Abstract
Treatment of structural heart disease (SHD) represents a growing need and, with increasing device availability, an increasing number of SHD can be and will be treated percutaneously. However, interventional treatment of SHD is challenging. Long procedure times and steep learning curves are recognised obstacles. The main difficulties arise, however, from the inability to visualise simultaneously the anatomy and the devices using a single imaging technology. In fact, the majority of percutaneous interventions in SHD are guided by fluoroscopy. On the other hand, a multitude of imaging technologies are presently available to guide the interventionalist. Of these technologies, transoesophageal echocardiography (TEE), and particularly 3-D TEE, is rapidly becoming the imaging modality of choice for many of these procedures because it provides critical insights into soft tissue anatomy. However, adequate visualisation and appreciation of the relationships between the cardiac structures and the devices using various imaging modalities remain a challenge. Hence, the interaction between the operator and imager is a crucial factor in attaining procedural success. Innovative technology that fuses live 3-D TEE with live x-ray in an intuitive way could have an important added value. This new imaging technology seeks to improve the communication between the echocardiographer and the interventionalist, to increase the confidence and anatomical awareness, to assist in guidance, and to increase procedural efficiency.
Introduction
Percutaneous treatment of structural heart disease (SHD) is often challenging. The main difficulties arise from the inability to visualise simultaneously the anatomy, the catheters and the devices using a single imaging technology. In fact, the majority of percutaneous interventions are guided by fluoroscopy. However, this limits our precision in targeting soft-tissue lesions, as is often the case in SHD interventions. For this reason, additional imaging technologies such as transoesophageal (TEE) or intracardiac (ICE) echocardiography are increasingly used1. Both echocardiographic techniques provide fantastic temporal resolution and detailed appreciation of the anatomy and the lesion to be treated. However, they are of limited value for following wires and catheters navigating within the heart. The introduction of real-time 3-D echocardiography, and in particular 3-D TEE, has added a new level of understanding of the anatomy and the relation between the lesion to be treated and the surrounding anatomy, and has rapidly found its way into the cathlab for the closure of patent foramen ovale, atrial and ventricular septal defects, closure of left atrial appendix and valvular interventions, including the treatment of paravalvular leaks2,3.
Recently, several SHD interventions have been reported as successful with echocardiographic guidance only (without the need for fluoroscopic guidance), an approach that appears particularly appealing in small children in order to avoid ionising radiation1,4,5. However, a single imaging modality is often insufficient to guide complex catheter interventions with precise placement of devices, and a simultaneous multimodality approach may be preferred. As a consequence, the interventional cardiologist needs to communicate effectively with the echocardiographer in the cathlab to obtain the appropriate projections that will guide his manipulations. Vice versa, the echocardiographer should be familiar with all steps of the procedure and the particular preferences and needs of the interventionalist.
Integrated real-time multimodality approach to guide SHD interventions
The optimisation of image guidance in SHD interventions requires an integrated, interactive approach merging both fluoroscopic and echocardiographic information in real time. Optimally, such a system should combine the anatomical information and soft tissue discrimination of echocardiography with the device and catheter visualisation of fluoroscopy.
This approach is technically very challenging. In order to be precise, real-time image co-registration of both imaging modalities is necessary. Furthermore, this fusing technique needs to provide an image orientation that is appropriate for guiding the intervention. One of the biggest difficulties that interventional cardiologists are facing regarding multimodality image guidance is that the image orientation on the separate screens is often not the same, requiring additional mental effort to comprehend three-dimensional structures fully and to be able to navigate interventional tools appropriately. Traditionally, the interventional cardiologist is more familiar with the standard fluoroscopy projections compared with the classical TEE orientations. Therefore, displaying the anatomy by echocardiography with the same orientations as by fluoroscopy could potentially help in guiding the wires and catheters within the heart.
Several technological solutions have recently been proposed to facilitate guidance of complex cardiac catheterisation procedures. Among them the combination of pre-acquired volumetric images using computed tomography (CT) or magnetic resonance (MR) images merged with fluoroscopy has found its application in the treatment of atrial fibrillation6 and percutaneous pulmonary valve replacement7. Other solutions are presently under investigation to facilitate aortic valve prosthesis placement in transcatheter aortic valve implantation (TAVI). In this case the volumetric images are segmented and reconstructed before the intervention and are then superimposed on fluoroscopy during the TAVI procedure. This real-time multimodality approach allows virtual planning of device implantation by 3-D visualisation of the aortic root, the aortic annular geometry and dimensions. It also allows an appreciation of the distribution of calcifications of the aortic cusps and the potential prediction of their interaction with the stent-mounted implants. However, for live guidance the technique is still limited by the fact that MR- or CT-derived roadmap images are static and not synchronised to respiratory motion or changes in fluoroscopy projections. Therefore, in case of more complex interventions, where careful and precise placement of the device is crucial, the use of static images as a roadmap for live guidance does not appear to be of particular help. In an effort to overcome this limitation, newer multimodality systems are being developed which harmonise TEE and fluoroscopy through continuous real-time updating of image co-registration based on the movement of predefined markers on the TEE probe. In particular, the EchoNavigator system (Philips Medical Systems, Best, The Netherlands) has been installed in the first two centres in the world (at the University of Colorado in Denver and at the University Hospital Zurich, Switzerland). The initial experience with this system is the subject of this review.
The EchoNavigator system
The EchoNavigator system synchronises echocardiography and fluoroscopy in real time by tracking the movement of the TEE probe on fluoroscopy. By this means, changes in angulation, rotation or position of the TEE probe are immediately registered and updated on fluoroscopy images. This allows for concordant views of TEE and fluoroscopy by presenting echocardiography images in the same anatomic alignment as the c-arm gantry. Conversely, if the c-arm is moved, echocardiography images are updated and reconstructed with the same angulation, allowing rapid understanding of the anatomy and the relation between cardiac structures and devices during complex interventions. Furthermore, the “marker” feature allows placing markers on regions of interest on echocardiography that are then displayed simultaneously and in real time in the co-registered fluoroscopy. This feature appears particularly appealing to identify targets on echocardiography (otherwise not visible on fluoroscopy, e.g., interatrial septum, left atrial appendage, mitral valve plane or valve lesions, vascular orifice, etc.) and to use these markers for adequate guidance of catheters and devices on fluoroscopy.
How does the co-registration of 3-D TEE and fluoroscopy work?
The EchoNavigator system is based on a fast and robust image algorithm for the registration of 2-D and 3-D TEE with x-ray fluoroscopy. This algorithm places the two image modalities in the same co-ordinate system and is based on the localisation and tracking of the TEE probe8. Although the TEE probe is designed to be flexible, its transducer is encapsulated in a rigid head. The transducer has been reconstructed using a nano-CT system capable of reconstructing ultra-high-resolution 3-D volumes. In order to determine the position of the TEE probe in the local 3-D co-ordinate of the x-ray system, the 3-D reconstructed TEE probe is used to fit into the x-ray images centring the probe using a 2-D/3-D image registration algorithm. In practice, at the beginning of a procedure the TEE probe is co-registered by the acquisition of two angulated fluoroscopy projections (Figure 1). Once the algorithm recognises the TEE probe and its orientation, the TEE and x-ray images are co-registered and can be oriented in the same plane or can be manipulated further. The system allows the simultaneous display of three different echocardiographic views: 1) the c-arm gantry view, in which the echocardiographic view is oriented in the same plane as the x-ray view (this view is automatically updated by each movement of the c-arm); 2) the echocardiographic view which is the standard TEE projection showing up on the echocardiographer’s screen; and 3) a free image that can be rotated or cropped by online user interaction at the table site using a sterile mouse pad. This feature is particularly appreciated as it allows the operator to manipulate the real-time images without needing to request further TEE projections from the echocardiographer. The ability to rotate the images at the table site allows the operator to understand better how his catheters and devices are interacting with the surrounding anatomy. This can potentially improve the communication between different specialists (i.e., interaction between operator and imager). In addition, manipulating the 3-D images can very quickly produce several new and unconventional views. These unconventional reconstructed views can be of particular help in understanding complex anatomy.
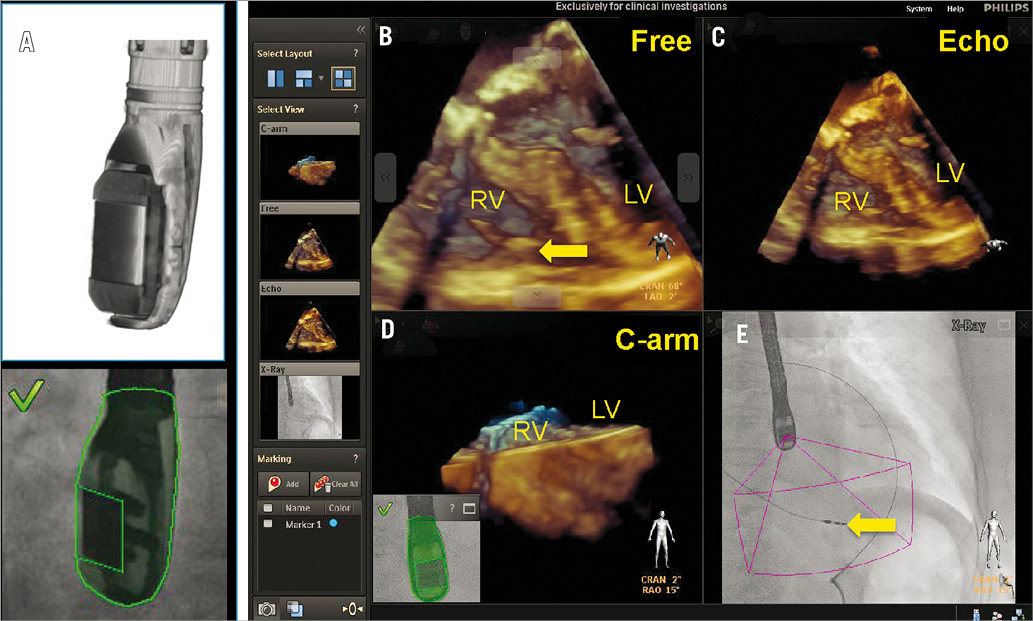
Figure 1. Co-registration of x-ray and TEE is performed by fluoroscopic acquisition of the TEE probe in two angulated projections. The algorithm recognises the position of the TEE probe transducer by comparing the x-ray data with the acquired ultra-high-resolution volumes from the 3-D model (A). Four images are displayed simultaneously (B-E) by the EchoNav system and are described as follows: (B) free rotated TEE image: this view can be freely manipulated by a mouse at the table site. C) Echo image: this is the standard TEE projection as it appears on the echocardiographer’s screen. D) The C-arm gantry view is the echocardiographic image oriented in the same plane as the x-ray view. E) Finally, the fluoroscopy shows the angiographic view with the sector volume displayed onto the x-ray view. The yellow arrow indicates the tip of a right ventricular pacemaker lead. RV: right ventricle; LV: left ventricle
Potential use and present experience
The EchoNavigator system has been tested during its development phase in percutaneous interventions such as atrial septal defect (ASD) and left atrial appendix (LAA) closure, paravalvular leak closure and percutaneous valve interventions such as TAVI and percutaneous mitral valve repair (MVR) using the MitraClipTM system (Abbott Vascular Structural Heart, Menlo Park, CA, USA).
The ability to introduce markers on TEE images that simultaneously appear in the co-registered fluoroscopy images appears particularly useful for guiding complex catheter or device manipulations. In fact, once the target is defined in TEE images, the precise placement of the catheter is easier and faster by manipulating the catheter under fluoroscopic guidance targeting the displayed marker.
In our experience the EchoNavigator system is particularly appreciated in procedures involving the atrial septum as is the case with interventions requiring a precise transseptal puncture or in the presence of multi-fenestrated ASD. In this case the precise placement of the device is at least as important as the choice of the appropriate device size, and the simultaneous visualisation of the echocardiographic and x-ray images facilitates passage of the largest defect. Similar considerations apply to the closure of the LAA. The selective intubation of the LAA orifice, optimal alignment of the catheter and precise positioning of the occluding device can be challenging. In our experience, the possibility of introducing markers in the echocardiographic 2-D and 3-D images that simultaneously appear in the co-registered x-ray images facilitates positioning and alignment of the catheter in the LAA and the placement of the occluder device (Figure 2). This is especially true in cases where LAA closure is performed immediately after percutaneous MV repair, where the septal puncture site chosen for optimal MV repair is by nature suboptimal for guidance of the catheter during LAA closure.
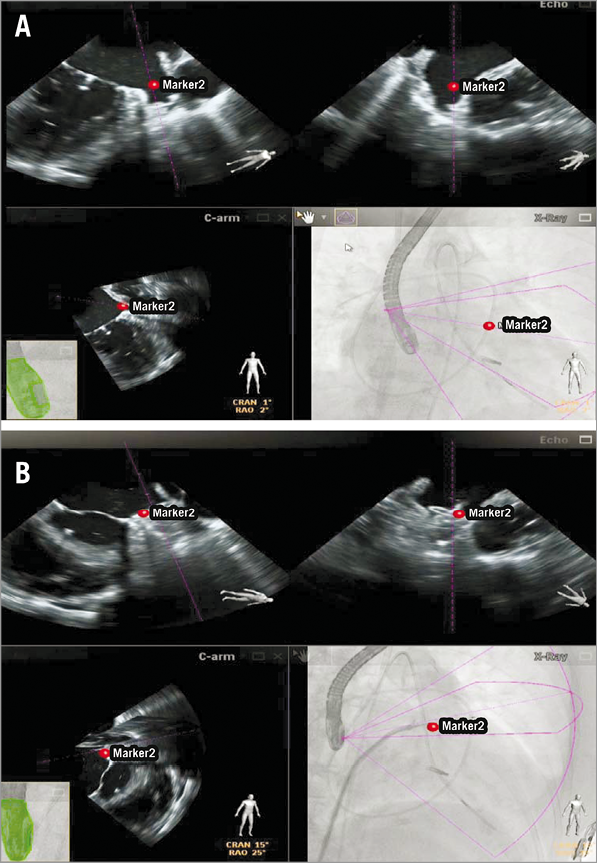
Figure 2. Percutaneous left atrial appendage (LAA) closure after percutaneous mitral valve repair using the MitraClip system. The LAA orifice is indicated by a red marker in the echocardiographic images (upper panels in A), which simultaneously appears on co-registered fluoroscopy (lower panel right in A), facilitating the navigation of the device within the left atrium and its placement in the LAA (B).
A multimodality approach combining reformatted CT images with fluoroscopy is under clinical investigation to guide placement and deployment of the aortic prosthesis during TAVI procedures. It is conceivable that in the near future we will be able to plan the TAVI procedure on the 3-D echo images by virtual introduction of simulated catheters and devices in a 3-D (“holographic”) reconstruction of echo and co-registered fluoroscopy images. At present we use the EchoNavigator to facilitate prosthesis placement by marking the level of the annulus on echo images. This reference has been used (similarly to the common practice of using the calcification on fluoroscopy) to target implantation depth for both the balloon-expandable as well as the self-expandable prosthesis. The hope is to be able to achieve more precise implantation and simultaneously to reduce the volume of contrast media injection.
The EchoNavigator system has potential value in all interventions requiring a precise transseptal puncture, such as during percutaneous mitral valve repair using the MitraClipTM system. The exact site of transseptal puncture within the fossa ovalis is crucial for the success of percutaneous MVR, where the distance from the puncture site to the valvular plane should not exceed or be much lower than 3.5-4.0 cm. This distance allows the MitraClipTM device comfortably to reach and grasp the mitral leaflets while it provides enough space for manipulations of the delivery system. Using the EchoNavigator system the optimal location for the transseptal puncture can be marked on the TEE images, and the same marker simultaneously appears on fluoroscopy (Figure 3), facilitating precise targeting of the predefined puncture site. Furthermore, the navigation of the steerable catheter within the left atrium is facilitated by the use of markers defining the target for the clip at the level of the mitral valve (Figure 4). In this case the origin of the maximal regurgitant jet can be marked and, accordingly, the device is steered towards this marker on fluoroscopy images. In our initial experience the use of markers has turned out to be immensely helpful for the closure of paravalvular leaks. The intubation of such a lesion can be very challenging and time-consuming by using either echocardiography or fluoroscopy guidance alone. By integrating both technologies, the localisation of the lesion and the evaluation of the surrounding structures are simpler, and the introduction of markers on fluoroscopy images facilitates targeting the lesion (Figure 5).
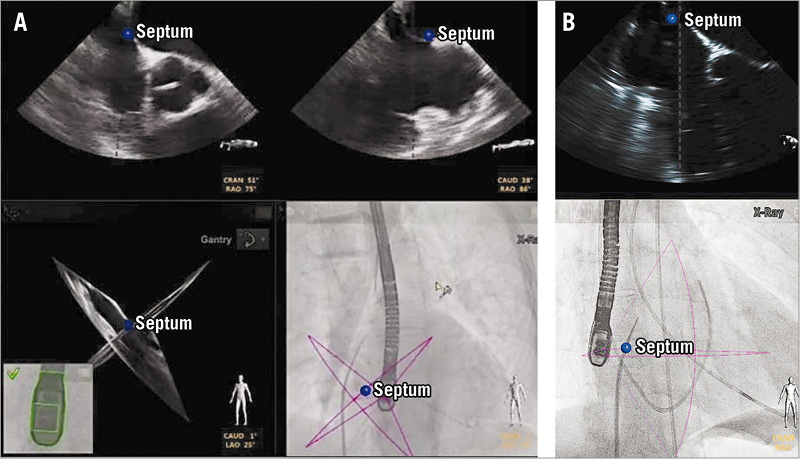
Figure 3. To perform a precise transseptal puncture, the desired location for puncture is defined on echocardiography and marked with a marker (blue dot), which simultaneously appears in the co-registered fluoroscopic image. The fluoroscopic view is then mainly used to orient the transseptal needle to the marker.
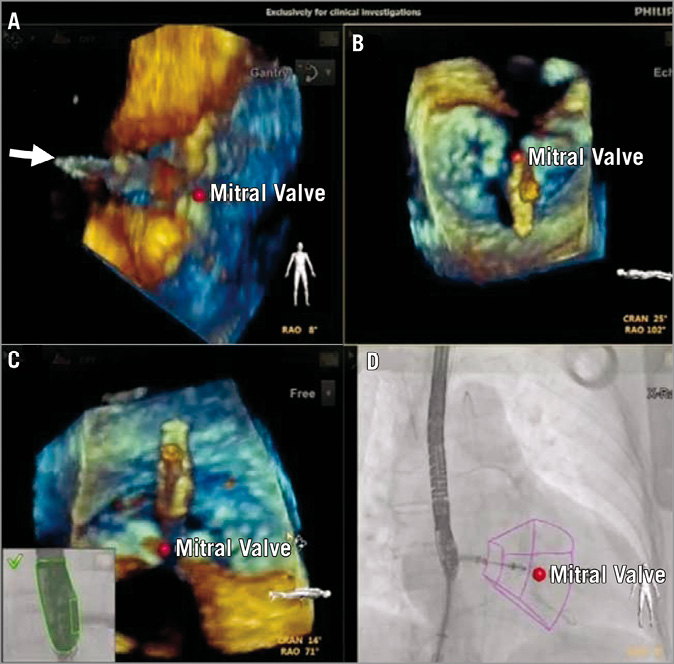
Figure 4. Percutaneous mitral valve repair using the MitraClip system. The simultaneous display of different oriented echocardiographic views allows different manoeuvres in targeting the lesion without the need to manipulate the TEE probe or the images by the echocardiographer. In this case, the 3-D TEE in the same orientation as the x-ray (A) allows fast appreciation of the perpendicularity of the MitraClip in approaching the target. The echocardiographer (B) and free (C) views allow orienting the clip within the valve (D), aiming at the predefined target (visualised as red point) and with the clip arm perpendicular to the line of leaflet coaptation.
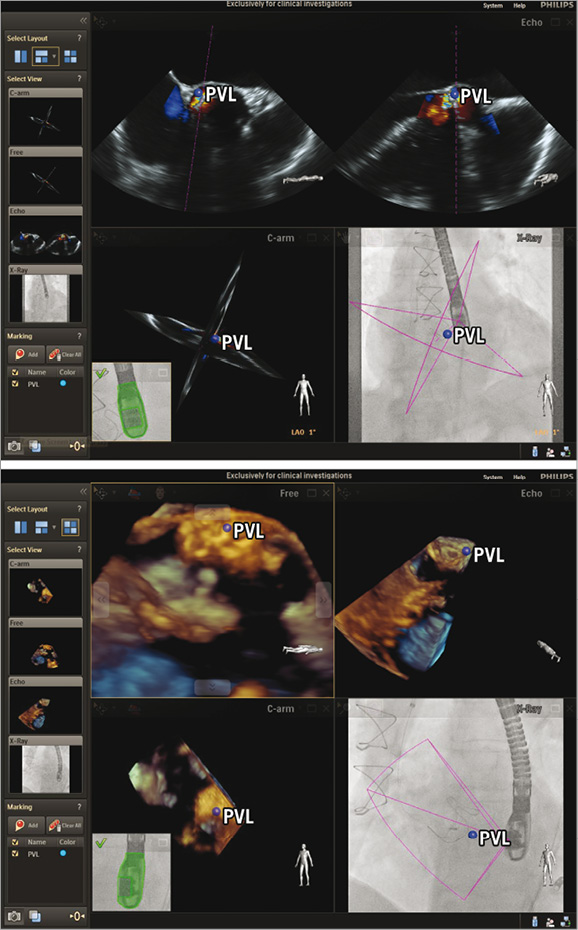
Figure 5. Percutaneous closure of a paravalvular leak in a biological aortic valve prosthesis. The paravalvular leak is marked with a blue dot in the x-plane echocardiographic images (upper panel). This marker is simultaneously displayed in all echocardiographic views as well as in the co-registered x-ray and allows easier targeting of the leak and placement of the occluder. The implanted Amplatzer Vascular Plug III is visualised with 3-D echocardiography confirming the accuracy of the marker.
Conclusion
The EchoNavigator system is the first multimodality system conceived to integrate and synchronise echocardiography and fluoroscopy images in real time and thereby facilitate SHD interventions (such as percutaneous MVR, PVL closure, LAA closure). It provides the freedom to explore cardiac structures and their interaction with catheters and devices at the table site, opening new frontiers in interventional cardiology and the use of 3-D echocardiography. During its initial development and validation period, we used the system in a broad spectrum of SHD interventions to understand its potential application. SHD interventions can often be complex and demanding, and they require a lot of interaction between the operator and the echocardiographer. In our experience, the EchoNavigator system facilitates our understanding of the anatomy by providing the ability to reproduce new and unusual echocardiographic projections by manipulating the images at the table site. The main advantages are the simultaneous display of real-time multimodality images, and the co-registration of echocardiographic and fluoroscopic images. This improves the communication between the team performing the intervention and the imaging specialist. In addition, the use of the marker feature, which allows the placement of markers on echocardiography and displays those simultaneously on co-registered fluoroscopy images, facilitates targeting of cardiac structures and reduces procedure time and radiation exposure. A potential disadvantage is an excess of visual information that could distract an inexperienced operator during the intervention.
Further research is necessary to demonstrate the benefit of fused imaging for interventions in SHD with respect to procedure and fluoroscopy time as well as procedural safety and success.
Conflict of interest statement
P. Biaggi, O. Gaemperli, and R. Corti have received speaker fees from Abbott Vascular. R. Corti and P. Biaggi are faculty at the Crossroad Institute in Brussels (Belgium). O. Gaemperli is further supported by a research grant from Abbott Vascular, Brussels, Belgium. R. Corti and J. Grünenfelder received proctor fees from Edwards Lifesciences. V. Falk received an unrestricted grant from Philips. R. Corti received a consultancy fee from Philips. O. Kretschmar has received consultant honoraria from Medtronic. The other authors have no conflicts of interest to declare.




