Abstract
Aims: The currently stated optimal catchment population for a pPCI centre is 300,000-1,100,000, resulting in 200-800 procedures/year. pPCI centres are increasing in number even within small geographic areas. We describe the organisation and quality of care after merging two high-volume centres, creating one mega centre serving 2.5 million inhabitants, and performing ~1,000 procedures/year.
Methods and results: In this descriptive cohort study, we linked individual-level data from the national Central Population Register holding survival status with our in-hospital dedicated PCI database of baseline, organisational and procedural characteristics. Quality measures were treatment delays and 30-day all-cause mortality. In the three-year study period, 2,066 consecutive pPCIs were performed. After the fusion of the two centres, pPCI procedures increased by 102%, while door-to-balloon remained stable at 32 minutes. Up to 75.1% of patients were directly transferred by pre-hospital triage, of whom 82.7% had ECG-to-balloon <120 min, 92.6% had door-to-balloon <60 min. Thirty-day all-cause mortality remained low at 6.3%.
Conclusions: This study challenges the stated maximal pPCI centre volume. The quality of a centre reflects governance, training, resources and pre-hospital triage, rather than catchment population and STEMI incidence, as long as a minimum volume is guaranteed. Resources can be utilised better by merging neighbouring centres, without negative effects on quality of care.
Introduction
Ischaemic heart disease remains one of the biggest burdens on Europe’s hospital system with an incidence of ST-segment elevation myocardial infarction (STEMI) ranging from 44 to 142 per 100,000 inhabitants1. Denmark provided one of the first pivotal trials demonstrating the superiority of pPCI compared to fibrinolytic therapy and, immediately following the result of the DANAMI II trial2, implemented pPCI as a national reperfusion strategy3. Initially, there were five pPCI centres serving the country’s 5.5 million inhabitants. Worldwide, the capacity for treating patients with STEMI has increased as pPCI has become the dominant therapy. Current guidelines stress the importance of establishing STEMI networks between hospitals and ambulance services4,5, and professional organisations coming together to improve the access to timely effective pPCI using focused implementation programmes such as the “Stent for Life” initiative6.
Based on the number of pPCI/year/country and the number of non-stop 24/7 pPCI centres/country reported by Widimsky et al in 2010 on behalf of the European Association for Percutaneous Cardiovascular Interventions (EAPCI)1, the calculated mean volume of pPCI procedures is 295 per pPCI centre in 33 European countries. It is currently stated that the optimal catchment population for a pPCI centre ranges from 300,000-1,100,000 inhabitants, resulting in ~200-800 pPCI procedures/year/centre1, while a higher volume could result in an overload of the centre. However, current guidelines4,5 are not specific regarding the optimal pPCI centre size and are based on consensus and clinical practice1 rather than specific studies. The overwhelming clinical data demonstrating better survival for patients reperfused with pPCI7,8 combined with less well documented statements from authorities regarding the optimal catchment area have led to a marked increase in the number of pPCI centres in many regions with often quite a low patient volume9. Furthermore, the current European and American guidelines4,5 favour a pharmacoinvasive approach for patients in whom pPCI is not possible within 120 minutes after first medical contact, even suggesting that this time limit be reduced to one hour among early presenters. Other, more operational recommendations have been issued by national bodies such as the British National Institute for Health and Care Excellence (NICE) guidelines10, recommending pPCI if delivered within 120 minutes of the time when fibrinolysis could have been given.
Throughout this development, Denmark kept its organisation and recently even decreased the number of pPCI centres. The purpose of the present report is to describe the organisation and quality of care in terms of treatment delay and 30-day mortality after merging two high-volume pPCI centres of the Copenhagen capital region, creating one mega centre in 2011, serving 2.5 million inhabitants and performing approximately 1,000 pPCIs per year.
Methods
POPULATION
During a three-year study period from 1/12/2009 to 1/12/2012, 2,066 consecutive STEMI patients were treated with pPCI in this Danish cohort. Patients were grouped according to two 18-month study periods, one before and one after the official date of the merger of two high-volume pPCI centres. We linked individual-level data from national registries, using the personal registration number provided to all Danish residents. The data were obtained from the Central Population Registry, registering survival status, and linked to our in-hospital dedicated PCI database holding baseline and procedural patient characteristics, enabling follow-up of all patients who had not emigrated. Follow-up was 99.7% complete.
GEOGRAPHY
During the first 18 months of the study period, the catchment area of our institution, Rigshospitalet, consisted of the urban Copenhagen area and the former counties of Storstrøm and Western Zealand, as well as the island of Bornholm, which covered a total area of ca. 7,000 km2 with a catchment population of ~1,250,000 citizens. Gentofte Hospital was the other pPCI centre on Zealand covering a catchment area of ~2,750 km2, constituted by the former counties of suburban Copenhagen, Fredensborg and Roskilde with a catchment population of ~1,250,000 citizens. Following a political decision, all cardiothoracic surgery and acute invasive cardiology in these regions were moved to Rigshospitalet on June 1 2011, making it responsible for all pPCI patients from Denmark’s main island, Zealand, and its neighbouring islands (Lolland, Falster and Bornholm), serving a population of 2.5 million inhabitants and a total catchment area of almost 10,000 km2 (Figure 1).
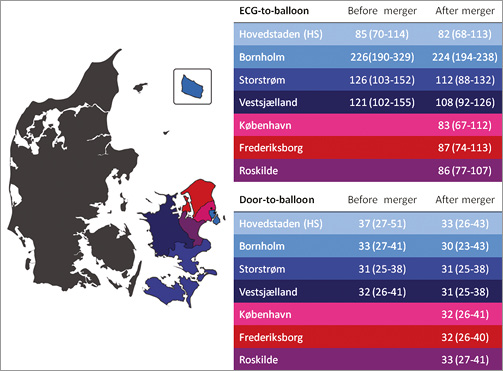
Figure 1. Map of Denmark. Eastern Denmark is marked by a colour code where the blue shades indicate the catchment area before the centre merger and the red shades indicate the catchment area added to Rigshospitalet after the merging of the two pPCI centres on Zealand (indicated by the black dots on the map, the southern dot being Rigshospitalet). The tables illustrate how ECG-to-wire and door-to-balloon times remained the same or decreased after the centre merger, stratified by county.
LOGISTICS OF PRE-HOSPITAL TRIAGE AND IN-HOSPITAL ORGANISATION
The logistics of pre-hospital triage on Zealand have previously been described in detail11-13. Briefly, all ambulances in Denmark are by law equipped with defibrillators and a device for 12-lead ECG recording and transmission. Usually, the first emergency medical services (EMS) to arrive at the scene are the primary ambulances staffed by paramedics or emergency medical technicians. In order to initiate advanced stabilising treatment, primary ambulances are supported by emergency medical units manned by physicians, which are fast vehicles without the space to transport patients, dispatched simultaneously with the primary ambulance to the injury site or on the way (en route) to the hospital, for a so-called “rendezvous” with the primary ambulance.
The on-call cardiologist at the pPCI centre interprets the digitally transmitted 12-lead ECG and calls the ambulance to conduct a short health personnel interview. In case of a likely STEMI5, the ambulance is re-routed directly to the pre-alerted catheterisation laboratory. Patients from remote injury sites in the catchment area are transferred to the rooftop helipad at Rigshospitalet by helicopters staffed with trained physicians. During the inclusion period, ~300 STEMI patients were transferred by helicopter at a cost of approximately 4,500 euros/airlift. The median time saved by air transfer was 20 min for patients transported over a median of 97 km14.
This pre-hospital organisation refers to a 24/7 catheterisation laboratory with one primary on-call team, consisting of one out of a total of eight PCI operators, as well as three nurses. The interventional cardiologist is reimbursed with a fixed amount for each day on call plus an hourly rate for actual duty, including time spent on transport. As a consequence of the volume increase, the operator is now more often continuously present in the hospital, which is why hospital cost has not increased proportionally to the increase in pPCI volume. In order to accommodate the increased patient influx, a second pPCI team is activated on an ad hoc basis to avoid treatment delays. This occurs four to nine times per month. Team 2, when called, is reimbursed by a fixed amount. The centre has four dedicated PCI suites and one fully integrated hybrid catheterisation laboratory/operating room for PCI/CABG, and performs approximately 2,200 PCI per year, including ~1,000 STEMI patients. The pPCI centre is backed up by a cardiac ICU and an in-hospital high-volume cardiothoracic surgery department performing ~800 isolated CABG per year15.
As a consequence of the Danish national reperfusion strategy, no fibrinolytic or pharmacoinvasive therapy was administered during the study period3. Pre-hospital adjunctive pharmacological treatment consisted of 300 mg ASA, either 600 mg clopidogrel or 60 mg prasugrel and 10,000 U of unfractionated heparin. The in-catheterisation laboratory treatment consisted of bivalirudin with provisional use of glycoprotein IIb/IIIa inhibitors. During the study period, pre-hospital loading with prasugrel increased, while ticagrelor only came in at the end of the period.
Low-risk STEMI patients, i.e., patients without cardiac arrest, cardiogenic shock, left ventricular ejection fraction ≤40%, atrioventricular block, continuous post-reperfusion malignant arrhythmias, bleeding complications or recurrent angina16, constitute the majority of patients (>65%) and are handled in a “fast track” programme with a maximum length of stay of three days. Before discharge to the secondary district hospital, a transfer of low-risk patients from the cardiac care unit to the in-hospital step-down unit is performed. The latter was a direct consequence of the increased volume after the centre merger. Length of stay at the pPCI centre was not affected and was maintained at two to three days. Numbers of CCU beds did not increase after the merger, nor did the number of beds at the cardiac ICU. However, the number of cardiac nurses from 15:00 hrs to 07:00 hrs was increased. Another direct and planned consequence of this fusion of centres was the decrease in the number of NSTEMI and UAP cases admitted from referring hospitals. Conversely, the centre which no longer treats STEMI patients changed its case mix accordingly towards increased numbers of NST-ACS.
TIME MEASURES OF REPERFUSION DELAY
We used time from diagnostic ECG to balloon as a surrogate for the latest ESC definition of system delay, defined by first medical contact to wire.
STATISTICAL ANALYSIS
The distribution of continuous variables was tested by the Shapiro-Wilk test and was interpreted via a Q-Q plot. Continuous variables are presented as mean±standard deviation (SD) or median with interquartile range (IQR). Discrete data are presented as frequencies and percentages. Age, systolic/diastolic and heart rate were normally distributed, presented as mean±SD and a t-test was used. BMI, heart rate and all time variables (min) were continuous variables that had a non-Gaussian distribution and the Mann-Whitney test was applied. The χ2 test was used for comparison of categorical variables. Survival rates were evaluated by Kaplan-Meier curves and compared by log-rank (Mantel-Cox) test. In Cox regression analysis, all baseline variables shown in Table 1 were tested as mortality predictors in univariable models, and only significant predictors were entered in a manually entered multivariable model. Variables were retained in the model if p<0.1. The statistical significance level was p<0.05 (two-sided test). Data were analysed using the PASW Statistics software package, version 19 (IBM Corp., Armonk, NY, USA).
Results
The fusion with another high-volume pPCI centre resulted in a 102% increase of invasively treated STEMI patients. Baseline and procedural characteristics were well balanced when comparing an 18-month period before and after the centre merger. In the vast majority of cases (93.2%), only one vessel was reperfused and procedural success was high with 93.7% of patients obtaining full reperfusion with TIMI flow 3 (Table 1, Table 2).
Before the centre fusion, 23.7% (1,281/5,398) of all coronary angiograms (CAG) performed at Rigshospitalet had an acute indication and in 66.3% (849/1,281) of these pPCI was the indication. After the centre merger, 40.8% (2,539/6,216) of all CAGs were acute and 70.1% (1,780/2,539) had an indication for pPCI.
Time from diagnostic ECG to balloon decreased significantly by 18% to 93 min, and door-to-balloon times decreased slightly to 32 min after fusion of the two centres (Table 3). The proportion of patients treated within 120 min of the diagnostic ECG and within 60 min of arrival at the pPCI centre increased significantly to 74% and 93%, respectively (Table 3, Figure 2).
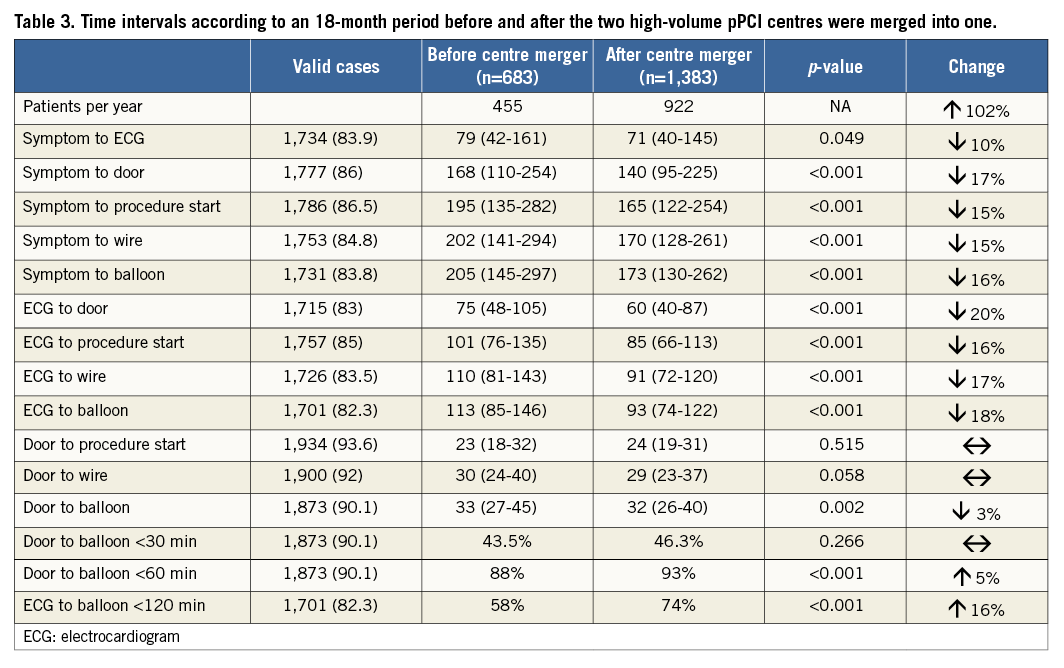
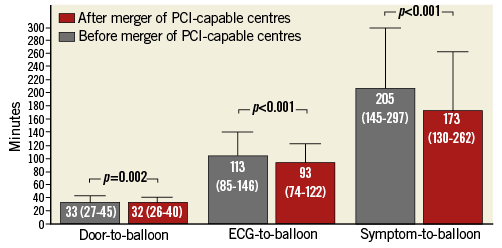
Figure 2. Bar plot (median, error bar depicts upper interquartile range) of overall reperfusion delays before and after the fusion of two high-volume pPCI centres into one mega pPCI centre.
Even after excluding the patients added from the other (and geographically closer) centre after the merger, ECG-to-balloon and door-to-balloon still decreased significantly from median 114 (87-148) to 105 (80-135) min (p<0.001) and 33 (26-43) to 32 (25-40) min (p=0.013), respectively.
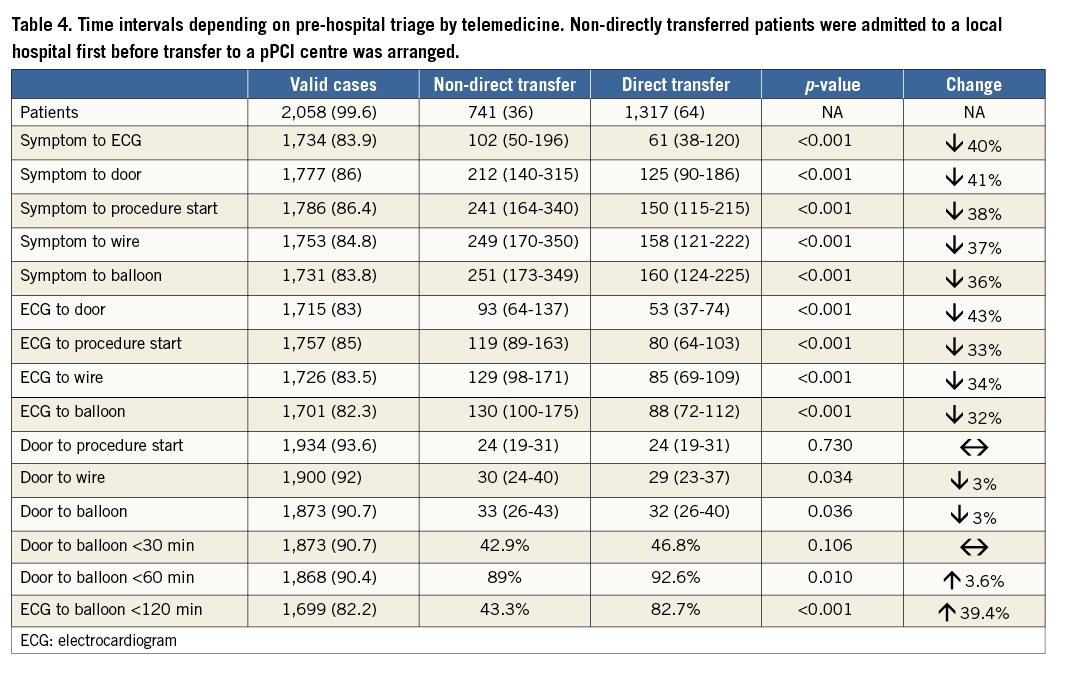
Throughout the entire observation period pre-hospital triage with direct transfer to the pPCI centre, bypassing local hospitals, was achieved in 64% of patients, resulting in a significant decrease in ECG-to-balloon interval from 130 to 88 min. Among patients who were transferred directly, 82.7% were treated within 120 min of pre-hospital ECG recording (Table 4). Rates of pre-hospital triage increased over time (38% in 2010, 68% in 2011 and 72% in 2012) and in the fourth quarter of 2012 were at 75.1%. However, these rates are subject to initial underreporting to the database in the early phase of the observation period and therefore do not represent real changes of pre-hospital triage patterns.
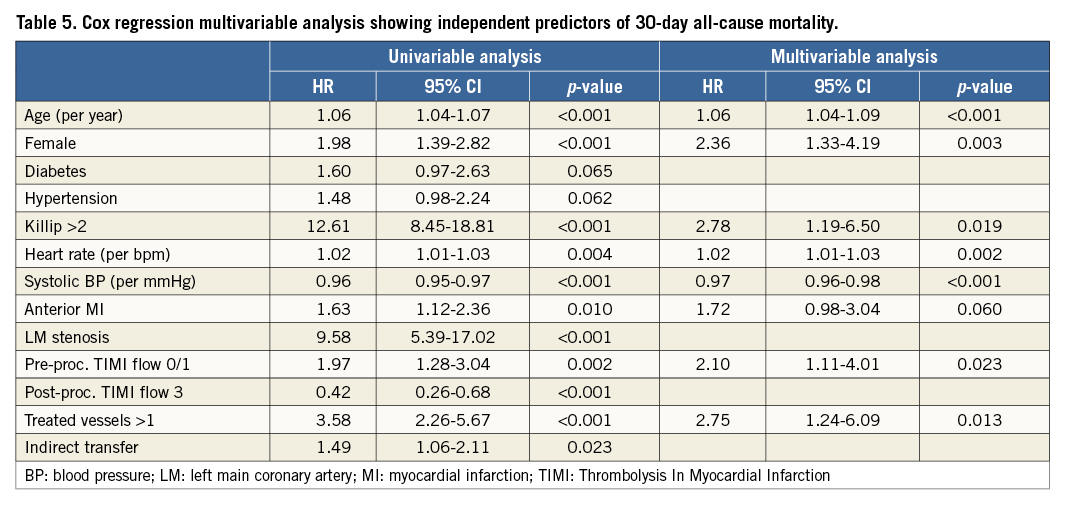
Thirty-day all-cause mortality was very similar before and after moving from a high-volume to a mega centre (44 [6.4%] vs. 86 [6.2%] events [log-rank p=0.525]) (Figure 3). Cox regression multivariable analysis identified age, female gender, systolic blood pressure, heart rate, Killip class >2, pre-procedural TIMI flow 0/1 and multiple treated vessels as independent predictors for mortality. All variables were evaluated at admission (Table 5). Indirect transfer was a univariable, but not multivariable predictor of 30-day all-cause mortality (8.0% vs. 5.4%, log-rank p=0.021). Formal interaction testing was carried out for direct/indirect transfer and before/after the merger regarding 30-day mortality. No significant interaction existed (p=0.641), and before/after merger was not an effect modifier. In univariable logistic regression analysis among variables to hand at the moment of triage, prior CABG was associated with indirect transfer (OR 2.13 [95% CI: 1.11-4.10], p=0.023), whereas an RCA culprit was associated with a trend towards a higher likelihood of field triage (OR 1.20 [95% CI: 0.98-1.44], p=0.054). Other baseline characteristics were not associated with the transfer path of admission. Killip >2 (OR 2.13 [95% CI: 1.33-3.40], p=0.002) and higher heart rate (per bpm) (OR 1.01 [95% CI: 1.01-1.02], p=0.001) evaluated at pPCI centre admission were associated with indirect transfer.
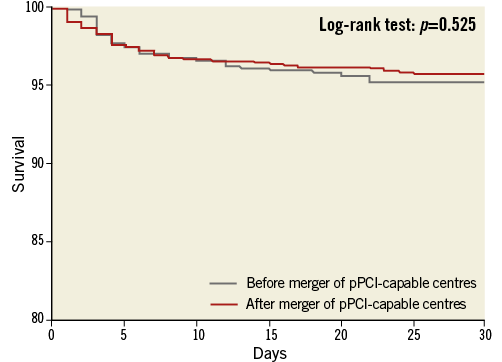
Figure 3. Kaplan-Meier curve. Thirty-day all-cause mortality after pPCI before and after the fusion of two high-volume pPCI centres into one mega pPCI centre.
Discussion
The main result of the present study is the finding that one mega pPCI centre with ~1,000 procedures per year, serving a catchment population of 2.5 million people, provides high-quality treatment, measured by internationally recognised standard quality parameters of pPCI.
Patient load at the present centre doubled after the fusion with the other major PCI centre from the urban Copenhagen region, thereby exceeding the currently stated ideal number for annual procedures in a high-volume pPCI centre1, while preserving standards of quality of care. Pre-hospital triage with direct transfer and post-procedural TIMI flow 3 reperfusion within in- and pre-hospital ESC guideline-recommended time frames was achieved in the vast majority of patients. The rate of post-procedural TIMI flow 3 compared well with the recent reports from large randomised trials17 and reports from international registries18,19. The 30-day all-cause mortality rate for this consecutive population of unselected STEMI patients, including resuscitated STEMI patients and STEMI patients with periprocedural cardiogenic shock, was unaffected by the fusion of the two centres and low compared to international standards1,20.
Our results demonstrate that the feared overload of a pPCI centre by catchment populations >1 million people1 is unjustified, but rather is dependent on pre- and in-hospital organisation.
The decrease in delay in the pre-hospital phase after the centre merger is partly due to the catchment area being primarily expanded with patients living in the greater urban area, but the closed-down pPCI centre also served patients from rural areas up to 70 km away. Air transfer was not used for patients originating from the merged catchment area. Of note, pre-hospital times in patients living nearby were unaffected as a result of our unaltered STEMI network with pre-hospital triage in place, whereas ECG-to-wire times in remote patients were improved (Figure 1). This is due to the recent implementation of a physician-staffed helicopter for patients from these counties14. Furthermore, the proportion of acute CAGs performed under the indication of pPCI (and conversely the proportion of “false activations”) remained similar before and after the centre merger, indicating that criteria for patient referral were not altered after the merger.
We have recently shown that in consecutive CAGs pPCI was performed in 81% of patients triaged in the pre-hospital phase, which constitutes a high catchment rate with a low frequency of “false” catheterisation laboratory activations. In contrast, pPCI was only performed in 41% of patients transferred from emergency departments and cardiac care units without pre-hospital triage11. Acute CAGs in so-called false catheterisation laboratory activations yield important risk-stratifying information in patients with chest pain, cardiogenic shock or resuscitated out-of-hospital cardiac arrest. These results indicate that directly and indirectly transferred STEMI patients are probably not entirely comparable, although there were no differences in age, gender and the registered comorbidities. Patients who were directly transferred by pre-hospital triage had an unadjusted 2.5% absolute 30-day mortality risk reduction. Elevated heart rate and Killip >2 were associated with missing pre-hospital triage; however, these variables were evaluated at pPCI centre admission and therefore cannot be interpreted as causal for indirect transfer. Notably, indirect transfer remained an independent predictor of mortality in multivariable models incorporating all of the variables shown in Table 5, but lost significance when acute heart failure was added.
Our results indicate that efficient STEMI networks delivering pre-hospital triage in order to reduce reperfusion delay and lower false activations of the catheterisation laboratory are, together with in-hospital organisation, important conditions for allocating and providing quality care in high-volume pPCI centres with large catchment populations and areas. These results confirm previous national experiences, where direct transfer substantially reduced system delays by 60-100 min for ground ambulance21-23 and an additional 20-70 minutes by helicopter ambulance24, depending on injury site location.
International hospital settings are currently under reformation with small rural hospitals closing their doors or joining larger units resulting in highly specialised departments at greater distances from local injury sites. This calls for an efficient pre-hospital phase. In a recent North American based study, the benefits and costs of building new PCI capability at US hospitals versus diverting patients with STEMI to existing PCI-capable hospitals were compared. The authors found that emergency medical service (EMS) systems, in which STEMI detection and diversion are feasible, may be more effective and less costly than construction and staffing of new hospital PCI capability25,26.
Conclusion
This single-centre experience demonstrates the possibility of doubling the capacity of a pPCI centre without compromising the quality of care parameters of in-hospital delays, epicardial reperfusion rate and 30-day mortality. The present study challenges the concept of a maximum desirable capacity of a pPCI centre, as suggested in current European and American guidelines. Quality of care of a pPCI centre is more likely to reflect governance, proper training, resources and pre-hospital triage, rather than catchment population size and STEMI incidence, as long as a minimum volume is guaranteed. Whilst the spreading of pPCI initially demanded the creation of new centres, the present study suggests that resources can be utilised better by merging neighbouring centres, without negative effects on quality of care.
| Impact on daily practice The present study challenges the concept of a maximum desirable capacity of a primary percutaneous coronary intervention (pPCI) centre, as suggested in current international guidelines, and demonstrates that ≈1,000 annual pPCI procedures per centre with a catchment population of 2.5 million people can be performed efficiently and safely. Quality of care, as measured by treatment delays, coronary reperfusion and 30-day mortality, is more likely to reflect resources and pre-hospital triage, rather than catchment population size, as long as a minimum volume is guaranteed. The present study suggests that resources should be focused on efficient pre-hospital triage and in-hospital organisation, and resources can be utilised better by merging neighbouring pPCI centres. |
Conflict of interest statement
The authors have no conflicts of interest to declare.




