
Abstract
Aims: Our aim was to determine whether use of the filter-based Sentinel™ Cerebral Protection System (CPS) during transcatheter aortic valve implantation (TAVI) can affect the early incidence of new brain lesions, as assessed by diffusion-weighted magnetic resonance imaging (DW-MRI), and neurocognitive performance.
Methods and results: From January 2013 to July 2015, 65 patients were randomised 1:1 to transfemoral TAVI with or without the Sentinel CPS. Patients underwent DW-MRI and extensive neurological examination, including neurocognitive testing one day before and five to seven days after TAVI. Follow-up DW-MRI and neurocognitive testing was completed in 57% and 80%, respectively. New brain lesions were found in 78% of patients with follow-up MRI. Patients with the Sentinel CPS had numerically fewer new lesions and a smaller total lesion volume (95 mm3 [IQR 10-257] vs. 197 mm3 [95-525]). Overall, 27% of Sentinel CPS patients and 13% of control patients had no new lesions. Ten or more new brain lesions were found only in the control cohort (in 20% vs. 0% in the Sentinel CPS cohort, p=0.03). Neurocognitive deterioration was present in 4% of patients with Sentinel CPS vs. 27% of patients without (p=0.017). The filters captured debris in all patients with Sentinel CPS protection.
Conclusions: Filter-based embolic protection captures debris en route to the brain in all patients undergoing TAVI. This study suggests that its use can lead to fewer and overall smaller new brain lesions, as assessed by MRI, and preservation of neurocognitive performance early after TAVI. Clinical Trial Registration: Dutch trial register-ID: NTR4236. URL http://www.trialregister.nl/trialreg/admin/rctsearch.asp?Term=mistral
Introduction
Transcatheter aortic valve implantation (TAVI) is less invasive and results in faster recovery and improvement in quality of life as compared to surgical aortic valve replacement (SAVR)1-6. In selected patients TAVI also reduces one-year mortality7. Major stroke is still a vexing complication associated with aortic valve replacement8. Recent studies suggest similar stroke rates with SAVR and TAVI, varying between 2 and 10%9. Transcranial Doppler (TCD) and brain diffusion-weighted magnetic resonance imaging (DW-MRI) studies revealed, respectively, cerebral high-intensity transient signals (HITS) and new ischaemic brain lesions in up to 90% of all patients undergoing TAVI10-13. Approximately half of all strokes within 30 days after TAVI occur in the first 24 hours and are thus directly related to the procedure14-16. TAVI inevitably releases debris from the aortic wall, the aortic annulus and even from cardiac structures, and catheter-related foreign body particles17,18. Recently, the randomised DEFLECT III trial demonstrated fewer DW-MRI-detected ischaemic brain lesions and less cognitive decline with the use of the TriGuard™ cerebral embolic protection device (Keystone Heart Ltd., Caesarea, Israel)19. The Sentinel™ Cerebral Protection System (CPS) (Claret Medical Inc., Santa Rosa, CA, USA) provides filter protection to the brachiocephalic trunk and the left common carotid artery. The safety and efficacy of the device were demonstrated and the device obtained CE mark in January 201420. Furthermore, recent pathology studies have confirmed capture of debris with the Sentinel CPS in 75 to 86% of all patients undergoing TAVI17,21. The clinical impact of this embolised debris into the brain and consequent new ischaemic brain lesions by DW-MRI is controversial, but silent brain infarcts have been correlated with premature neurocognitive deterioration and dementia22,23. The aim of the randomised MRI Investigation in TAVI with Claret (MISTRAL-C) study (Dutch trial register-ID: NTR4236) is to determine whether use of the Sentinel CPS during TAVI can decrease the incidence of new brain lesions as assessed by DW-MRI, and can prevent neurocognitive decline.
Methods
The MISTRAL-C was a multicentre double-blind randomised trial. All eligible patients underwent multimodality imaging, including multislice computed tomography (MSCT) scan of the aortic valve and the arterial vasculature. Patients were deemed at high risk for SAVR and selected for transfemoral TAVI by Heart Team consensus. Aortic arch anatomy had to fit the sizing requirements for the Sentinel CPS: the brachiocephalic trunk and left common carotid artery should range between 9 and 15 mm and 6.5 and 10 mm, respectively, without excessive tortuosity or >70% obstructive atherosclerotic disease. Key exclusion criteria were the presence of a permanent pacemaker or automated internal cardiac defibrillator (AICD) at baseline, a history of prior stroke with sequelae and dementia. Patients were randomised 1:1 to TAVI with or without the Sentinel CPS. Per protocol, a DW-MRI scan and extensive neurological examination were performed one day before and planned again five to seven days after TAVI. One dedicated experienced neuroradiologist independently read all MRI studies. A trained neurology specialist performed a comprehensive neurological exam, including the National Institutes of Health Stroke Scale (NIHSS) and the modified Rankin Scale (mRS), and a neurocognitive evaluation with the Montreal Cognitive Assessment (MoCA) and the Mini-Mental State Examination (MMSE)24,25. The Center for Epidemiologic Studies Depression scale (CES-D) was used to rule out significant depression. The neuroradiologist and neurology specialists were blinded to the randomisation arm. The most recent Valve Academic Research Consortium definitions were applied to report relevant clinical endpoints26. The local institutional review board at each site approved the study protocol, all subjects provided written informed consent, and the study was conducted in accordance with the principles of the Declaration of Helsinki. The Erasmus Medical Center received a research grant from Claret Medical which partially covered study-related expenses. The authors are fully responsible for the study design, study execution and drafting of the manuscript.
SENTINEL CPS
The Sentinel CPS is a 6 Fr-compatible 100 cm coaxial, steerable sheath housing two cone-shaped filters made of a 140 um pore size biocompatible polyurethane film. The device is inserted using a right radial or brachial arterial access. The proximal filter is first deployed into the brachiocephalic trunk. The distal segment of the catheter can then articulate to navigate through the aortic arch and into the left common carotid artery where the distal filter is deployed (Figure 1). At the end of the TAVI procedure, the previous steps are reversed.
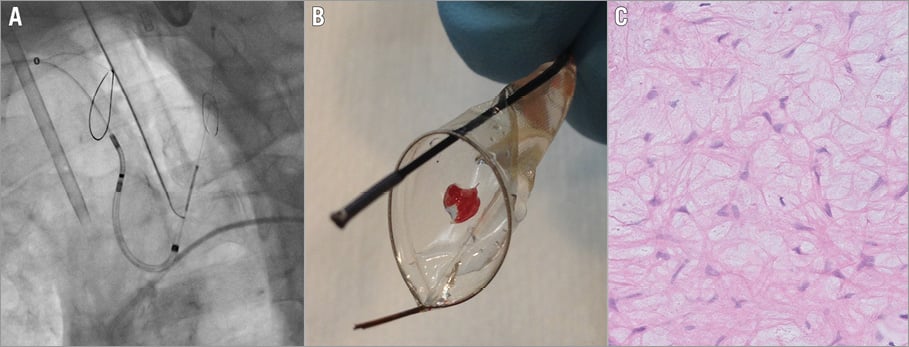
Figure 1. Sentinel dual filter system. A) Fluoroscopic image of the Sentinel CPS after deployment in the brachiocephalic trunk and left common carotid artery. B) Photograph of a retrieved filter containing embolic debris. C) Microscopic image showing the lamina spongiosa of the aortic valve (H&E staining, magnified ×20).
MAGNETIC RESONANCE IMAGING
The MRI exam was performed with a 3.0 Tesla scanner with an 8-channel head coil. The MRI protocol consisted of three sequences: 1) transverse DW-MRI sequence with a b-value of 0,500,1000 s/mm² (SE/EPI, TR 8,000 ms, TE 80 ms, FOV 24×24 cm, matrix 128×128, slice thickness 3.6 mm, 3 NEX); 2) sagital 3D-FLAIR sequence (TR 6,500, TE 115, FOV 26×26 cm, matrix 224×224, slice thickness 1.2 mm, NEX 1); 3) 2D-T2w TSE sequence (TR 5,000 ms, TE 105 ms, FOV 24×24 cm, matrix 416×384, slice thickness 3 mm, NEX 2). The number, location, and volume (cm) of new hyperintense lesions were recorded. New lesions were allocated to the cerebellum, or the left or right vascular territory of the anterior, medial or posterior cerebral artery. To calculate the volume of hyperintense lesions on DWI, a semi-automated segmentation method was developed using MeVisLab (MeVis Medical Solutions AG, Bremen, Germany)27. The brain was arbitrarily divided into Sentinel CPS protected and unprotected regions. Unprotected regions are vulnerable to embolisations coming from the unprotected left vertebral artery, which corresponds to the cerebellum and the vascular territory of both posterior cerebral arteries.
HISTOPATHOLOGY
Filters were retrieved and released from the delivery system, stored in a buffered formalin (4%) solution. Debris was dehydrated, embedded in paraffin and cut into 3 to 4 mm thick sections. Staining was done with haematoxylin and eosin and Movat pentachrome. Additional staining techniques were performed whenever applicable to identify specific tissue origin, as previously described17.
STATISTICAL ANALYSIS
Power analysis was based on the primary endpoint of new cerebral lesions by DW-MRI five to seven days after TAVI. To reach a reduction from 80% to 40% in volume of new ischaemic lesions by DW-MRI (standard deviation 50%) with the Sentinel CPS and based on the continuity-corrected chi-square test, we estimated that 54 patients (27 in each treatment arm) would be needed with an 80% power and a two-sided alpha of 0.05. To balance a potential 20% drop-out in MRI follow-up, 65 patients would be needed to obtain 54 patients with MRI before and after TAVI. Continuous variables were displayed as either mean±standard deviation or median with interquartile range, depending on distribution. Normality was tested by use of histograms and the Shapiro-Wilk test. Normally distributed variables were compared using a Student’s t-test, while non-normally distributed variables were compared using the Kruskal-Wallis test. Categorical variables were displayed as frequencies and percentages. A chi-square test for equality of proportions was used for trends. Between-group comparisons for new brain lesions and neurocognitive function were restricted to patients with MRI or neurocognitive testing pre and post TAVI. Binary outcomes were compared using log-linear regression and were displayed as relative risks. All statistical analyses were performed with SPSS, Version 22 (IBM Corp., Armonk, NY, USA).
Results
From January 2013 to July 2015, 65 patients were randomised 1:1 to transfemoral TAVI with or without cerebral protection with the Sentinel CPS (Figure 2) at four centres. Table 1 depicts baseline characteristics. The median age was 81 years (IQR 78-85) and 52% were male. The STS predicted risk of mortality was 4.8% (IQR 3.4-7.2), and appeared higher in the control cohort (STS 6.6 [IQR 3.8-9.9] vs. 4.6 [IQR 3.4-6.4]). Frailty was common (68%). A prior history of neurological events was present in 19% of patients. The distribution of the different transcatheter valve designs is displayed in Figure 3. The Sentinel CPS was successfully deployed in all but two patients. In one patient no Sentinel CPS was inserted because of protracted haemodynamic instability after induction of general anaesthesia. One patient was a screening failure and presented with an anatomic anomaly (arteria lusoria) that precluded Sentinel CPS placement. There were no device-related injuries.
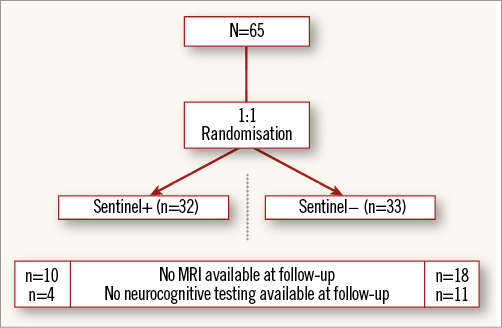
Figure 2. Patient flow diagram including follow-up missing for MRI and neurocognitive testing.
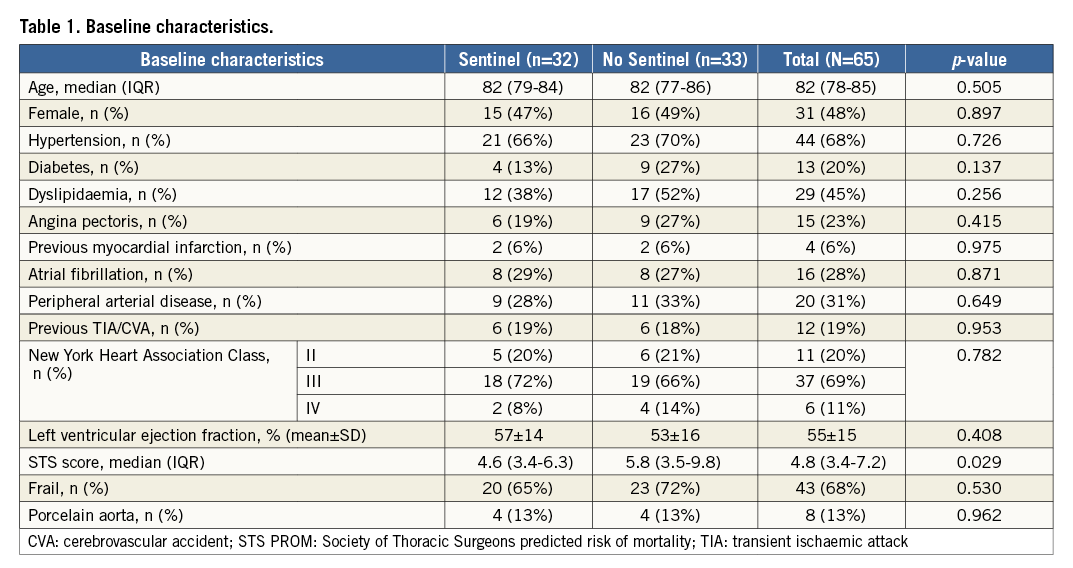
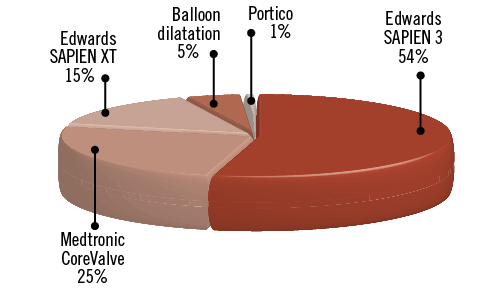
Figure 3. Relative proportion of various transcatheter heart valve designs used in the trial.
Clinical endpoints at 30-day follow-up are summarised in Table 2. Overall, all-cause mortality at 30 days was 3%. Two patients –both in the unprotected cohort– suffered a disabling stroke and died within 30 days. Twelve patients (19%) needed a new permanent pacemaker after TAVI.
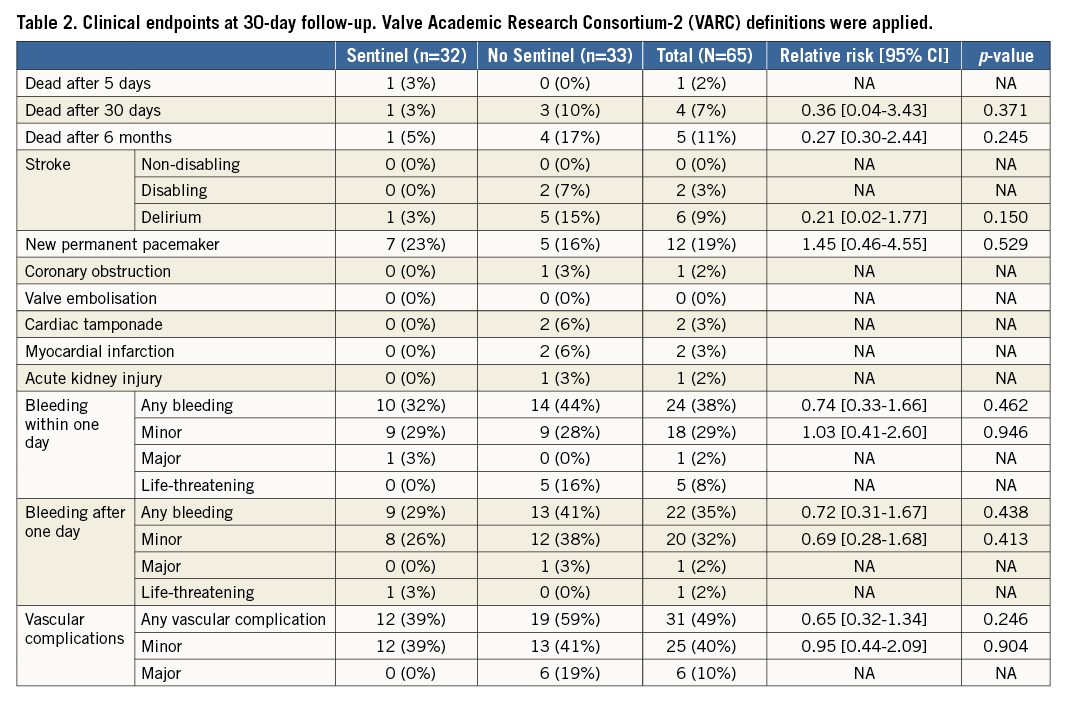
BRAIN MRI
Baseline brain MRI assessment confirmed ischaemic lesions in 11% of patients. Follow-up MRI was completed in 57% of the patients a mean of 5.0±1.1 days post TAVI. Twenty-eight patients did not undergo a follow-up MRI for the following reasons: implantation of a non-MRI-compatible pacemaker (n=10), patient refusal (n=6), unstable clinical condition/deceased (n=5), logistical challenges (n=4) and delirium (n=3). Overall, 78% of patients with follow-up MRI had new brain lesions. There were numerically fewer new lesions and a smaller total lesion volume (95 mm3 [IQR 10-257] vs. 197 mm3 [95-525]) in patients with Sentinel CPS protection (Figure 4, Figure 5). The difference was driven by fewer lesions and smaller total lesion volume (0 mm3 [IQR 0-102] vs. 76 mm3 [IQR 40-221, p=0.057]) in the protected lobes. No difference in single lesion volume was apparent (Figure 5). Overall, 27% of Sentinel CPS patients and 13% of control patients had no new lesions (Figure 4). Ten or more new brain lesions were found only in the control cohort (in 20% vs. 0% in the Sentinel CPS cohort, p=0.03). Half of the patients with Sentinel CPS protection had no new lesions in the protected lobes vs. 20% of patients without protection (p=0.04). There was no difference in the occurrence of new lesions in the unprotected lobes. Total lesion volume was greater in patients with self-expanding TAVI vs. balloon-expandable TAVI (693 mm3 [IQR 459-744] vs. 266 mm3 [IQR 155-358], p=0.067). In particular, the lesion volume in the posterior lobes was significantly greater with self-expanding THVs (405 mm3 [IQR 332-530] vs. 92 [IQR 40-240], p=0.037).
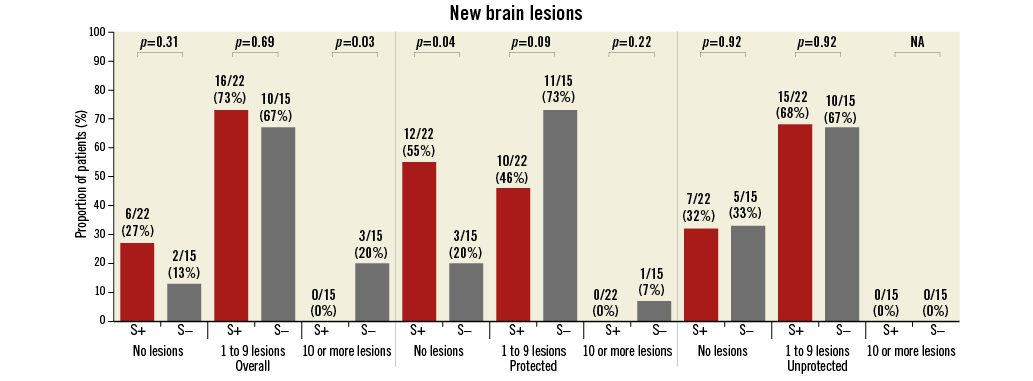
Figure 4. Occurrence and distribution of brain lesions by MRI.
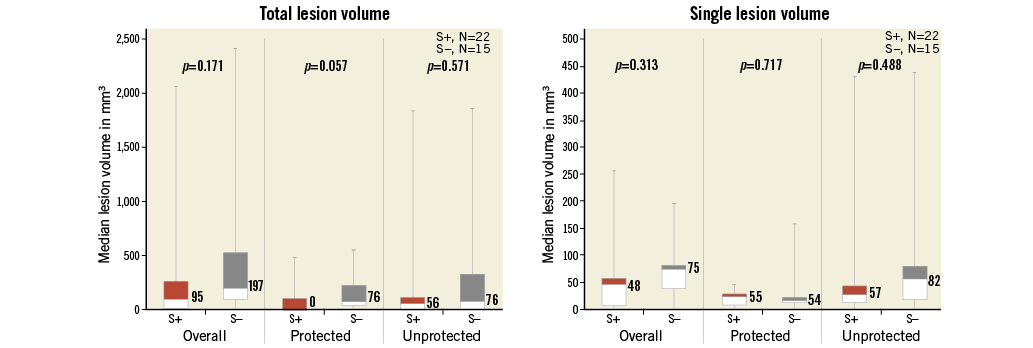
Figure 5. Brain lesion volumes at follow-up MRI. Left: overall lesion volume. Right: volume per lesion.
NEUROCOGNITIVE PERFORMANCE
Neurocognitive assessment was complete for all patients at baseline and for 80% at follow-up at a mean of 5±1.0 days after TAVI. Fifteen patients did not undergo follow-up neurocognitive testing, due to logistical issues (11 cases), delirium (two cases) and clinically unstable condition (two cases). Changes in neurocognitive performance were mainly identified through MMSE. MMSE score increased by 0.25±1.6 in patients with Sentinel CPS and decreased by 0.77±2.5 in the control group (p=0.086). Neurocognitive deterioration was present in one patient (4%) with the Sentinel CPS vs. six patients (27%) without (p=0.017) (Figure 6).
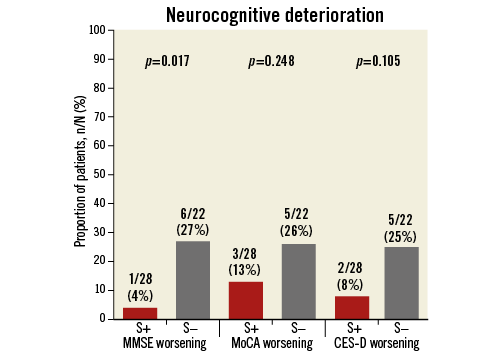
Figure 6. Relative proportion of patients with deterioration in neurocognitive performance after TAVI.
HISTOPATHOLOGY
Debris was found in all patients who were treated with the Sentinel CPS (Figure 7). Thrombotic material and tissue-derived material were present in 87% and 100% of patients, respectively. Tissue stemmed from the myocardium, aortic valve and/or atherosclerotic arterial plaques. Foreign body polymer material stemming from catheters and valve delivery systems appeared in 30% of all patients.
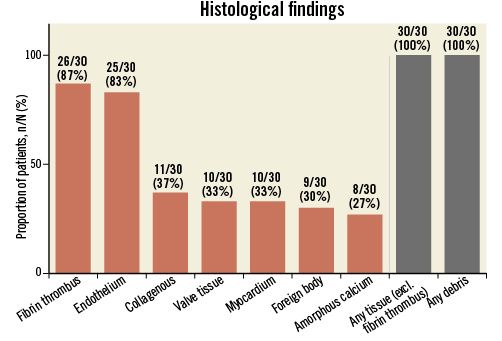
Figure 7. Frequency and characterisation of captured debris in all patients undergoing TAVI with Sentinel CPS protection.
Discussion
The MISTRAL-C trial is a mechanistic study that underscores the potential value of filter-based cerebral embolic protection with TAVI. Filters capture thrombotic and/or tissue-derived debris in all patients undergoing TAVI and will result in fewer and overall smaller ischaemic brain lesions in the protected brain areas and consequently preserve neurocognitive performance.
The primary endpoint of MISTRAL-C was the presence and volume of new ischaemic brain lesions as assessed by sequential (pre- and post-TAVI) MRI. Unfortunately, compliance with follow-up MRI appeared challenging in this population of octogenarians at high operative risk. A total of 43% of patients did not complete the follow-up MRI study, mainly because of the need for PPI and patient refusal. This loss to MRI follow-up parallels the 41% and 33% in the DEFLECT III and PROTAVI trials19,28. In MISTRAL-C, 78% of patients had new brain lesions at a median of five days after TAVI. This finding reconciles the previously reported 60-90% incidence of new brain lesions by MRI within one week after TAVI29. The use of filter protection did reduce the total number and the total volume of lesions. These benefits clustered in the areas irrigated by the carotid arteries and seem to fit with the fact that the current Sentinel CPS version does not protect the left vertebral artery. Over a quarter of patients undergoing TAVI with Sentinel CPS protection had no new brain lesions, while half had no new lesions in the protected lobes. In the PROTAVI pilot study, all patients developed new brain lesions post TAVI and use of Embrella embolic protection (Edwards Lifesciences Ltd, Irvine, CA, USA) did not affect lesion characteristics28. The randomised DEFLECT III trial reported freedom from ischaemic brain lesions in 21% of patients undergoing TAVI with TriGuard embolic protection and, furthermore, there were numerically fewer and smaller lesions19. A more detailed comparison between the various MRI studies evaluating different embolic protection devices is hazardous because of MRI field strength (1.5 vs. 3 Tesla), MRI analysis methodology and because the timing of MRI follow-up after TAVI was not uniform. DWI at a higher field strength is more sensitive, can detect smaller lesions, allows shorter acquisition time and has a higher signal-to-noise ratio18. Baseline mapping may be important to address existing lesions properly. Also, the number and size of detected lesions can change considerably within the first week post procedure. A short interval following TAVI is logistically and clinically challenging, yet longer intervals may miss transient brain injuries and new lesions may appear that are not immediately procedure-related. Kahlert et al demonstrated that 80% of the newly acquired brain lesions by MRI at a median of 3.4 days after TAVI had resolved three months later and thus represent ischaemic but not infarcted areas10.
In MISTRAL-C, two neurological events were described, both in patients without Sentinel CPS. This 3% disabling stroke rate fits with contemporary published TAVI data8,30. Paired neurocognitive testing comprised three screening tests and was complete in 80% of patients. Neurocognitive performance deteriorated more often in patients without Sentinel CPS. Only the MMSE showed significant dynamic changes around the TAVI procedure. In DEFLECT III, MoCA neurocognitive testing was performed, and paired assessments with baseline were available for 88% and 74% of patients at a mean of 5.6±2.2 days and 30 days, respectively19. Patients who underwent TAVI with TriGuard protection appeared to have less worsening in MoCA assessment. Ghanem et al used the repeatable battery for the assessment of neuropsychological status (RBANS) in 111 patients undergoing TAVI31. Procedural testing three days after TAVI could not be completed in 13% of patients because of critical illness, and transient early cognitive decline was detected in 6% (6/97).
Neurocognitive performance was similar to baseline at later time points up to two years. In the absence of solid guidelines, results of serial neurocognitive assessments need to be interpreted with caution. In fact, MMSE has not been developed for frequent serial testing, and changes of <2 points may still represent measurement error, regression to the mean, or a practice effect24. The comparison of TAVI studies involving serial brain MRI and neurocognitive assessment requires caution in the absence of uniformity in the timing and methodology of these tests18. Initiatives to harmonise further research in this field and provide guidance based on expertise and consensus are underway.
The current-generation Sentinel CPS offers filter protection to three of the four major arterial conduits to the brain, leaving the left vertebral artery unprotected. In general, the left vertebral artery is more dominant than the right vertebral artery and therefore has a larger vascular territory32. Filter effects should therefore predominantly manifest in the vascular territory of the anterior and medial cerebral arteries. Indeed, half of all patients with Sentinel CPS protection did not have new lesions in the protected brain regions and new lesions appeared smaller. The appearance of new subclinical ischaemic brain lesions and microinfarcts may pose meaningful threats to neurocognitive function and psychosocial wellbeing in lower-risk and younger patients with symptomatic severe AS who may arguably become candidates for TAVI in the near future22,23.
Limitations
Our study had a small sample size and was underpowered due to a higher than expected MRI drop-out rate. Also, despite randomisation, the STS score was significantly higher in patients treated without Sentinel CPS, who also had more major vascular complications. Yet, patients with major vascular complications did not complete MRI or neurocognitive follow-up and therefore did not affect our findings in terms of brain lesions and neurocognitive performance. We only assessed the early postoperative timeframe. The longer-term significance of early neurocognitive deterioration and transient ischaemic brain lesions that may not result in permanent infarcts is unsettled. The MISTRAL-C results should be considered hypothesis-generating and justify the larger randomised SENTINEL trial (NCT02214277) evaluating the Sentinel CPS that is currently recruiting patients in the USA and Germany.
Conclusion
Filter-based embolic protection captures debris en route to the brain in all patients undergoing TAVI. This study suggests that its use can lead to fewer and overall smaller new brain lesions as assessed by MRI and preservation of neurocognitive performance early after TAVI. These hypothesis-generating findings need confirmation in a larger randomised trial.
| Impact on daily practice Embolisation of thrombus and tissue debris to the brain is omnipresent with transcatheter aortic valve implantation. Filters may capture this material en route to the brain, potentially decreasing new brain insults and preserving neurocognitive performance. |
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Département de Cardiologie, Hôpital Xavier Bichat, Faculté Paris Diderot, DHU FIRE, Paris, France.
Funding
The Erasmus Medical Center received a research grant from Claret Medical that partially covered study-related costs.
Conflict of interest statement
P. de Jaegere is a proctor for Boston Scientific. N. Van Mieghem has received research grants from Boston Scientific, Medtronic and Edwards Lifesciences. The other authors have no conflicts of interest to declare. The Guest Editor declares low-level consultancy work for Medtronic.




