
Abstract
Aims: We aimed to examine the prevalence, clinical outcomes and procedural characteristics of percutaneous coronary intervention (PCI) complicated by coronary artery perforation (CAP) in a contemporary patient population.
Methods and results: Procedural records of 39,115 patients undergoing PCI between 2005 and 2016 were reviewed. CAP affected 149 cases (0.37%). The prevalence of CAP increased from 0.31% in 2005 to 0.45% in 2016 (p=0.03), reflecting an increase in more complex PCI (from 14% in 2005 to 21% in 2016; p<0.0001). CAP was associated with increased all-cause mortality (23.1% vs. 9.4% in those without perforation; p=0.0054) and was an independent predictor of mortality (HR 2.55; 95% CI: 1.34-4.78). In-patient mortality was 4% (6/149). In 43 of 149 (28.9%) cases, a significant pericardial effusion ensued and mortality rates were higher in this subgroup. Thirty-one patients had covered stents (CS) inserted and five did not survive to discharge. Of the 26 patients with a CS who survived to hospital discharge, six (23.1%) had definite stent thrombosis, and two (7.7%) had possible/probable stent thrombosis.
Conclusions: CAP remains uncommon but the prevalence is increasing. CAP is associated with significant short- and long-term mortality, particularly when there is haemodynamic compromise necessitating pericardiocentesis. Covered stents are a valuable tool but they are associated with a high risk of stent thrombosis.
Abbreviations
ARC: Academic Research Consortium
BCIS: British Cardiovascular Intervention Society
CABG: coronary artery bypass grafting
CAP: coronary artery perforation
IVUS: intravascular ultrasound
MI: myocardial infarction
NICOR: National Institute for Cardiovascular Outcomes Research
NSTEACS: non-ST-elevation acute coronary syndromes
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
STEACS: ST-elevation acute coronary syndromes
Introduction
Coronary artery perforation (CAP) is a recognised complication of percutaneous coronary intervention (PCI), with a reported prevalence of up to ~1%. CAP is commonly caused by angioplasty wire exit, balloon or stent inflation or rotational atherectomy and can rapidly lead to death secondary to cardiac tamponade1-7. Prompt recognition and treatment are essential to prevent immediate life-threatening complications. Covered stents, prolonged balloon inflation, fat/coil embolisation and surgical repair are often used to seal the perforation with reasonable short-term results7. CAP is associated with a worse long-term outcome but there are limited data examining the relation of outcomes to patient-level clinical and procedural characteristics8.
The aims of this study were to examine the prevalence, clinical outcomes and procedural characteristics of PCI complicated by CAP in a large contemporary patient population.
Methods
STUDY DESIGN AND PATIENT POPULATION
This was an observational cohort study of 39,115 patients undergoing 40,746 PCI procedures in three interventional centres in London (UK): Royal Free Hospital (8,643 procedures), The Heart Hospital (7,758 procedures) and London Chest Hospital. In May 2015 the London Chest Hospital and The Heart Hospital merged to form Barts Heart Centre at St Bartholomew’s Hospital (Barts Health NHS Trust, 24,345 procedures). The study period spanned 11.5 years, from January 2005 to May 2016. Patients with stable angina, unstable angina, non-ST-elevation acute coronary syndromes (NSTEACS) or ST-elevation acute coronary syndromes (STEACS) were included.
ETHICS
Data were collected as part of a national cardiac audit and all patient identifiable fields were removed prior to analysis. The local ethics committees advised that formal approval was not required.
PROCEDURAL DETAILS
The interventional strategy was at the discretion of the operator, including the use of direct stenting, predilatation/post-dilatation, intravascular imaging, and use of ablative devices. All patients received aspirin 300 mg and either clopidogrel 300 mg or 600 mg or ticagrelor 180 mg prior to the procedure. All patients were prescribed 75 mg aspirin and 75 mg clopidogrel daily or 90 mg ticagrelor twice daily maintenance therapy. ADP receptor antagonist maintenance therapy was recommended for one month in the bare metal stent (BMS) group, 12 months in the drug-eluting stent (DES) group and 12 months for patients treated for STEACS/NSTEACS. Unfractionated heparin was given during the procedure at a loading dose of 70 U/kg and the activated clotting time (ACT) was maintained at >250 sec. Glycoprotein IIb/IIIa inhibitors were used at the operator’s discretion and according to local guidelines.
DATA COLLECTION
Data were prospectively entered into a clinical PCI database at the time of the procedure in accordance with the British Cardiovascular Intervention Society (BCIS) standards. Data collected included patient characteristics (age, prior myocardial infarction [MI], PCI and coronary artery bypass grafting [CABG], hypertension, diabetes mellitus, hypercholesterolaemia, smoking status, and cardiogenic shock) and procedure-related data (indications for PCI, target vessel, number of diseased vessels, use of intravascular ultrasound [IVUS], optical coherence tomography [OCT], pressure wire, use of DES and GP IIb/IIIa inhibitors). Patients with CAP were identified from the BCIS database, and all available clinical/procedural records and angiograms were reviewed.
CORONARY ARTERY PERFORATIONS
The BCIS definition of CAP was used8. Coronary perforations were classified according to Ellis9, from an extraluminal crater without extravasation (class I) to pericardial/myocardial blush (class II) to extravasation through more than 1 mm perforation (class III) to extravasation into a chamber or coronary sinus (class IV)9.
PERICARDIAL TAMPONADE
Pericardial tamponade was diagnosed using conventional criteria in the presence of systemic hypotension (systolic blood pressure <90 mmHg), with echocardiographic/fluoroscopic evidence of pericardial effusion (and/or evidence of pulsus paradoxus, major respiratory variation in transmitral Doppler velocity, dilated inferior vena cava failing to collapse with inspiration, right ventricular free wall collapse in diastole)7. Pericardiocentesis was performed utilising fluoroscopy and/or echocardiography.
OUTCOME DATA
All-cause mortality data were recorded as of April 2016 and obtained via the BCIS national database, part of the National Institute for Cardiovascular Outcomes Research (NICOR). This national database is linked to the UK Office of National Statistics and provides systematically updated alive/dead status of treated patients. Only patients who had complete database records and National Health Service unique numbers (allowing alive/dead status to be assessed) were included in the analysis. A retrospective data quality audit of 100 randomly selected medical records established that 94.8% of data fields, including complications, were entered correctly into the database.
Stent thromboses were defined according to the Academic Research Consortium (ARC) definition as angiographic or pathologic confirmation of partial or total thrombotic occlusion within the peri-stent region plus at least one of acute ischaemic symptoms, ischaemic ECG changes or elevated cardiac biomarkers10. Repeat PCI rates due to target vessel revascularisation were identified from the PCI database.
STATISTICAL ANALYSIS
Baseline patient, procedural, and post-procedural characteristics were compared between the two groups. Categorical data are summarised using absolute values (percentage). Normally distributed, continuous data are presented as mean±SD or, where skewed, as median (25th to 75th percentile). Normally distributed continuous variables were compared using Student’s t-tests, and the Mann-Whitney U test was used to compare non-normally distributed continuous variables. Categorical data were compared using the Pearson chi-squared test. Multivariable logistic regression analysis was used to determine independent predictors of coronary perforation, using selected covariates. Variables associated at univariate analysis with coronary perforation (all with p<0.05) and those judged to be of clinical importance from previously published literature were eligible for inclusion into the multivariable model. Candidate variables included sex, circumflex artery lesion, chronic total occlusions, rotational atherectomy use, calcified lesion, IVUS use, diabetes mellitus, tortuous vessel, cutting balloon predilation, and previous CABG. Model discrimination was measured by the C-statistic and calibration by the Hosmer-Lemeshow goodness-of-fit test. The Cochran-Armitage test for trend was used to test the changes over time. Long-term survival was described by the Kaplan-Meier method, and comparisons in survival between groups were made using the log-rank statistic. A two-sided p<0.05 was considered statistically significant. All statistical analyses were performed using SPSS, Version 21.0 (IBM Corp., Armonk, NY, USA).
Results
During the study period, 39,115 patients were treated with 40,746 PCIs. Coronary perforation occurred in 149 patients (0.37%). The prevalence of coronary perforation increased over time (Figure 1A) from 0.31% in 2005 to 0.45% in 2016 (p=0.03). During the same time period, there was an increase in PCI involving CTO, rotational atherectomy and in patients with previous CABG, LVEF <30% and multivessel disease, from 13.9% in 2005 to 21.2% in 2016 (p<0.0001). This trend is illustrated in Figure 1B.
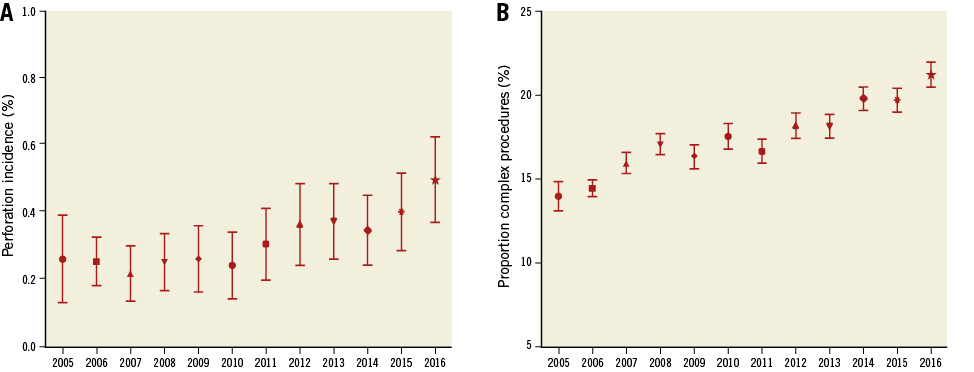
Figure 1. The prevalence of coronary perforations and complex PCI per year. A) Incidence of coronary perforations over time. Data shown as mean±SEM. B) Proportion of elective PCIs performed for complex procedures (CTO, previous CABG, LVEF <30% and multivessel disease rotational atherectomy) over the same time period.
BASELINE AND PROCEDURAL CHARACTERISTICS
The baseline clinical demographics are presented in Table 1. Patients with perforations tended to be older (p=0.01), female (p=0.005) and have higher rates of previous CABG (p=0.005) compared to those patients without coronary perforation. Grades of perforation ranged from 1-4 (grade 1: 30 patients (20.1%), grade 2: 44 patients (29.5%), grade 3/4: 75 patients (50.4%).
In absolute numbers, most perforations involved the LAD (n=67, 45%), followed by the RCA (n=48, 32.2%) and the Cx (n=29, 19.5%). The remaining five CAP involved vein grafts (n=4) or the left main stem (n=1). When CAP were considered as a proportion of treated vessels, 0.39% (95% CI: 0.35-0.43) of Cx interventions were complicated by CAP compared to 0.37% (95% CI: 0.32-0.40) of RCA and 0.34% (95% CI: 0.28-0.37) of LAD interventions (p=0.24).
A high proportion of lesions complicated by CAP were complex, with type B2 lesions in 44.6% and type C lesions in 51.8% of cases. Small calibre vessels (<2.5 mm) were involved in 29.5% of perforations. In addition, 20.1% were chronic total occlusions. Six patients (4.0%) underwent rotational atherectomy. GP IIb/IIIa inhibitors were used in 33 patients (22.1%), and 25 patients (16.8%) had undergone intracoronary imaging (IVUS/OCT).
CAUSES OF CORONARY ARTERY PERFORATION
The angioplasty balloon was identified as the cause of the perforation in 41 cases (27.5%), with cutting balloons making up 9.8% (n=4/41). The angioplasty wire was responsible in 57 cases (38.3%), with the remaining 51 cases (34.2%) secondary to stent inflation. Rotational atherectomy devices were not directly involved in causing perforation.
PREDICTORS OF CORONARY ARTERY PERFORATION
Factors predictive of CAP included age (odds ratio [OR] per year 1.04; 95% CI: 1.01-1.05; p<0.001), previous coronary artery bypass graft (OR 1.64; 95% CI: 1.17-2.57; p<0.001), use of IVUS (OR 2.89; 95% CI: 1.60-4.51; p<0.001), and chronic total occlusions (OR 4.96; 95% CI: 2.08-6.78; p<0.001). The C-statistic for the propensity score model was 0.79 (95% CI: 0.76-0.81), indicating good discrimination. The Hosmer-Lemeshow goodness-of-fit test p-value was 0.97, confirming good calibration and model fit.
CARDIAC TAMPONADE AND PERICARDIOCENTESIS FOLLOWING CORONARY ARTERY PERFORATION
In 43 of 149 patients (29%), a haemodynamically significant pericardial effusion ensued requiring drainage (all percutaneous). Nine of the 43 (20.9%) cases occurred more than two hours post procedure; four of these patients had received GP IIb/IIIa inhibitors during the procedure and two had received bivalirudin. The use of GP IIb/IIIa inhibitors or previous CABG was not associated with the development of haemodynamically significant pericardial effusion. Six of the 43 patients were referred for emergency bypass surgery and surgical repair of the perforation (13.9%), two of whom subsequently died during the same admission. Patients treated with a covered stent were referred for surgical repair if there was failure to seal the perforation.
ACUTE TREATMENT TO SEAL CORONARY ARTERY PERFORATIONS AND IN-HOSPITAL MORTALITY
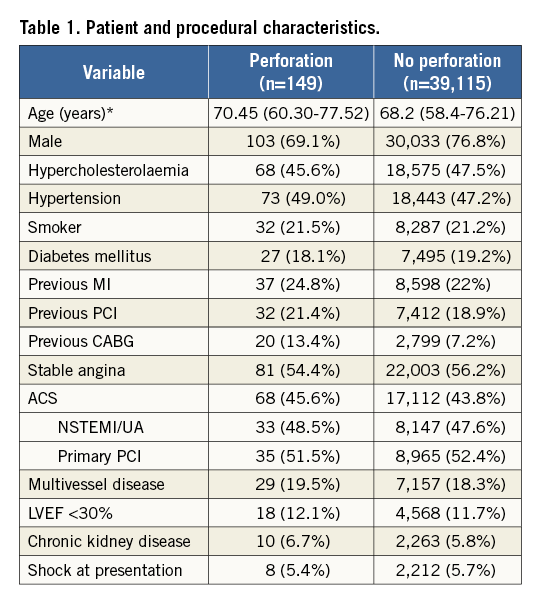
Thirty-one patients had covered stents inserted. The remaining patients were managed with balloon tamponade or fat embolisation (no coil embolisation used) and pericardial drainage alone. Six patients died during their inpatient stay (in-hospital mortality rate=4.0%). The treatments received and in-patient mortality are summarised in Figure 2.
Figure 2. Flow chart demonstrating treatment of CAP and in-patient mortality.
LONG-TERM OUTCOMES
During the study period, a total of 25 patients died following CAP (six during acute admission and 19 post discharge). Coronary perforation was associated with an increased all-cause mortality over the follow-up period (23.1%, 95% CI: 10.1-33.6) in CAP patients vs. 9.4% (95% CI: 8.2-10.7) in patients without CAP (p=0.0054) (Figure 3). Cox analysis revealed coronary perforation to be a predictor of mortality compared with no perforation (HR 2.34; 95% CI: 1.29-4.26), and this difference was maintained with multiple adjustments (HR 2.55; 95% CI: 1.34-4.78). Mortality rates were higher if pericardiocentesis was required (Figure 4).
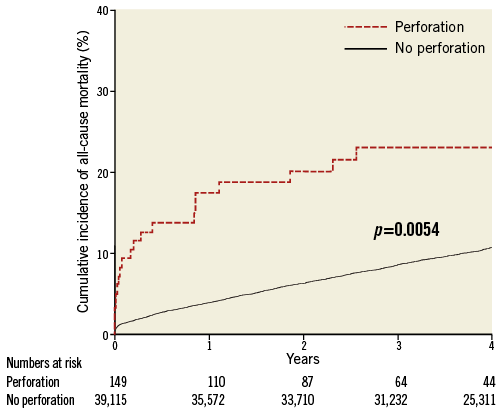
Figure 3. Kaplan-Meier curves showing cumulative probability of all-cause mortality following perforation as compared to no perforation.
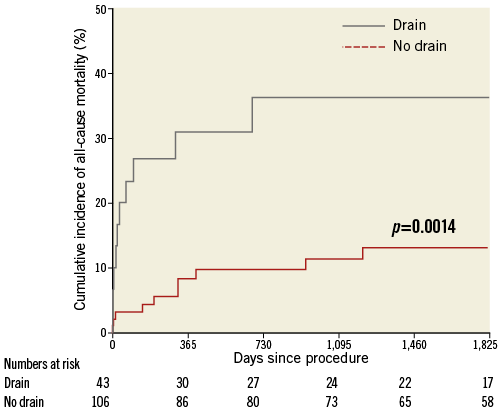
Figure 4. Kaplan-Meier curves showing cumulative probability of all-cause mortality following pericardiocentesis versus no pericardiocentesis following coronary perforation.
COVERED STENT THROMBOSIS
The 26 patients treated with covered stents who survived to hospital discharge were followed up for a median of 520 (31-1,843) days. During this period, a total of eight patients (29.6%) presented with stent thrombosis as per the ARC definition. Angiographically confirmed stent thrombosis was observed in six (23.1%) patients. Two (7.7%) had possible/probable stent thrombosis presenting with sudden death 23 and 31 days post procedure. Most stent thromboses (7/8) occurred within one year of CAP. A Kaplan-Meier curve illustrating the incidence of stent thrombosis is shown in Figure 5. All of the patients with stent thrombosis were prescribed dual antiplatelet therapy and there were no data to suggest premature discontinuation. Four of the stent thromboses were treated percutaneously with restoration of coronary flow, whereas two were treated medically. Of the patients with CAP who died during follow-up, 31.6% of deaths were following stent thrombosis.
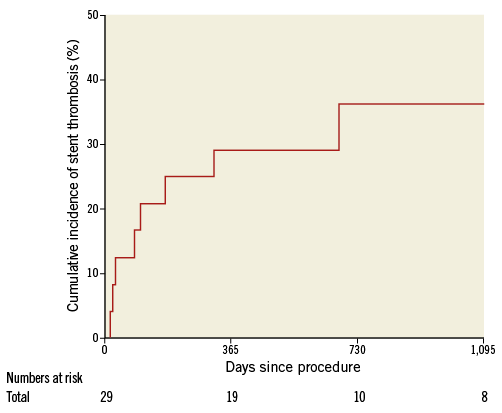
Figure 5. Kaplan-Meier curves showing cumulative probability of stent thrombosis following PCI with covered stent (follow-up includes hospital stay; number at risk therefore totals 29).
Discussion
This study shows that CAP is an uncommon complication of PCI but the prevalence is increasing in parallel with an increase in complex PCI. Perforations most commonly involve balloons or stents and frequently result in cardiac tamponade. Even though most coronary artery perforations can be successfully sealed percutaneously, CAP is associated with significant acute and long-term mortality, and covered stents appear to be prone to thrombosis.
As a consequence of the continuing expansion of interventional techniques, technology and range of equipment, coupled with the increasing age of the population, more tortuous, calcified and occluded vessels are being treated by PCI11,12. The temporal increase in complex PCI is likely to be directly responsible for the increasing prevalence of CAP observed in this study. The rapid improvements in PCI techniques, especially CTO revascularisation, may offset some of the inherent risks of complex PCI, and help to keep the prevalence of CAP low. In this large contemporary patient population, the overall prevalence of CAP was low (0.37%), and development of tamponade in ~1/3 of patients is in keeping with previous reports3,13-15. These data should reassure cardiologists and patients that PCI remains a safe procedure despite increased procedural and clinical complexity.
Similar to previous reports, most CAP in this study were caused by the advancement, inflation or rupture of balloons or coronary stents13,16,17. About a quarter of CAP involved intracoronary imaging, and in particular intravascular ultrasound. Intracoronary imaging devices were not the physical cause of perforation; the association with increased CAP risk is likely to reflect lesion complexity. Other predictors of coronary artery perforation such as older age, hypertension, previous CABG, history of congestive cardiac failure (CCF), and lower creatinine clearance18-20 also probably reflect clinical and lesion complexity. This study suggests that, even though previous CABG increases the risk of perforation, postoperative pericardial adhesions may contain bleeding from a perforated artery and protect against cardiac tamponade.
Once a CAP occurs, it is imperative to seal the coronary artery defect, minimise extravasation of blood to the pericardial space and prevent cardiac tamponade. Prolonged balloon inflation is often effective in sealing the defect but this technique is limited by the development of cardiac ischaemia unless a perfusion balloon catheter is used. If prolonged balloon inflation fails, a covered stent(s) can be deployed over the perforation to form a seal. Several studies have reported encouraging short-term outcomes21,22. Despite their obvious utility, covered stents compromise side branch flow and, as they are bulky, can only be delivered to the proximal and mid segments of coronary arteries. This study highlights another major limitation of covered stents, namely thrombosis. Covered stent thrombosis occurred in 30% of recipients and may relate to delayed endothelialisation23. Smaller studies in the past have also reported covered stent thrombosis. A study of 24,465 patients undergoing PCI reported definite stent thrombosis in 8.6%24 of 56 patients with a grade III coronary perforation, whereas a Japanese study of 57 patients who were treated with a covered stent for coronary perforation reported definite stent thrombosis in 2% at one year and 5% at three years25. The rate of stent thrombosis is higher in this study but the reasons are unclear. The type of covered stent used may also have influenced the rate of stent thrombosis but, as the type was not always reported, outcomes according to stent type could not be investigated.
Covered stent thrombosis highlights the need for optimal deployment during the procedure and consideration of more aggressive antiplatelet therapy after the procedure. The use of ticagrelor or prasugrel may help to reduce covered stent thrombosis but data are lacking. Since most stent thromboses occurred within one year of deployment, it is highly unlikely that prolonged dual antiplatelet therapy (beyond one year) will have a significant impact. Elective surgical revascularisation of the affected vessel is another option in selected patients treated with a covered stent when there are concerns about optimal deployment or tolerability of antiplatelet therapy. Further studies are needed to determine which covered stents are the least thrombogenic.
Patients who experience CAP and survive the complication have worse long-term survival than their peers, especially if pericardiocentesis is required. The increased mortality is only partially explained by covered stent thrombosis and is likely to relate to the advanced age and comorbidities of this patient population. CAP should act as a red flag for adverse prognosis. Such patients deserve closer follow-up and assiduous medical care.
Limitations
Our study is a consecutive observational analysis from a multicentre experience. As this was an observational study, the findings may have been subject to confounding factors for which we have been unable to control. However, our data set includes all major clinical variables known to affect outcome, which would support the validity of our results. The subtle nature of type I perforations means that the true incidence of coronary artery perforation may have been underestimated. The main outcome of this study was all-cause mortality rather than cardiac specific mortality and not all deaths can be attributed to the coronary artery perforation.
Conclusions
Coronary artery perforation is an uncommon but important complication of percutaneous coronary intervention, especially when complex lesions are treated. In most cases, perforations can be managed percutaneously and covered stents are a valuable tool in this respect. However, covered stents are associated with a high risk of stent thrombosis, and more aggressive dual antiplatelet therapy or elective surgical revascularisation needs to be considered. CAP is associated with increased short- and long-term mortality, particularly when there is haemodynamic compromise necessitating pericardiocentesis.
| Impact on daily practice CAP remains uncommon but the prevalence is increasing probably due to an increase in complex PCI. CAP is associated with significant short- and long-term mortality, particularly when there is haemodynamic compromise necessitating pericardiocentesis. Covered stents are a valuable tool but they are associated with a high risk of stent thrombosis. More aggressive dual antiplatelet therapy or elective surgical revascularisation needs to be considered following implantation of a covered stent. |
Conflict of interest statement
The authors have no conflicts of interest to declare.




