Abstract
Aims: The first-in-man ABSORB Cohort A trial demonstrated the bioresorption of the ABSORB BVS (Abbott Vascular, Santa Clara, CA, USA) at two years. This report describes the 4-year clinical outcomes.
Methods and results: The ABSORB Cohort A trial enrolled 30 patients with a single de novo native coronary artery lesion. Clinical follow-up was available in 29 patients since one patient withdrew consent after the six month follow-up. At four years, the hierarchical ID-MACE of 3.4% remained unchanged. Clopidogrel therapy had been discontinued in all patients.
Conclusions: Four-year clinical results demonstrate a sustained low MACE rate (3.4%) without any late complications such as stent thrombosis.
Introduction
Bioresorbable polymeric drug-eluting scaffolds were developed as an alternative to metallic drug-eluting stents with the aim of providing transient vessel support combined with drug delivery capability without the long-term limitations of permanent metallic stents.
The ABSORB everolimus-eluting bioresorbable vascular scaffold (Abbott Vascular, Santa Clara, CA, USA) was tested in the first-in-man ABSORB Cohort A trial enrolling 30 patients with a single de novo coronary artery lesion. The two- and three-year follow-up of the trial were previously reported.1-3
We report the four-year clinical results of the ABSORB Cohort A trial.
Methods
Study design, device and endpoint definitions
The study design and device is reported elsewhere.1-3 Clinical endpoints assessed at four years included ischaemia-driven major adverse cardiac events (ID-MACE) and individual components of MACE. All MACE events were adjudicated by an independent Clinical Events Committee.
Statistical analysis
For binary variables, percentages are calculated. The analysis presented in this report is based on the intention-to-treat population.
Role of funding source
This study was sponsored by Abbott Vascular (Santa Clara, CA, USA) and registered with ClinicalTrials.gov, number NCT00300131.
Results
One patient withdrew consent from the study after the six-month follow-up, but his vital and clinical status remained available through his referring physicians. Two patients died from non-cardiac causes and 4-year clinical follow-up was obtained in the remaining 27 patients.
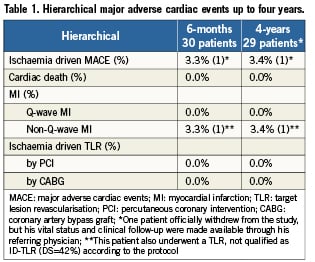
Baseline demographic, clinical, and angiographic characteristics are described in full elsewhere3. Clinical outcomes are shown in Table1. Up to four years, there was only one non-Q-wave MI related to the treatment of a non-flow-limiting stenosis (quantitative coronary angiography [QCA] diameter stenosis, 42%) of an ABSORB BVS at 46 days post-procedure2. Overall, there were no new ID-MACE events between six months and four years. In the entire cohort, there was no instance of stent thrombosis according to either the protocol or ARC definitions.4 At one year, fifteen patients were on dual antiplatelet therapy, while at four years, clopidogrel therapy had been discontinued in all patients.
Discussion
This study represents the longest available follow-up of ABSORB BVS and, thereby, provides important data on the long-term efficacy and safety of the ABSORB BVS. This short report on 4-year clinical outcomes of the ABSORB Cohort A trial demonstrates a low ischaemia-driven MACE rate of 3.4% without any scaffold thrombosis. There were no additional ID-MACE between six month and four years. The favourable clinical results demonstrated in this study need to be further confirmed in a large study.
Appendix
Clinical Events Committee: C. Hanet, MD, PhD, Brussels, Belgium; D. McClean, MD, Christchurch, New Zealand; V. Umans, MD, PhD, Alkmaar, The Netherlands
Data Safety Monitoring Board: J. Tijssen, PhD, Amsterdam, The Netherlands; T. Lefèvre, MD, Massy, France; P. Urban, MD, Geneva, Switzerland
Conflicts of interest statement
D. Dudek and J. A. Ormiston are members of the advisory board of Abbott Vascular; K. Miquel-Hebert is an employee of Abbott Vascular. The other authors have no conflicts of interest to report.




