-16. The current study showed one-year clinical outcomes which were comparable with these historical data with regard to death (3.1%), cardiac death (2.0%), and TV-MI (1.3%), while definite/probable ST (3.5%) and definite ST (2.8%) rates were somewhat higher, especially when compared with the newest-generation DES treatment arms. In patients in whom post-dilation was performed, one-year cardiac death (1.7%), recurrent MI (0.8%), probable/definite ST (2.5%), and definite ST (1.9%) rates compared well with the historical data, although it has to be pointed out that definite ST rates were considerably lower in the everolimus-eluting stent group of the EXAMINATION trial (0.5%) and in the biolimus-eluting stent group of the COMFORTABLE-AMI trial (0.9%) (definite ST rates in the BMS arms of the same trials were 1.6% and 2.1%, respectively)14-16.
Comparing the results of the current study with historical data should be done with caution, since most STEMI trials allowed the inclusion of patients with poor left ventricular function and patients who were in cardiogenic shock (i.e., Killip class IV), both major determinants of clinical outcomes, while in the current study left ventricular function and Killip class were unknown (and patients in cardiogenic shock excluded). Data from randomised trials directly comparing STENTYS with balloon-expandable stents are needed.
THE NEED FOR POST-DILATION
The thirty-day results of the APPOSITION III study have demonstrated that post-dilation was beneficial in STEMI patients treated with the STENTYS stent. Although these improvements in clinical outcomes could have been a result of selection bias, selecting those patients with a presumably lower risk of distal embolisation (i.e., low thrombus burden, for instance), it was found that the HR of post-dilation for the occurrence of MACE remained unaltered after multivariable adjustment, suggesting that the benefit of post-dilation remained irrespective of other confounding factors. As a consequence, post-dilation with normal-sized (i.e., balloon-to-reference vessel ratio <1.1:1), non-compliant balloons starting at relatively low pressures (8 atm) became strongly recommended in every STENTYS procedure during the study.
Whereas first-generation self-expanding stents, such as the WALLSTENT™ (Boston Scientific, Marlborough, MA, USA), with a stainless steel braided design, caused chronic outward force, resulting in chronic vessel wall injury with a subsequent increase of neointimal hyperplasia17, the newer-generation STENTYS Self-Apposing nitinol stent is designed in such a way that the chronic outward force is low and progressively diminishes as the stent grows. As a consequence, the self-expanding force of the device alone is probably not sufficient to treat a stenotic lesion, preventing adequate stent expansion when post-dilation is not performed. However, the resistive outward force against vessel compression is high, allowing low residual stenosis after balloon post-dilation. Previous intravascular imaging studies have shown that inadequate stent expansion, including stent eccentricity, is strongly associated with the occurrence of ST18,19. One might speculate that the higher ST rate we found in patients without post-dilation may have been caused by stent underexpansion rather than stent malapposition. Gentle post-dilation with normal-sized balloons in the current study most likely enabled adequate stent expansion, preventing underexpansion and stent eccentricity, and thus resulted in definite ST rates as low as 1.6% at 30 days and 1.9% at one year. This hypothesis is further supported by the fact that the benefit of post-dilation was limited to the first 30 days of follow-up, whereas no differences in clinical outcomes were observed between 30 days and one year. This again suggests that the early events occurring in patients without post-dilation are related to problems arising acutely, such as inadequate expansion.
The need for post-dilation, even with normal-sized balloons starting at low pressures, will probably result in less benefit, or even a complete lack of benefit, for the prevention of distal embolisation of thrombotic material when using STENTYS. The APPOSITION IV and APPOSITION V trials both include post-procedural myocardial blush grade as one of the secondary endpoints, and the results of these trials will therefore give us further insight as to whether the STENTYS stent could indeed prevent distal embolisation in STEMI patients, even if the stent is systematically post-dilated.
DES VERSUS BMS
Interestingly, we did not find differences in clinical outcomes between the BMS and the paclitaxel-eluting versions of the STENTYS. This might be explained by patient selection, due to the non-randomised nature of this comparison, the play of chance (small sample in the DES group), and/or a true lack of efficacy due to an unfavourable interplay between drug, polymer and tissue. Future studies with the STENTYS DES version are needed to confirm a superior efficacy with DES versus BMS, as has been shown with balloon-expandable stents. It is noteworthy that a sirolimus-eluting version has been developed and is currently under clinical investigation.
Limitations
Being a registry, a selection bias has occurred. Left ventricular function and Killip class, both important predictors for outcome, were not collected. Therefore, any comparison with historical data from previous STEMI trials needs to be done with great caution. Although baseline and angiographic characteristics seem to be comparable with previous primary PCI studies20,21, we cannot exclude that patients with a relatively better prognosis were included in the current study. Furthermore, the recommendations for post-dilation were changed during the course of the study, but not made binding (i.e., did not become mandatory). Therefore, post-dilation was still left to the discretion of the operator. As a result, it is unclear whether the clinical outcomes in the post-dilation group can be generalised to the general population since selection of patients at lower risk cannot be excluded. However, a multivariate adjustment for 30-day outcomes has shown an unaltered hazard ratio of post-dilation for the occurrence of MACE. The use of both a paclitaxel-eluting STENTYS and a bare metal STENTYS may have made clinical outcome results of the current study difficult to interpret. Moreover, QCA analyses were not performed systematically, which would have given us more insight into the relationship between residual stenosis after STENTYS placement and the occurrence of (early) clinical events, especially in the group which did not receive post-dilation. Furthermore, a systematic assessment of the blush grade would have given us more insights into whether post-dilatation resulted in an increased risk of distal embolisation of thrombotic material or plaque debris. Moreover, in the setting of primary PCI, it is difficult to detect the occurrence of periprocedural MI. We found some discrepancies between the definite ST (2.8%, 27 patients) and TV-MI (1.3%, 12 cases) rates. An explanation for this discrepancy is that 13 of the 27 definite ST cases occurred the same day as the index procedure. The CEC did not adjudicate these events as recurrent MI in 12 cases, most likely because they did not fulfil the criteria for recurrent MI (“Recurrent MI was defined as stable or decreasing values on two samples and 20% increase three to six hours after the second sample”) (Online Appendix 3). However, this limitation could be applied to every stent study performed in a STEMI population. Finally, in some centres patients were included in the registry after the procedure. Since the stent obtained a CE mark during the course of the study, the interventional cardiologists were formally allowed to implant the STENTYS without specific consent from the patient prior to the procedure and some centres asked for informed consent after the procedure for the collection of the data. As a consequence, the cases of unsuccessful STENTYS placement are most likely underreported (10 cases) (Figure 1), and therefore procedural success cannot be reliably calculated.
Conclusions
The use of the STENTYS Self-Apposing® stent was feasible in the setting of primary PCI with an acceptable cardiovascular event rate at one year. Clinical outcomes improved considerably when post-dilation was performed, and post-dilation became strongly recommended to ensure adequate stent expansion. Randomised trials comparing the STENTYS stent with contemporary balloon-expandable DES in the setting of primary PCI are needed.
| Impact on daily practice With the current study we have shown that the use of STENTYS in the setting of primary PCI was feasible, with acceptable one-year cardiovascular outcomes, but future randomised trials comparing STENTYS with second- or third-generation DES are needed before its use can be accepted as the standard of care. However, in certain anatomic subsets in which good apposition is unlikely to be achieved with conventional balloon-expandable stents, such as lesions with a very high thrombotic burden, lesions located in ectatic/aneurysmal vessels, or bifurcation lesions with large differences between the proximal and distal diameters (including left main), it seems reasonable to consider the use of STENTYS. When STENTYS is used, it should be post-dilated to ensure adequate stent and vessel expansion. |
Acknowledgements
The authors would like to thank Katherin Awad for her contribution to the manuscript.
Funding
The APPOSITION III study and associated data analysis were funded by STENTYS S.A., Paris, France.
Conflict of interest statement
M. Grundeken, K. Koch, J. Tijssen, G. Montalescot, J. Silvain, and G. Amoroso have received research grants to their institutions from STENTYS. R-J. van Geuns and R. Wessely are members of the scientific advisory board of STENTYS. G. Amoroso, R-J. van Geuns and R. Wessely have received speaker’s fees from STENTYS. A. IJsselmuiden has received consultancy fees from STENTYS. R. Spaargaren is an employee of STENTYS. The other authors have no conflicts of interest to declare.
Online data supplement
Online Appendix 1. Detailed list of investigators and institutions (51 sites in 14 countries) enrolling patients in the APPOSITION III study
The Netherlands: (total enrolment=426): OLVG, Amsterdam - G. Amoroso (102); AMC, Amsterdam - K.T. Koch (100); Albert Schweitzer Hospital, Dordrecht - A. IJsselmuiden (90); Erasmus Medical Centre, Rotterdam - R-J. van Geuns (65); St. Antonius Ziekenhuis, Nieuwegein - M.J. Suttorp (39); Vumc, Amsterdam - N. van Roijen (17), Scheper Ziekenhuis, Emmen - G. Jesserun (8); Amphia Ziekenhuis, Breda - P. den Heyer (5).
Germany: (total enrolment=177): SLK-Kliniken Heilbronn, Heilbronn - T. Dengler (43); Evang. Bethesda Johanniter, Duisburg - R. Wessely (43); Klinikum Neuperlach, Munich - H. Mudra (22); Elisabeth-Krankenhaus, Essen - C. Naber (21); Klinikum Coburg - H. Rittger (19); Universitätsklinikum Giessen und Marburg - H. Nef (16); Herz- und Diabeteszentrum NRW, Bad Oeynhausen - M. Wiemer (8); Klinikum der Universität, Munich - V. Klauss (4); Klinikum Bogenhausen, Munich - J. Rieber (1).
France: (total enrolment=136): CHU La Pitié-Salpêtrière, Paris - G. Montalescot (40); Clinique Saint-Hilaire, Rouen - J. Berland (32); Institut Hospitalier Jacques Cartier, Massy - T. Lefevre (28); CHU Henri Mondor, Créteil - E. Teiger (19); Centre Cardiologique d’Evecquemont - P. Brenot (9); Clinique de l’Europe, Amiens - A. Py (5); La Roseraie, Aubervilliers - H. Benamer (2); Centre Hospitalier Victor Dupouy, Argenteuil - T. Carreres (1).
Belgium: (total enrolment=53): Ziekenhuis Oost-Limburg, Genk - M. Vrolix (22); ZNA Middelheim, Antwerp - S. Verheye (18); Universitair Ziekenhuis, Brussels - D. Schoors (13).
Italy: (total enrolment=50): Ferrarotto Hospital, Catania - C. Tamburino (40); Ospedale della Misericordia, Grosseto - U. Limbruno (7); Complesso Ospedaliero San Giovanni Addolorata, Rome - F. Prati (3).
Switzerland: (total enrolment=48): Kantonsspital St. Gallen - D. Weilenmann (19); University Hospital Basel - C. Kaiser (12); CHUV Lausanne - E. Eeckhout (10); HFR Université de Fribourg - S. Cook (7).
Poland: (total enrolment=48): Klinika Kardiologil Wojskowy, Wroclaw - K. Reczuch (26); Bieganski Hospital, Łódź - Z. Peruga (13); University Hospital Krakow - D. Dudek (6); American Heart of Poland S.A, Bielsko-Biala - P. Buszman (2); WIM, Warsaw - W. Wąsek (1).
Spain: (total enrolment=30): Hospital Universitario La Paz, Madrid - R. Moreno (9); Hospital Clinic i Provincial, Barcelona - M. Sabaté (8); Hospital Clínico Universitario San Carlos, Madrid - R. Hernández-Antolín (7); Complejo Hospitalario Universitario de Vigo - Meixoeiro, Vigo - A. Iñiguez (4); Hospital Puerta de Hierro-Majadahonda, Majadahonda (Madrid) - J. Goicolea (2).
Sweden: (total enrolment=12): University Hospital, Orebro - O. Frøbert (12).
Finland: (total enrolment=8): Satakunta General Hospital, Pori - P. Karjalainen (8).
Czech Republic: (total enrolment=5): University Hospital Brno, Brno - P. Kahla (5).
United Kingdom: (total enrolment=4): East & North Hertfordshire NHS Trust, Welwyn Garden City - N. Kukreja (4).
Denmark: (total enrolment=3): Rigshospitalet, Copenhagen - T. Engstrøm (3).
Austria: (total enrolment=2): Landesklinikum Thermenregion Mödling, Mödling - F.X. Roithinger (2).
Online Appendix 2. Detailed description of study organisation and management
Baseline clinical, angiographic (visual estimates), and procedural characteristics were prospectively collected and stored in an electronic database (e-capture, genae associates). Data collection was coordinated by an external data coordinating centre (genae associates, Antwerp, Belgium). Every site was periodically monitored with 100% source verification. In cases of repeat revascularisation of the target vessel, coronary angiograms were collected and quantitative coronary angiography (QCA) analyses were performed by an independent core lab (Cardialysis, Rotterdam, The Netherlands) to assess lesion severity. In case of a potential major adverse cardiac event (MACE), all source documentation, including the QCA core lab report in case of a repeat revascularisation, was collected and assessed by an independent clinical events committee (CEC) for endpoint adjudication.
The following individuals and organisations contributed to the APPOSITION III study:
Steering Committee: G. Amoroso, OLVG, Amsterdam, The Netherlands; G. De Luca, Azienda Ospedaliera “Maggiore della Carità”, Novara, Italy; U. Limbruno, Ospedale della Misericordia, Grosseto, Italy; M. Wiemer, Herz- und Diabeteszentrum NRW, Bad Oeynhausen, Germany.
Clinical Events Committee: P.F. Agostoni, UMC Utrecht, Utrecht, The Netherlands; G.J. Laarman, Tweesteden Ziekenhuis, Tilburg, The Netherlands.
Site management and data monitoring: genae associates, Antwerp, Belgium.
Data analysis: M.J. Grundeken & J.G.P. Tijssen, AMC, Amsterdam, The Netherlands.
Online Appendix 3. Detailed endpoint definitions
All deaths were considered cardiac unless they could unequivocally be attributed to a non-cardiac cause. Spontaneous MI was defined as any rise in creatine kinase isoenzyme MB (CKMB) above the upper limit of normal. Recurrent MI was defined as stable or decreasing values on two samples and 20% increase three to six hours after the second sample. All recurrent MIs were considered to be target vessel-related unless the MI could unequivocally be related to a non-target-vessel culprit lesion. If CKMB was not available, troponins were used for adjudication.
TVR was defined according to the ARC definitions as any revascularisation performed on the target vessel either by PCI or by coronary artery bypass graft (CABG) surgery. TLR was defined as any percutaneous or surgical revascularisation of the target vessel to treat a stenosis of the target lesion (from 5 mm proximal to 5 mm distal to the stent). Clinically indicated TLR was defined as any TLR involving a percent diameter restenosis ≥70% of the target lesion on coronary angiography. If the percent diameter restenosis was between 50% and 70%, a TLR was considered clinically indicated if one of the following applied: 1) a positive history of recurrent angina pectoris, presumably related to the target vessel; 2) objective signs of ischaemia at rest (ECG changes) or during an ischaemia detection test, presumably related to the target vessel; or 3) abnormal results of any invasive functional diagnostic test of the target lesion (e.g., Doppler flow velocity reserve, fractional flow reserve). A TLR involving a percent diameter restenosis <50% was considered not to be clinically indicated. ST was defined according to the ARC definitions11.