Abstract
Aims: Next to patient characteristics, the lack of a standardised approach for bioresorbable vascular scaffold (BVS) implantation is perceived as a potential explanation for the heterogeneous results reported so far. To provide some guidance, we sought to find a consensus on the best practices for BVS implantation and management across a broad array of patient and lesion scenarios.
Methods and results: Fourteen European centres with a high volume of BVS procedures combined their efforts in an informal collaboration. To get the most objective snapshot of different practices among the participating centres, a survey with 45 multiple choice questions was prepared and conducted. The results of the survey represented a basis for the technical advice provided in the document, whereas areas of controversy are highlighted.
Conclusions: Consensus criteria for patient and lesion selection, BVS implantation and optimisation, use of intravascular imaging guidance, approach to multiple patient and lesion scenarios, and management of complications, were identified.
Introduction
Current-generation drug-eluting stents (DES) hold several advantages over previous-generation DES and bare metal stents1. However, permanent metallic caging of the coronary vessel by contemporary DES is still perceived as a limitation, in that it abrogates vasomotion, thereby preventing late expansive remodelling.
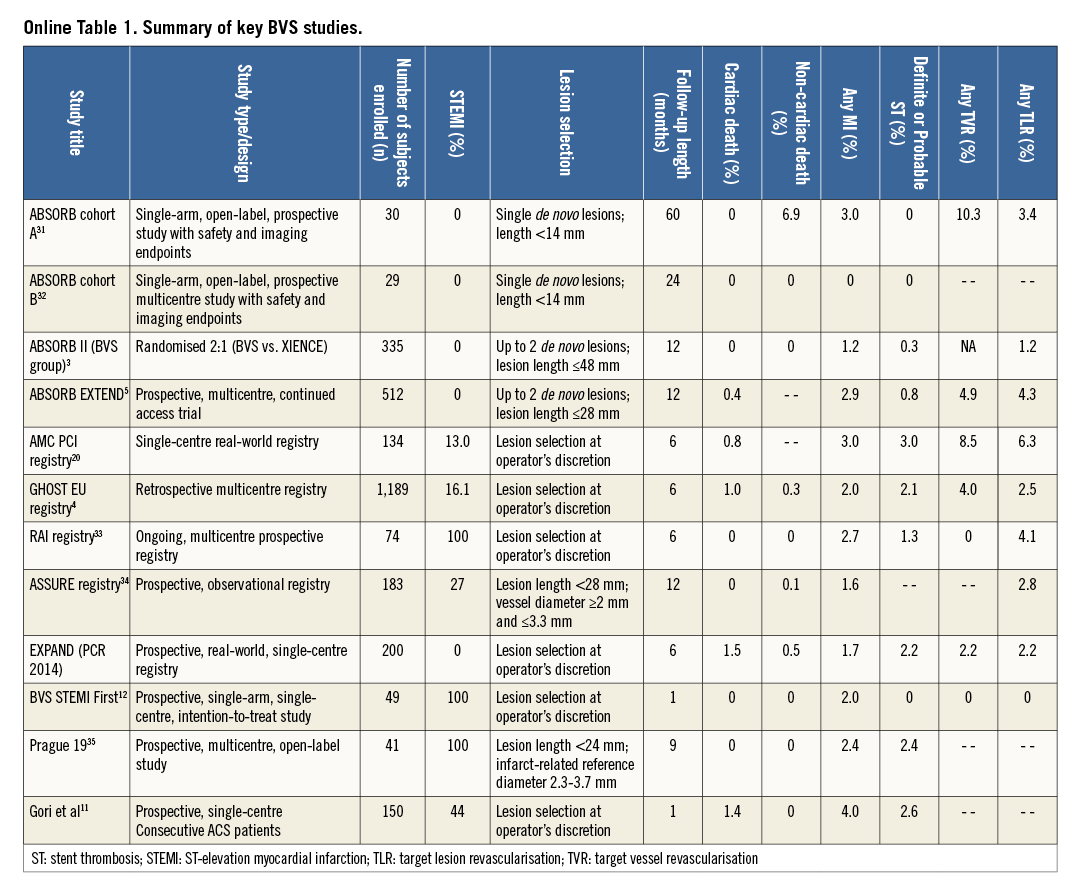
Bioresorbable scaffolds promise complete bioresorption after two to three years, vessel lumen enlargement, reduction of the plaque:media ratio, and restoration of vasomotion2. Most of the available experience and data regarding these devices have so far been generated with the everolimus-eluting Absorb bioresorbable vascular scaffold (BVS) (Abbott Vascular, Santa Clara, CA, USA). In the ABSORB II trial, BVS showed similar one-year composite secondary clinical results compared to everolimus-eluting DES in relatively simple lesions3. Conversely, heterogeneous outcomes have been reported from registries including more complex populations than those investigated in ABSORB II (Online Table 1)4,5.
Next to patient characteristics, the lack of a standardised approach when implanting BVS is perceived as a potential explanation for the varying outcomes reported so far6. To provide some guidance, a group of interventional cardiologists from high-volume centres across Europe, all with specific expertise and experience regarding PCI with BVS, sought to find a consensus on the best practices for BVS implantation and management across a broad array of patient and lesion scenarios (details on the objectives and methodology can be found in the Online Appendix).
Device description
A full description of the device is given in the Online Appendix.
General implantation rules
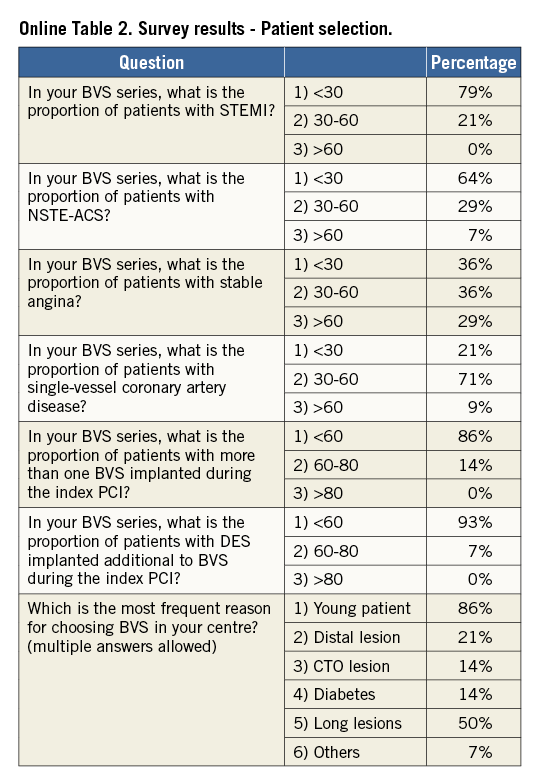
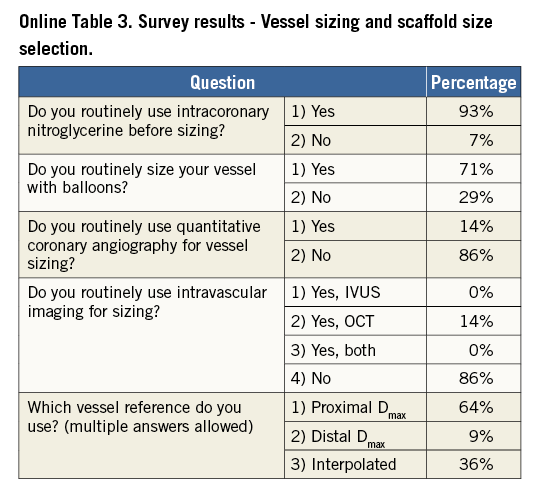
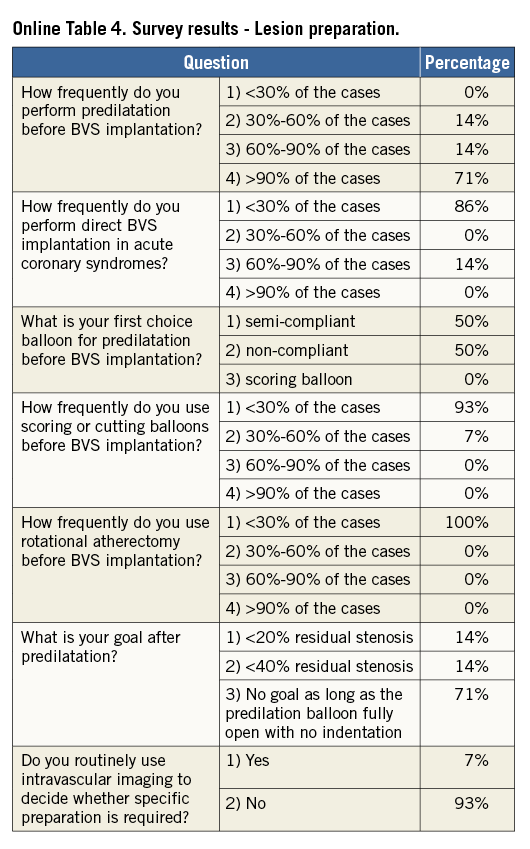
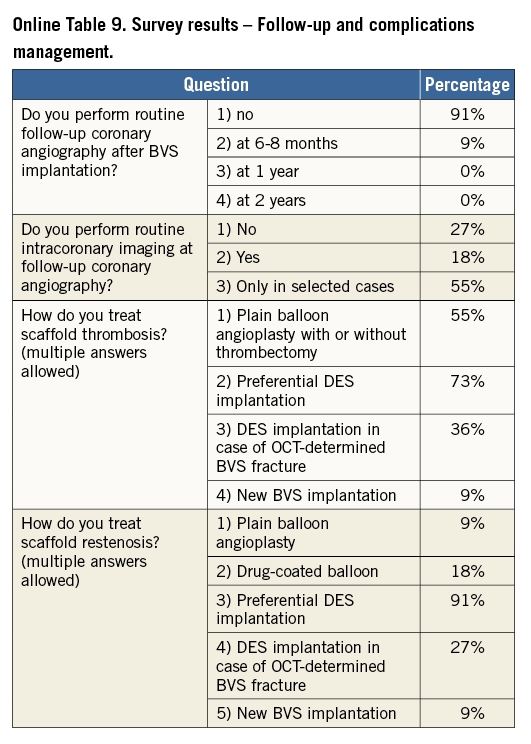
Online Table 2-Online Table 9 illustrate the results of a survey conducted across the participating centres to facilitate reaching a consensus on the most accepted and advisable practices for BVS use. The results of this survey constitute the background for the advice provided in the present document, with areas of controversy highlighted where necessary. A standard operating protocol for BVS use is provided as a practical guide for new users of BVS (Figure 1).
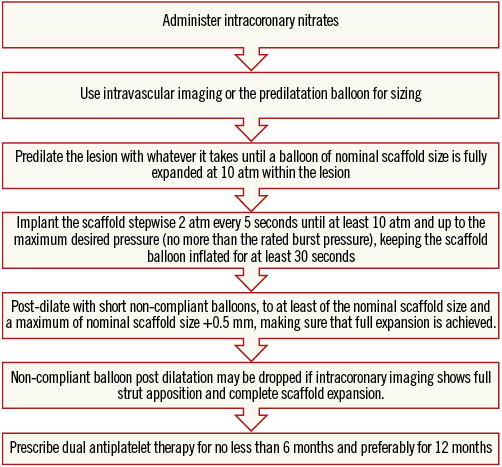
Figure 1. Practical operating protocol for new users of BVS.
EVALUATION OF PATIENT AND LESION SUITABILITY
New users of BVS should ideally build up their experience gradually, starting with simple lesions, and, as experience is gained, more complex lesions can be treated. In the authors’ opinion, patients who may particularly benefit from BVS implantation are typically young and/or present with long lesions, as long chains of metallic stents increase the risk for late failure. As a general rule, BVS should not be implanted into lesions that cannot be adequately prepared with balloon inflations, particularly when the balloon used for predilatation cannot be fully expanded or when the result of dilation is unsatisfactory (i.e., residual stenosis >40%). Indeed, due to the relatively thick struts, underexpansion of BVS represents a potential risk for scaffold thrombosis or restenosis. Considerations on lesion suitability should also include a careful evaluation of the vessel location (proximal versus distal), vessel size and plaque morphology. The ability to reach the target lesion with the device can be jeopardised by extreme vessel tortuosity before the stenosis, especially in the presence of heavy calcifications. Finally, it should be emphasised that the 3.5 mm BVS should not be expanded above 4.0 mm, a threshold above which the polymeric struts might break, which limits current use in coronary segments with a large diameter such as the left main coronary artery.
VESSEL SIZING AND SCAFFOLD SELECTION
The polymeric nature of BVS leads to physical limitations that should be taken into account before deployment, since improper handling of their limited ability for overexpansion can lead to disruption of scaffold integrity within the vessel. Hence, meticulous vessel sizing before BVS implantation is crucial for procedural success.
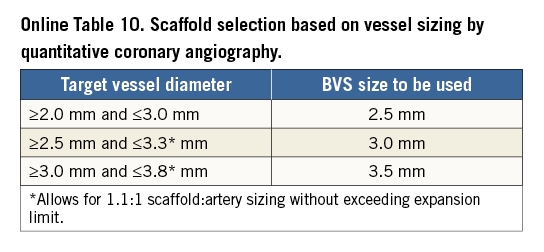
Conventional practice is based on a careful review of the angiogram comparing the vessel lumen with the known dimensions of the guiding catheter after administration of an intracoronary bolus of nitroglycerine. If sufficiently large balloons are used for predilatation, which is advisable, they can also help sizing. In case of doubt regarding vessel size, it is advisable to upsize the scaffold (Online Table 10). As compared to visual estimation, quantitative coronary angiography provides information that is more objective, but it also tends to underestimate the lumen diameter, offering no information on the true vessel size (Online Appendix). Intravascular imaging with intravascular ultrasound (IVUS) or optical coherence tomography (OCT) is widely considered the gold standard for scaffold size selection, although in the authors’ experience it is not routinely used and not absolutely required for this purpose (Figure 2). Pragmatically, except in case of extreme vessel tapering, scaffold selection should follow the proximal maximal vessel diameter or should be based on the interpolated method. Sequential implantation of scaffolds with different diameters can also help to accommodate different vessel reference diameters at the proximal and distal ends of the lesion when required.
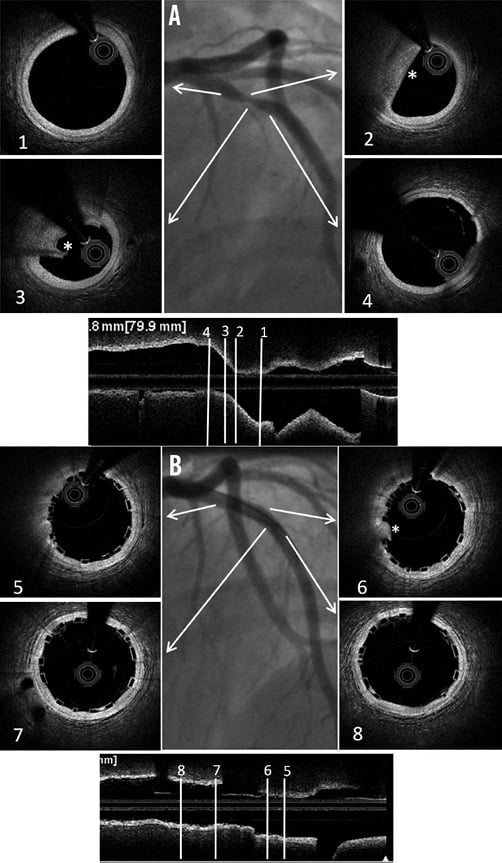
Figure 2. Sizing and post-implant OCT evaluation. Case example. A) Baseline angiogram showing a severe stenosis at the proximal LAD. Boxes 1 and 4: OCT proximal and distal references (proximal mean diameter=3.21 mm, distal mean diameter=3.12 mm). Boxes 2 and 3: OCT lipid rich plaque (*). B) Angiogram showing the final result after deployment of a 3.5×12 mm BVS post-dilated with a 3.5 mm NC balloon at 12 atm. Boxes 5 and 8: proximal and distal edges with well apposed struts. Box 6: limited tissue prolapse (*) not affecting the lumen area significantly. Box 7: Mid portion of the BVS with well apposed struts.
LESION PREPARATION
Lesion preparation before deployment is an essential step of any stent implantation procedure. This is particularly crucial for patients receiving BVS, where the goal of lesion preparation is to facilitate scaffold delivery, reduce plaque shift, and, most importantly, to allow optimal scaffold expansion (Online Table 11). Effective plaque cracking, especially in patients with long tapered lesions, ostial lesions, chronic total occlusions, bifurcations, and calcified lesions, is essential to avoid underexpansion. For this reason, direct BVS placement is typically not recommended, even in acute coronary syndromes.
For lesion preparation, it is generally advisable to use semi- or non-compliant balloons with a diameter equal to or only minimally undersized compared to the diameter of the selected BVS delivery system. Short (6 to 10 mm long) high-pressure balloons should be used after predilation of the segments when lesions show focal residual underexpansion due to the presence of highly calcific atherosclerotic plaques. Although no supporting data are available, the use of plaque modification devices (i.e., cutting/scoring balloons, rotational atherectomy) is encouraged when necessary to enable the BVS to cross and better expand calcified stenoses, but in the authors’ experience this is rarely required. In the event that one cannot achieve full opening of the (last) predilatation balloon with no indentation, it is advisable not to implant a BVS.
SCAFFOLD IMPLANTATION AND OPTIMISATION
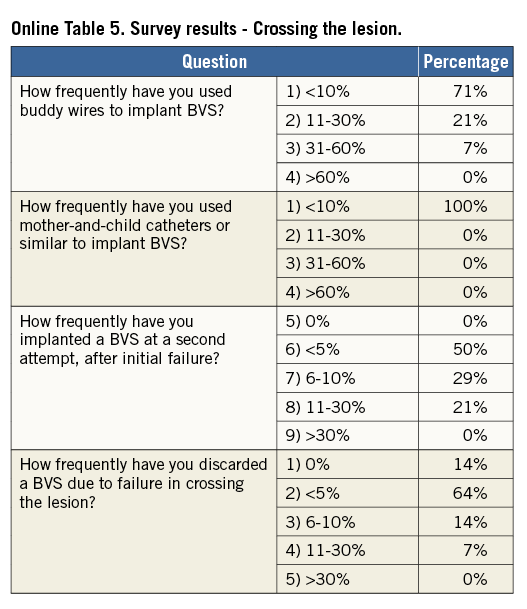
When difficulties are anticipated during crossing of the lesion, an extra back-up support guiding catheter or a guide extension (i.e., GuideLiner®; Vascular Solutions, Inc., Minneapolis, MN, USA, or Guidezilla®; Boston Scientific, Marlborough, MA, USA), a more supportive guidewire or a buddy wire may be considered. All sizes of BVS can be delivered through a 7 Fr guide extension (i.e., 6 in 7 Fr). 6 Fr guide extension systems (i.e., 5 in 6 Fr) can accommodate 2.5 mm and 3.0 mm BVS, but require preloading into the extension tube outside the patient’s body. A 3.5 mm BVS cannot be used with a 5-in-6 Fr guide extension.
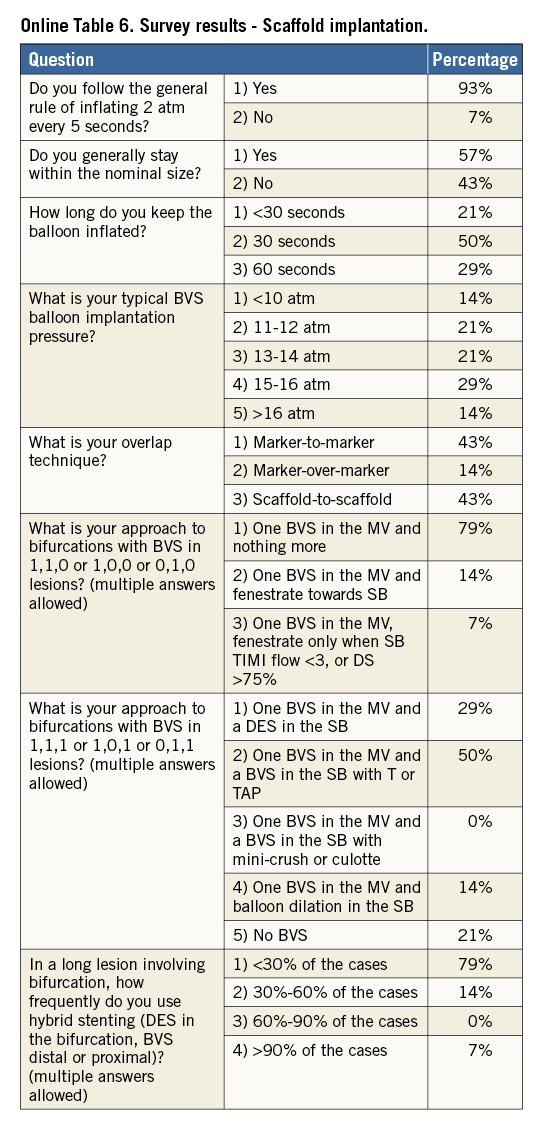
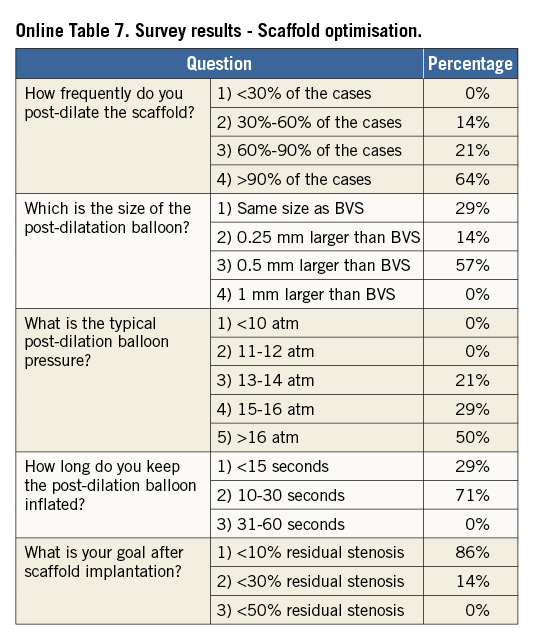
The scaffold should cover ≥2 mm of the healthy vessel at either edge of the lesion. Since overlapping of struts should be minimised, too short BVS should be avoided, and the BVS size should allow sufficient margins for post-expansion to optimise apposition proximally. Deployment must occur gradually, pressurising the delivery system in 2 atm increments every five seconds until complete expansion of the scaffold. Some operators prefer to increase the inflation pressure beyond nominal (10 atm) and up to the rated burst pressure (16 atm) to achieve full scaffold expansion. Upon device deployment, target pressure should be maintained for at least 30 seconds. Due to the polymeric material, the BVS has a maximum scaffold expansion limit of 0.5 mm above its nominal diameter, which should be respected strictly during implantation. Upon scaffold deployment, one should aim to obtain <10% residual stenosis, full scaffold expansion and optimal wall apposition. Therefore, routine post-dilatation for 10-30 seconds using a high-pressure non-compliant balloon is advisable unless intracoronary imaging confirms full expansion and apposition.
INTRAVASCULAR IMAGING
Anecdotal cases of BVS thrombosis following inadequate expansion and apposition of the BVS have indirectly supported an extensive application of intravascular imaging techniques7. In the absence of clear evidence of benefit from large controlled trials, there is agreement among the authors that new users of BVS especially should have a low threshold for the use of imaging before and after BVS implantation.
IVUS has the advantage of visualising the total vessel diameter and area, which allows optimisation of scaffold size while reducing the risk of disruption with oversized balloons. OCT has higher resolution, so that scaffold integrity, apposition to the underlying wall, presence of thrombus, edge dissections, and changes in strut characteristics over time can be easily studied (Figure 2, Online Figure 1). Subintimal calcifications detected with OCT may call for pre-intervention rotational atherectomy or the use of scoring devices. Incomplete scaffold expansion detected either by OCT or by IVUS suggests that further steps should be taken for lesion optimisation with higher pressure or balloons of larger diameter (preferably with short lengths). Small edge dissections identified by OCT at the scaffold edges can often be left untreated in the absence of severe plaque burden or vessel recoil.
Specific subsets and technical limitations
BIFURCATIONS
When treatment of bifurcation lesions is desired with BVS, the provisional stenting technique seems to be a logical approach. However, the BVS is a breakable device, thus the proximal optimisation technique, side branch (SB) fenestration, and kissing balloon inflation should be performed with caution. For one-stent approaches, two very simple strategies could be considered: i) one BVS across the bifurcation and nothing else (preferred by most of the authors), and ii) SB predilation and one BVS across the bifurcation. These strategies have the advantage of avoiding scaffold deformation, but they cannot always be used, and lateral dilation of SB through the BVS struts may be desired. Two issues should be borne in mind when this manoeuvre is considered: i) the maximum tolerated balloon diameter and inflation pressure used to dilate the SB, and ii) the maximum tolerated balloon diameter used to correct the induced BVS deformation at the main vessel. In a recent in vitro study, the authors observed no fractures when the balloon used for fenestration towards the side branch was ≤2.0 mm, but 16% of fractures were noted when a 3.0 mm BVS had side dilation with 2.5 or 3.0 mm balloons8. This fracture rate was reduced to 6% when a 3.5 mm BVS was evaluated with the same technique. In case of fracture, prolonged balloon inflation of the main vessel could partially restore the geometry of the BVS, with uncertain outcomes. With respect to the correction of BVS deformation, the classic kissing balloon inflation is not recommended due to the possibility of proximal strut fracture. Several alternatives to the classic kissing can be used: i) single balloon post-dilation at the nominal value of the BVS; ii) sequential balloon inflations; iii) undersized kissing with the proximal theoretical mean hugging balloon diameter (calculated by the Mitsudo formula9) smaller than the BVS nominal value +0.5 mm; iv) kissing with minimal balloon overlap (“snuggle or mini-kissing”). Benchtop data suggest that the fracture threshold for mini-kissing balloon inflation is 5 atm8. When a second stent is required in the SB, a second BVS or a metallic stent can be advanced and implanted through the main vessel BVS, although the latter might be easier as crossing profiles are generally smaller. Techniques with least strut layering should be preferred (i.e., T, T and small protrusion) when a second stent or scaffold is needed. Complex bifurcation scaffolding procedures should ideally be avoided with BVS or even undertaken under intravascular imaging guidance to detect and correct possible scaffold fractures or deformations.
LONG LESIONS
Since the longest scaffold length currently available is 28 mm, stenting longer segments requires overlapping of two or more scaffolds. The relatively thick struts of BVS mandate keeping the overlap to a minimum to avoid delays in healing10. For an optimal overlap technique, it should be noted that the scaffold marker beads are not located at the edges of the scaffold. The scaffold edges fall within the 1 mm balloon markers on each end and the scaffold marker beads are placed approximately 1 mm from the scaffold end. Therefore, if the balloon marker lines up with the scaffold marker, the result will be ~1 mm of overlap. Against this background, there are many possible overlap techniques (Figure 3). The two most advised by the authors are the “marker-to-marker” (~1 mm of overlap) and “scaffold-to-scaffold” (no overlap) techniques. In the marker-to-marker configuration, which appears to be the best one to avoid gap restenosis, the second scaffold is advanced until the distal balloon markers line up with the proximal marker beads of the implanted scaffold. As such, the markers of the second scaffold will be adjacent to the markers of the deployed scaffold. Attention should be paid to scaffold size selection and placement order (i.e., starting with the distal scaffold is preferred) to avoid damage at the overlap site. Working in orthogonal views helps to avoid markers on the second device appearing closer than they actually are due to foreshortening.
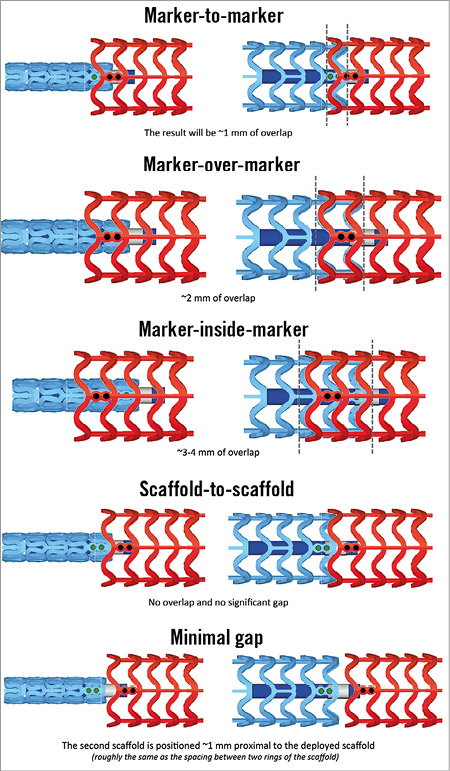
Figure 3. BVS overlapping techniques.
THROMBOTIC LESIONS
Recent reports have shown that BVS may be safely implanted in the context of thrombotic lesions in acute coronary syndromes11-13. A theoretical advantage of BVS implantation in thrombotic lesions could be the reduction in distal embolisation due to the larger strut width, with a possible increased capacity of the BVS to entrap thrombotic material between the scaffold and the vessel wall. In addition, device undersizing facilitated by acute phase vasoconstriction, while being permanent when using metal stents, could be counterbalanced by bioresorption and positive vessel remodelling in the BVS scenario. In ST-elevation myocardial infarction, predilation is sometimes perceived as more risky than in stable lesions, and accurate evaluation of lumen diameter is hampered by vasospasm and the thrombotic nature of the lesion. In this setting, thrombectomy and intracoronary nitrates administration may represent two important steps before deciding whether a BVS can be implanted. The use of thrombectomy devices prior to scaffold implantation may also help to foresee the crossability of the lesion and potential difficulties to be encountered during implantation.
CALCIFIED LESIONS
In pivotal trials of BVS, severely calcified lesions were excluded. While the positive effect of BVS on vasomotion may not materialise in these lesions, other effects such as conformability, late lumen enlargement, disappearance of late uncovered struts and late inflammatory processes might still apply. However, with the larger crossing profile of BVS, lesion preparation, as well as sufficient guide catheter and guidewire support, is even more essential. With proper lesion preparation, if necessary using debulking strategies, lumen gain similar to the gain achieved in other non-calcified lesions can be achieved. Full dilatation of the lesions with non-compliant balloons or cutting balloons is a prerequisite for success.
CHRONIC TOTAL OCCLUSIONS
Data on the performance of BVS in chronic total occlusion (CTO) lesions are sparse. However, the biomechanical properties of BVS make these platforms suitable for recanalised CTO lesions, with the following considerations. First, the chain of scaffolds required to cover these long lesions fully could chronically influence the vessel rheological properties and increase the risk of restenosis. Second, particularly the use of dissection/re-entry techniques to achieve successful guidewire passage is associated with higher probability of SB occlusion and subintimal haematomas, which can lead to reduced outflow and aneurysm formation. These result in higher risk of late stent malapposition leading to stent thrombosis and restenosis14. On the other hand, later disappearance of the scaffold struts, allowing early recovery of vessel motility and late liberation of caged SB, promises longer-term benefits.
The frequent presence of calcifications as well as the hypoplastic transformation of the distal bed of the occluded artery due to the chronic low flow state makes implantation of BVS in these lesions a challenge15. As a result, optimal lesion preparation and BVS selection become even more necessary for CTO PCI. Due to the lack of long-term data, the authors do not recommend covering long subintimal channels with multiple scaffolds.
IN-STENT RESTENOSIS
Currently, PCI using DES or drug-coated balloons is the most accepted approach for treating in-stent restenosis. From a theoretical standpoint and despite currently representing an off-label indication, BVS might be of potential advantage in this setting for two reasons. First, when compared with a drug-coated balloon, a BVS provides a more prolonged drug delivery capability. Most drug-coated balloons are coated with paclitaxel; however, the superiority of sirolimus and everolimus has been reported in several clinical trials, which might be an additional advantage of everolimus-eluting BVS for this specific indication. In addition, the scaffold struts provide vessel scaffolding at the edge of the former stent, which is also not provided by drug-coated balloons. Second, disappearance of the BVS may avoid long-term strut layering (“onion skin”), although caging of the vessel persists and late luminal enlargement is prevented. If the reason for restenosis is neoatherosclerosis, debulking devices should be considered.
POST-TREATMENT MANAGEMENT
Considerations on post-treatment management of BVS are provided in the Online Appendix.
How to prevent and manage BVS complications
SCAFFOLD DISRUPTION
In vivo disruption of a BVS is a rare complication when the recommendations of the manufacturer are followed strictly (Online Figure 2). The exact incidence of scaffold disruption is unknown but appears to be associated with post-dilatation above the recommended limit, kissing balloon inflation, strut opening towards the side branch with balloons larger than 2.0 mm, and implantation in areas of excessive torsion or flexion.
SCAFFOLD MALAPPOSITION, RESTENOSIS, MULTIPLE INTERSTRUT HOLLOWS
Data on the incidence of acute, persistent, and late acquired malapposition of BVS are still very limited16,17. The malapposition rate seems to drop over time due to physiological healing phenomena (i.e., endothelialisation). The incidence of late acquired malapposition, possibly due to overstretching at implantation, is unknown. The presence of fibrocalcific plaques, lack of adequate lesion preparation (i.e., insufficient predilatation), and post-dilatation with excessively short balloons may all be associated with BVS malapposition16,17. Importantly, malapposition has been reported to be associated with the presence of uncovered struts.
Current knowledge regarding BVS restenosis is also at an early stage. Small retrospective studies suggest restenosis rates of 6% at three years of clinical follow-up18, mostly associated with incomplete scaffold expansion. Optimal management of BVS restenosis, including the chance for different strategies based on the predicated integrity of the scaffold in relation to the time to restenosis, is undefined and warrants dedicated studies. Most of the authors agree that metallic stent implantation should be considered in case of BVS restenosis.
In a minority of cases, peri-stent contrast staining (defined as contrast staining outside the stent contour extending to >20% of the stent diameter measured by quantitative coronary angiography) and multiple interstrut hollows (defined as multiple hollows with maximum depth >0.5 mm existing between and outside well-apposed stent struts), which have previously been associated with abnormal vascular reactions to the eluted drug and/or the polymer, have also been observed19.
Illustrative case examples of scaffold malapposition, restenosis and multiple interstrut hollows are provided in Online Figure 3.
EARLY SCAFFOLD THROMBOSIS
Early thrombotic events following BVS implantation have been reported in some series4,20. This may reflect the high complexity of the lesions treated and/or reasons linked to suboptimal implantation. As a general rule, one should exclude two potential causes of early scaffold thrombosis: mechanical issues or suboptimal antithrombotic therapy. Whenever thrombosis occurs, use of intravascular guidance is recommended to rule out and guide correction of any mechanical causes.
Another important point is whether scaffold thrombosis should be treated with metallic stent implantation or not21. Treatment by metallic stent implantation should be reserved for cases of documented fracture or when the operator wants to achieve a final diameter beyond the maximum permitted ranges of the BVS22. Metallic stent implantation should aim to cover at least the fractured or malapposed struts. A proposed algorithm for management of early scaffold thrombosis is displayed in Figure 4.
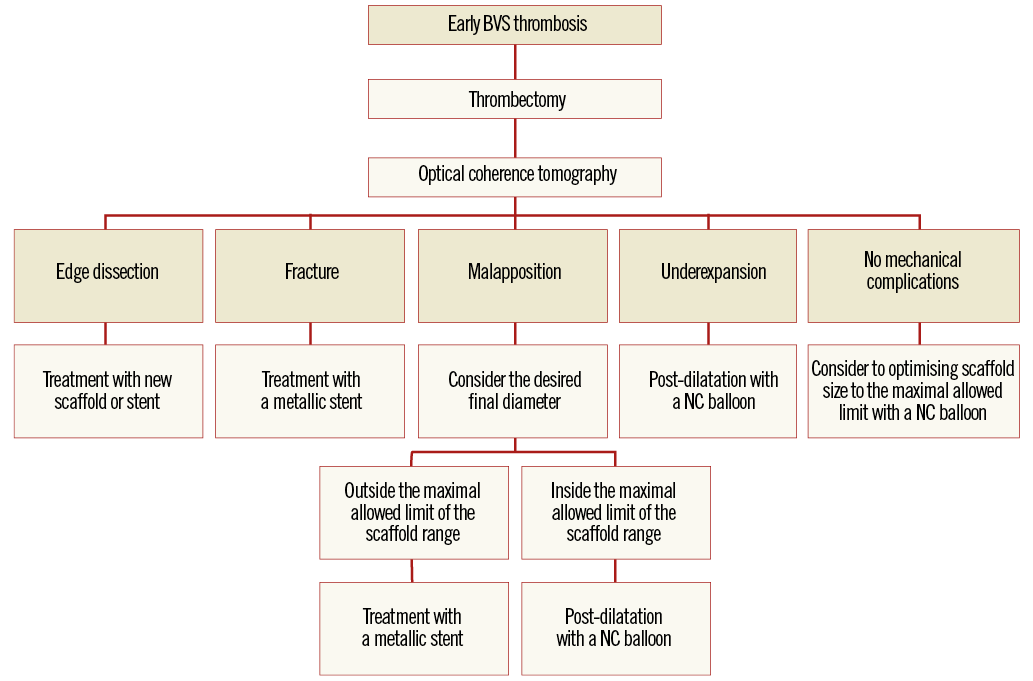
Figure 4. Proposed algorithm for the treatment of early scaffold thrombosis.
Limitations
BVS therapy is not free from limitations. Apart from some considerations outlined above, such as the challenges in bifurcation and calcified lesions, some further limitations exist that may warrant careful consideration as to whether or not to use a BVS in a specific patient or lesion (Online Table 12).
Conclusions
Within a short time, BVS have become an accepted therapy for selected patients undergoing PCI. Current BVS devices differ significantly from DES and require special procedural considerations to achieve optimal short- and long-term results.
| Impact on daily practice Despite the lack of compelling long-term data from large randomised clinical trials, BVS implantation is now frequently undertaken in many European and non-European catheterisation laboratories. Appraising the knowns and unknowns of a new technology is critical, particularly in the earlier phases of its introduction and implementation in daily practice. The present document aims to disseminate harmonised criteria for BVS use, and to provide education and practical advice in a field where evidence is rapidly accumulating. The impact of a standardised approach for BVS use on clinical outcomes remains unknown, but optimal implantation techniques may have an impact on blunting the rates of early and late scaffold failure. |
Conflict of interest statement
C. Tamburino, R-J. van Geuns, J. Mehilli, T. Gori and H. Nef have received speaker’s honoraria from Abbott Vascular. R-J. van Geuns, S. Achenbach and H. Nef have received research grants (institutional) from Abbott Vascular. E. Boone is an employee at Abbott Vascular. D. Capodanno has received consulting honoraria from Abbott Vascular. The other authors have no conflicts of interest to declare.
Appendix
CONSENSUS METHODOLOGY
Fourteen European centres with a high volume of PCI procedures with BVS combined efforts in an informal collaboration with the following objectives: i) to explore different contemporary practices for the use of BVS; ii) to build a consensus on accepted technical approaches for BVS implantation; iii) to prepare a document summarising the results of this joint effort. One meeting which also included representatives of the device manufacturer was held in Frankfurt, Germany, in March 2014. To get the most objective snapshot of different practices among the participating centres, a survey with 45 multiple choice questions was prepared and conducted in October 2014, the results of which are summarised below. The results of the survey are illustrated in Online Table 2-Online Table 9, which represent a basis for the technical advice provided in the document, whereas areas of controversy are highlighted. Phone conferences and e-mail exchanges followed, as necessary, to finalise the manuscript. The manufacturer supported the organisation of the face-to-face meeting and participated in the revision of the final document, but was not involved in the conduct of the survey and the elaboration of the consensus. The charge for the consortium was to identify and disseminate appropriate criteria for patient and lesion selection, scaffold implantation and optimisation, use of intravascular imaging guidance, approach to multiple patient and lesion scenarios, and management of complications. Importantly, the mission was not to provide formal recommendations in a field where the evidence basis is at an early stage, but to provide education and practical advice to those who want to accrue more experience with the BVS device.
DEVICE DESCRIPTION
The Absorb BVS (Abbott Vascular, Santa Clara, CA, USA) consists of four components: the polymer scaffold, the polymer drug reservoir, the antiproliferative drug everolimus, and the XIENCE RX delivery system (Abbott Vascular). The scaffold is based on a semi-crystalline poly (L-lactide) acid (PLLA) backbone having a corrugated ring pattern similar to that of the MULTI-LINK® BMS (Abbott Vascular), coated with an amorphous matrix composed of everolimus and polymer poly-DL-lactide (PDLLA) in a 1:1 ratio. As the scaffold itself is radiotransparent, there are two radiopaque platinum markers at either end of the scaffold. Both PLLA and PDLLA are fully bioresorbable, with complete bioresorption expected by approximately 24 to 36 months. The degradation process happens gradually with minimal inflammation23. The polymer (matrix) breaks down via hydrolysis ultimately leading to CO2 and H2O. The Absorb BVS has the same drug dose density and release rate as the metallic counterpart XIENCE V® (Abbott Vascular), i.e., 100 μl/cm2, with 80% of the drug eluting in the first 30 days, and the remainder eluting over 120 days. The larger size of the Absorb struts, however, results in a larger eluting surface per unit of wall surface. The currently available size matrix includes lengths of 8, 12, 18, 23 and 28 mm at diameters of 2.5, 3.0 and 3.5 mm. Nominal strut thickness is 157 µm and the 3.0 mm scaffold has a crossing profile of 0.056 inches. The use of 6 Fr guiding catheters is recommended but BVS can also be implanted via 5 Fr guiding catheters.
QUANTITATIVE CORONARY ANGIOGRAPHY
As compared to visual estimate, quantitative coronary angiography (QCA) provides information which is more accurate, but it also tends to underestimate the lumen diameter, offering no information on the true vessel size. Taking this limitation into account, three different parameters should be used for vessel diameter sizing with two-dimensional QCA: i) Dmax (proximal/distal), defined as the largest vessel diameter at the level of intended implantation zone, representing the landing zone in the peri-lesion segment within 5 mm of the segment to be scaffolded; ii) the interpolated reference vessel diameter (RVD), defined as a virtual diameter at the level of minimal lumen diameter, representing an average of many vessel diameters between the two healthy segments at the lesion edges; and iii) lesion length. The interpolated RVD could be influenced by the presence of side branches and, especially in tapering vessels and for long lesions, it underestimates the maximal lumen diameter within the scaffolded region. Conversely, Dmax is a real measurement which helps to select the appropriate BVS diameter to match the lumen diameter in the “landing zone”. In general, except in case of extreme vessel tapering, the scaffold selection should match the proximal Dmax. When expanded at 6-8 atm it is unlikely that a BVS creates major vessel disruption at the distal end, and the size used offers a buffer proximally to avoid critical underexpansion. The relatively small length of the available BVS devices (28 mm at most) can also help when dealing with different vessel sizes proximally and distally, if needed. The considerations above are supported by a substudy of the ABSORB cohort B and ABSORB EXTEND trials, which showed that visual vessel estimation led to sizing error in over half the cases, while QCA measurements led to a significant increase in correct vessel sizing, when compared to the interpolated RVD or visual estimation24. In another substudy of the ABSORB cohort B trial, Dmax would seem to have a bearing on the appropriate deployment of the Absorb BVS as assessed by optical coherence tomography criteria22.
POST-TREATMENT MANAGEMENT
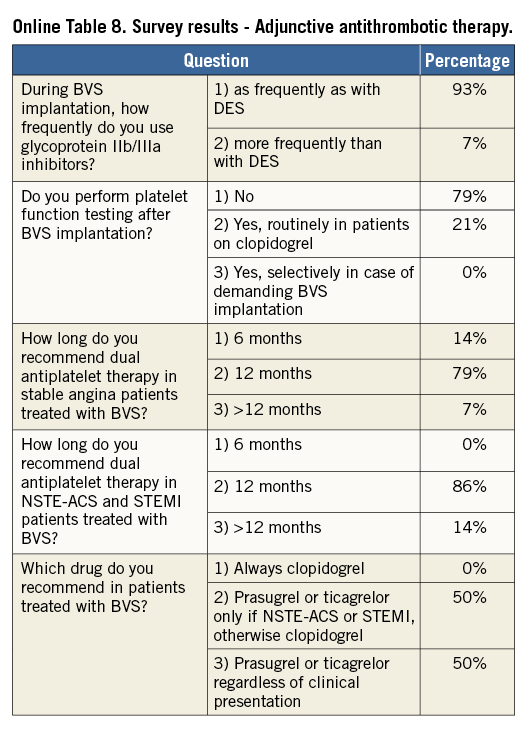
In current clinical practice, dual antiplatelet treatment after BVS implantation is recommended for at least 12 months in patients with acute coronary syndromes or six to 12 months in elective patients based on the guidelines for antiplatelet therapy after permanent metallic DES implantation25,26. Of note, while a minimum dual antiplatelet therapy of six months was recommended in both the ABSORB A and B cohorts, the effective duration was left to the operators’ discretion and thus the mean DAPT duration in cohort B of the ABSORB trial was 403 days27. However, OCT at six months suggests that scaffold coverage occurs in 98.9% of struts16. This phenomenon is significantly delayed in cases of scaffold overlap, due to increased shear stress and subsequent enhanced platelet aggregation28,29. As a result, some authors suggest longer dual antiplatelet therapy duration regimes (i.e., 18-24 months) and/or more potent agents (e.g., ticagrelor or prasugrel), particularly in the first months after BVS implantation because of their thicker struts30.
Current guideline recommendations on follow-up management and strategies after permanent DES implantation also apply to BVS patients and there appear to be no specific precautions. As for permanent metallic DES, the type and timing of anatomical/functional assessments after BVS implantation should be based on overall clinical risk evaluation, symptom status, stent diameter and length, treated lesions, results of previous procedure, residual stenosis and overall burden of coronary artery disease. Routine follow-up at six to 12 months with non-invasive coronary computed tomography angiography or with invasive angiography is not recommended and should be restricted to individual cases (i.e., bifurcations, CTO, multiple overlaps).
Supplementary references
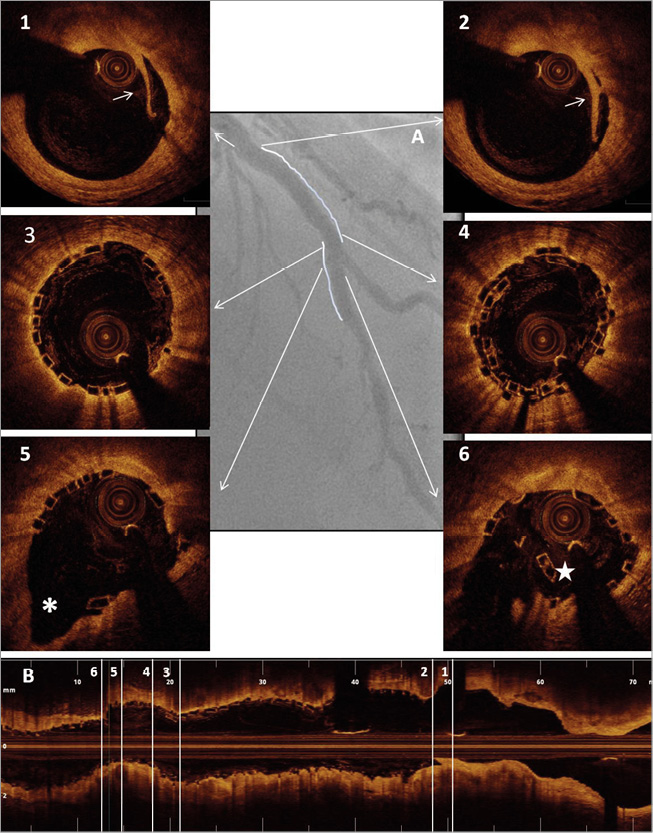
Online Figure 1. Scaffold optimisation with optical coherence tomography. Case example. A) Final angiographic result following two BVS (3.0*28 mm proximally and 2.5*18 mm distally) implantation on the mid left anterior descending across the origin of a diagonal branch. B) Longitudinal optical coherence tomography image following optimisation with side branch dilatation with a 2.0 mm semi-compliant balloon (8 atm) and final post-dilatation with a 3.5 mm non-compliant balloon (16 atm) proximally to the bifurcation (sequential dilatation with final proximal optimisation technique). Panels 1 & 2: limited proximal edge dissection (arrows). Panel 3: optimal strut apposition immediately proximal to the overlap. Panel 4: short struts overlap. Panel 5: well-opened scaffold struts at the bifurcation site towards the side branch (*). Panel 6: scaffold distortion distally to the carina (star).
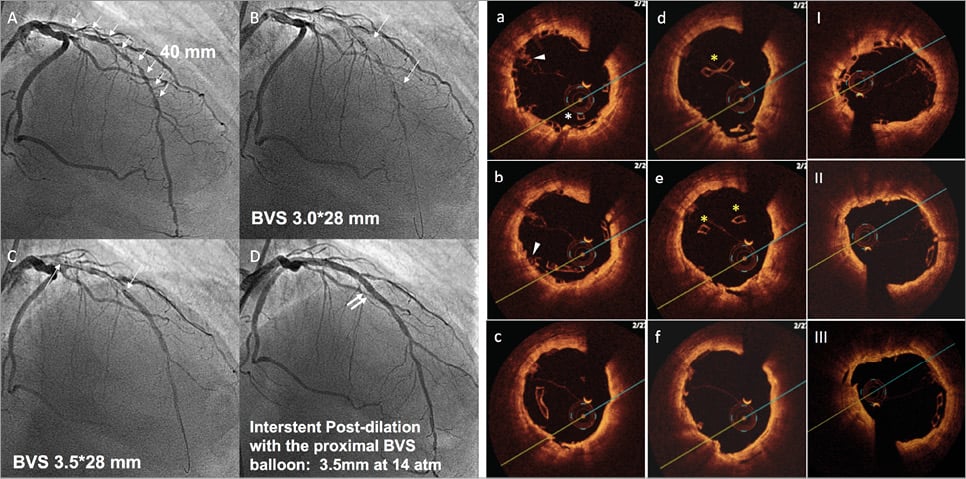
Online Figure 2. Scaffold disruption. Case example. A patient with a long diffuse lesion, treated with two long BVS and disruption of the proximal segment of the distal BVS. This complication occurred after dilation of the inter BVS coronary segment with a balloon size above that of the manufacturer’s recommendations (panels A to D). The diagnosis of the complication was made with OCT criteria (panels a to f) based on the presence of struts protruding into the centre of the lumen (*), overlapped struts (arrow), and struts not orientated perpendicular to the light source (yellow *). Prolonged inflation with a non-compliant balloon of a diameter within the expansion limits of the device resolved the complication (panels I to III).
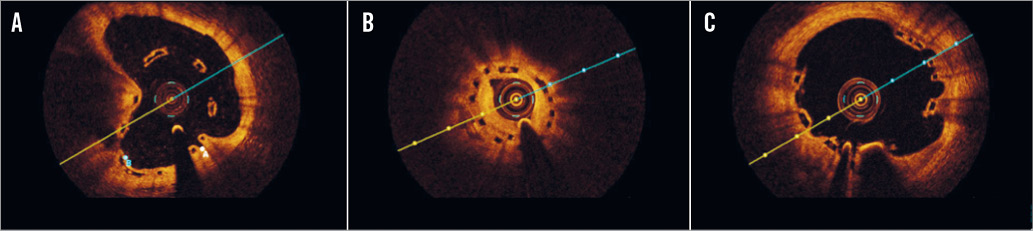
Online Figure 3. Illustrative case examples of malapposition, restenosis and multiple interstrut hollows by optical coherence tomography cross-sections. A) BVS malapposition at six-month control. The BVS was implanted and post-dilated at high pressure at the end of a complex procedure in a very calcific plaque. B) BVS restenosis 12 months after implantation. The lesion involved a small diagonal branch, and the scaffold appeared to be underexpanded (minimum lumen area 3 mm2). C) OCT performed 12 months after BVS implantation on the LAD during a STEMI demonstrating multiple interstrut hollows.