Abstract
Individualised, patient-centred care is a central tenet of modern medicine. The variety of transcatheter heart valves currently available affords the opportunity to select the most appropriate device for each individual patient. Prosthesis selection should be based on operator experience and pre-procedural multimodal three-dimensional imaging. Herein, we outline a number of clinical scenarios where specific transcatheter heart valve technologies have the potential to optimise clinical outcome.
Introduction
Until recently, most centres performing transcatheter aortic valve implantation (TAVI) used a single prosthesis type. While this practice appears somewhat restrictive given the fact that the same centres probably implant a variety of drug-eluting stents, pacemakers, and surgical heart valves, the relative infancy of TAVI has mandated that centres become proficient in one technology at a time.
Of course, the transcatheter heart valve (THV) landscape has changed considerably in recent years and TAVI is now considered to be a standardised, mature therapy, with hundreds of thousands of treated patients worldwide. Refined patient selection, pre-procedural anatomic assessment with multislice computed tomography (MSCT), growing operator experience, the emergence of new valves supported by prosthesis-specific education and proctoring, and relentless iteration of the foundation devices have manifested impressive reductions in procedural mortality, vascular complications, paravalvular leak, and other adverse events. In particular, the availability of an array of new THV systems (Figure 1), each with unique technical features and potential advantages, coupled with a much greater understanding of individualised patient anatomy, has afforded the TAVI operator the opportunity to develop a more patient-centric approach to TAVI.
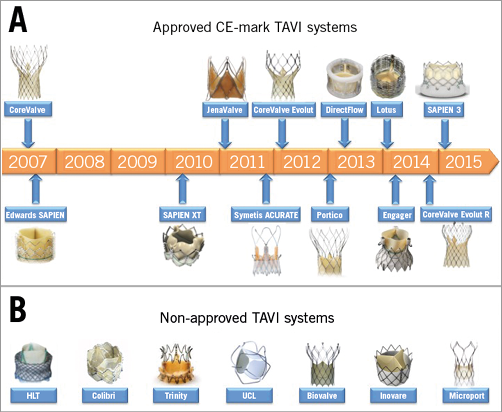
Figure 1. Approved and non-approved TAVI devices. A) CE-mark TAVI systems. CoreValve® (Medtronic, Dublin, Ireland); SAPIEN (Edwards Lifesciences, Irvine, CA, USA); SAPIEN XT (Edwards Lifesciences); JenaValve (JenaValve Technology GmbH, Munich, Germany); Symetis ACURATE™ (Symetis SA, Ecublens, Switzerland); CoreValve® Evolut™ (Medtronic); Portico™ (St. Jude Medical, St. Paul, MN, USA); Direct Flow Medical Aortic Valve™ (Direct Flow Medical Inc., Santa Rosa, CA, USA); Lotus™ (Boston Scientific, Marlborough, MA, USA); Medtronic Engager™ (Medtronic); SAPIEN 3 (Edwards Lifesciences); CoreValve Evolut R (Medtronic). B) Non-approved TAVI systems. HLT valve (Heart Leaflet Technologies Inc., Maple Grove, MN, USA); Colibri valve (Colibri Heart Valve, LLC, Broomfield, CO, USA); Trinity valve (Transcatheter Technologies GmbH, Regensburg, Germany); TRISKELE valve (University College London, London, UK); Biovalve (Biotronik AG, Bülach, Switzerland); Inovare® (Braile Biomedica, São José do Rio Preto, Brazil); MicroPort® (MicroPort Medical Group, Shanghai, China).
It is important to acknowledge that introducing a second or third transcatheter technology to a centre can be challenging. Unlike coronary stents, each THV system has unique features that must be mastered, and the devices can react differently in diverse anatomies and clinical situations. Device-specific education and training for physicians and allied staff is required, and a period of proctoring is essential. Nevertheless, a learning curve can be expected as staff acclimatise to the new technology. It is also questionable whether low-volume centres (<30 TAVI per annum) have sufficient experience to use multiple technologies safely.
While most patients can be treated successfully with a single balloon-expanding, self-expanding, or mechanically expanding THV system, specific situations do arise where the availability of an alternative valve is beneficial. In such cases, it is difficult to justify a suboptimal result simply because an alternative technology was not accessible. Herein, we detail clinical situations where specific TAVI devices may be beneficial.
Iliofemoral anatomy
Transfemoral vascular access for TAVI is considered to be the route of choice. This technique reduces morbidity and even mortality compared to transthoracic TAVI1, and expedites patient functional recovery. The ratio of the outer diameter of the vascular access sheath to the femoral artery is the most powerful predictor of major vascular complications2. The downsizing of delivery catheter dimensions of several THV systems has therefore increased the proportion of patients suitable for the transfemoral approach. The CoreValve® Evolut™ R (23, 26, 29 mm; Medtronic, Dublin, Ireland) and SAPIEN 3 (23, 26 mm; Edwards Lifesciences, Irvine, CA, USA) THVs can be implanted using 14 Fr InLine™ (Medtronic) or expandable-sheath technology, respectively. These low-profile systems can facilitate transfemoral TAVI in vessels as small as 5.5 mm (SAPIEN 3) or even 5.0 mm (Evolut R).
Aortic root anatomy
Most patients considered for TAVI undergo multimodal imaging assessment of the aortic root. Multislice computed tomography (MSCT) and/or three-dimensional echocardiography are the imaging modalities of choice (according to availability and local expertise) to evaluate the annular dimensions (diameters, area, perimeter) and unique anatomical features of each patient. A variety of adverse anatomical features may complicate THV implantation, and in such cases the availability of multiple TAVI technologies can facilitate procedural safety.
BORDERLINE SIZING
All THVs have specific manufacturer guidelines for valve sizing. Each device covers a range of annular sizes and, therefore, varying degrees of THV oversizing for an annular range. For example, a 26 mm CoreValve Evolut R is suitable for annular diameters between 20 and 23 mm and yields perimeter oversizing between 13 and 30%. Not infrequently therefore, clinicians encounter cases where the annular dimensions fall between two valve sizes for a specific THV system. In these “borderline” cases, there is an often difficult choice between accepting less oversizing and the risk of paravalvular leak (PVL), or opting for more oversizing and the risk of annular rupture. In such instances, the availability of both balloon-expandable and self-expanding TAVI systems can be of considerable benefit, as annular sizes considered borderline for balloon-expandable devices (e.g., SAPIEN XT; Edwards Lifesciences) are within a favourable range for a self-expanding THV (e.g., CoreValve; Medtronic Inc.). In a multicentre study including 615 TAVI recipients, Dvir et al demonstrated that significant oversizing with either the SAPIEN XT or CoreValve was associated with adverse outcomes, and suggested that more favourable outcomes could have been achieved in such cases by switching to an alternative device3. These authors concluded that an individualised approach to device selection (not just device size) has the potential to improve outcome.
The introduction of the SAPIEN 3 valve, with the option to “undersize” the valve relative to the annulus or “overexpand” with extra contrast in the balloon, has reduced the frequency of truly borderline SAPIEN cases. Similarly, “undersizing” the THV has been more frequently observed with the Lotus™ valve (Boston Scientific Corporation, Marlborough, MA, USA).
CALCIUM QUANTIFICATION AND DISTRIBUTION
Left ventricular outflow tract (LVOT) calcification has been identified as the most important anatomic predictor of aortic root rupture during balloon-expandable TAVI4 (Figure 2). In such cases, physicians can opt to use a smaller THV size and risk greater PVL, or can remove contrast from the balloon to minimise the risk of rupture. An alternative solution is to use a self-expanding or mechanically expandable THV for these patients. While this strategy may indeed result in a greater risk of PVL, it avoids the potential for catastrophic aortic root rupture and appears to be gaining popularity worldwide.
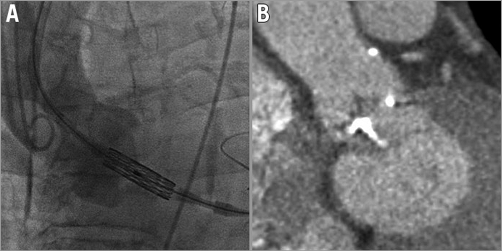
Figure 2. Anatomical hurdles in TAVI. A) Left transcarotid implantation of the SAPIEN 3 valve in a vertical annulus facilitated by the deflectable Certitude delivery catheter. B) Left ventricular outflow tract calcification presenting a significant risk of annular rupture with a balloon-expandable system.
AORTIC ROOT ANGULATION
Highly angulated aortic root anatomy (“vertical annulus” or “horizontal aorta”) can render THV implantation extremely challenging as coaxial implantation of the valve is compromised (Figure 2). Even when the aortic root is not excessively angulated, accurate implantation can be difficult due to the stiffness of the delivery system, tortuosity of the peripheral vasculature, or if right common carotid/right subclavian artery vascular access is used. Indeed, aortic root angulation >30° is considered to be a relative contraindication for CoreValve implantation via the right subclavian artery.
Non-coaxial valve deployment can result in valve embolisation, limited control of implantation depth, repeated valve resheathing and recapture, and the consequences of inaccurate deployment: moderate-severe PVL, new pacemaker implantation, mitral regurgitation, coronary occlusion, and requirement for a second valve. The availability of deflectable delivery catheters, such as the Edwards Certitude and Commander systems, and the ability to resheath and reposition the valve can greatly facilitate THV implantation, even in the most angulated anatomies.
CORONARY ARTERY OCCLUSION
Coronary artery occlusion is a rare but devastating complication of TAVI5 whose incidence has decreased since the advent of MSCT sizing and the recognition of anatomic risk factors: low height coronary ostia (<10 mm); narrow aortic sinuses (<2 mm diameter on either side of the THV); bulky and heavily calcified leaflets; and specific valve-in-valve procedures (e.g., Mitraflow and Freedom valves [Sorin Group, Milan, Italy]). Nevertheless, the availability of recapturable and repositionable TAVI systems should be considered in cases where the possibility of coronary occlusion is considered significant.
Bicuspid aortic valve
In patients with bicuspid aortic valve (BAV) stenosis, abnormal cusp fusion, asymmetry of the valve orifice and annulus, heavily calcified and fibrotic leaflets, and calcified raphe have the potential to yield suboptimal results with TAVI. Indeed, an early study of TAVI in BAV using first-generation devices reported a higher incidence of moderate PVL relative to tricuspid aortic valve stenosis6. Although MSCT imaging was sporadically applied in this nascent study (thereby making the results difficult to interpret), there is clearly a suggestion that BAV presents unique procedural challenges for TAVI. Overcoming these obstacles is of paramount importance as TAVI extends to younger patient cohorts with a higher prevalence of BAV morphology.
Recently, Perlman et al reported a multicentre experience of the SAPIEN 3 valve in 51 patients with BAV stenosis7. In this small series, there was no valve embolisation, no requirement for a second valve, and no patient had greater than mild PVL. The requirement for a new pacemaker was relatively high (23.5%), perhaps reflecting the proximity of the non-right commissure to the membranous septum.
It is also likely that the Lotus valve, Direct Flow valve (Direct Flow Medical, Santa Rosa, CA, USA), and repositionable self-expanding valves (Evolut R [Medtronic] and Portico [St. Jude Medical, St. Paul, MN, USA) will overcome some of the limitations of the foundation THV devices in treating BAV anatomy. In particular, the ability to reposition the device and the presence of a sealing skirt or membrane appear central to improving results. It is also notable that in China, where up to 50% of all TAVI recipients have BAV, self-expanding devices with significantly enhanced radial force have been specifically developed to manage the greater burden of calcium seen in patients with BAV (e.g., MicroPort®; MicroPort Inc., Shanghai, China).
Accumulating experience with TAVI in BAV stenosis has led to the emerging concept of supra-annular sizing. This technique refers to the selection of a THV sized not to the annulus (annular sizing), but rather to the area of maximal calcification and minimal aortic root diameter. This usually occurs 2-4 mm cranial of the annulus, and is more frequently observed in BAV stenosis due to the presence of calcified raphe and more diffusely distributed calcium throughout the body of the leaflets than in trileaflet aortic valve stenosis. It has been acknowledged for some time that THVs do not just anchor and seal at annular level, but along the entire leaflet length. It is likely that supra-annular anchoring and sealing is occurring in many cases of alleged THV “undersizing”. “Undersizing” is therefore an unhelpful misnomer which should be avoided as it has the potential to create uncertainty regarding THV sizing.
Aortic regurgitation
Pure aortic regurgitation is perhaps the least studied application of THV technology. An early assessment of foundation devices in this setting suggested an increased requirement for valve-in-valve procedures (18.6%), a higher incidence of PVL (≥ grade 2: 21%), and lower Valve Academic Research Consortium-defined success rates of 74.4%8. These suboptimal results relate to the absence of valvular calcification which impedes the ability of the THV to anchor, and perhaps instability of the prosthesis during deployment caused by the regurgitant jet. Interaction characteristics between the valve and anatomy differ between aortic stenosis and aortic regurgitation, and specific oversizing principles are required for non-calcified regurgitant valves (for self-expanding THV, >25% perimeter oversizing). Medical device companies and the interventional community must endeavour to develop this important information in the near future.
In September 2013, the JenaValve (JenaValve Technology GmbH, Munich, Germany) received CE-mark approval for the treatment of pure aortic regurgitation. This transapical system clips the native leaflets and does not rely on radial force for anchoring at the level of the aortic annulus. Results in a small multicentre study are encouraging but the requirement for transapical access has limited the adoption of this technology.
Several case reports have documented the use of repositionable devices in the setting of pure aortic regurgitation. Selection of these devices seems appropriate as they can be removed if suboptimal results are achieved.
Failing surgical aortic valves
The use of THV technology for the treatment of failing surgical aortic bioprostheses has greatly reduced the number of high-risk patients requiring redo surgical aortic valve replacement. All available THV systems have been successfully used in this setting, with universally acceptable results9. One exception, however, is in patients with small surgical bioprostheses (<21 mm). In such cases, the use of larger-than-necessary devices can lead to high transvalvular gradients (>20 mmHg), ongoing symptoms, and increased mortality. Therefore, while it remains feasible to implant any device in small anatomies, the best haemodynamic results can be expected when smaller devices or those with supra-annular leaflets are used. In the Valve-in-Valve International Data (VIVID) Registry, elevated post-procedural gradients were more common with balloon-expandable devices compared to self-expanding devices (HR 1.87, 95% CI: 1.21-2.9; p=0.005) for small and intermediate-sized surgical valves (41.2% vs. 23.4%, p=0.04; 35.8% vs. 19.4%, p=0.01, respectively)9. It is thus recommended that redo surgery be strongly considered in patients with internal surgical valve diameters <21 mm and that the 20 mm SAPIEN XT or 23 mm CoreValve Evolut R be used when redo surgery is not feasible. The ViV Aortic smartphone application provides an excellent resource for guiding device selection and implantation for these procedures.
One possible advantage of using repositionable (rather than balloon-expandable) technology in this setting is the potential to reduce the requirement for a second THV. In the VIVID Registry, a second device was implanted in 5.7% of patients (self-expanding 7.5% vs. balloon-expandable 4.1%; p=0.05)9. The option to recapture is also of particular relevance when the original surgical prosthesis presents a high risk of coronary occlusion or when control of implantation depth may be challenging (e.g., severe aortic regurgitation).
Non-aortic valve intervention
THV technology has also been applied to mitral and tricuspid valve-in-valve or valve-in-ring procedures and to the treatment of dysfunctional right ventricle-to-pulmonary artery conduits. These procedures were initially performed exclusively with the SAPIEN XT valve but the Lotus and Direct Flow prostheses have been successfully used more recently for mitral interventions. Again, the ViV Mitral smartphone application provides guidance for these procedures. Self-expanding prostheses are not applicable for non-aortic valve interventions.
Conclusion
A variety of clinical scenarios exists where the availability of multiple transcatheter devices in a single institution may enhance individual patient outcomes. An individualised approach to TAVI device selection based on three-dimensional pre-procedural imaging is therefore advocated.
Conflict of interest statement
D. Mylotte, T. Modine and N. Piazza are consultants for Medtronic and MicroPort. M. Lee has no conflicts of interest to declare.




