Biopsychosocial predictors of sexual function and quality of sexual life: a study among patients with colorectal cancer
Introduction
Oncological research primarily focuses on developing and implementing treatments that increase overall and disease-free survival. For colorectal cancer, the introduction of the total mesorectal excision procedure and the development of effective (neo)adjuvant treatments are important contributions in this regard (1,2). Due to the increased life expectancy for patients with colorectal cancer, more awareness arose for the potential side-effects of treatments and patients’ quality of life. Quality of life is a multi-dimensional construct, incorporating at least physical, psychological, and social well-being (3). Sexuality is considered central to a person’s well-being and is, as such, an important aspect of quality of life (4). However, sexuality itself is a broad concept as can be seen in the World Health Organization’s definition of sexuality: “…a central aspect of being human throughout life encompasses sex, gender identities and roles, sexual orientation, eroticism, pleasure, intimacy and reproduction. Sexuality is experienced and expressed in thoughts, fantasies, desires, beliefs, attitudes, values, behaviours, practices, roles and relationships. While sexuality can include all of these dimensions, not all of them are always experienced or expressed. Sexuality is influenced by the interaction of biological, psychological, social, economic, political, cultural, legal, historical, religious, and spiritual factors (5).” This definition establishes two important things. First, the definition entails that sexuality is more than sexual intercourse alone. Therefore, it is imperative to make a distinction between sexual function (SF) and the quality of sexual life (QoSL). SF refers to the normal performance standards of the sexual response cycle (i.e., desire, excitement, orgasm, and resolution) (6,7). A sexual dysfunction is characterized by a disturbance in this sexual response cycle or by pain associated with intercourse (8). QoSL takes into account the person’s subjective evaluation of his/her SF and, thus, concerns the extent to which someone is (dis)satisfied. One may assume that if patients experience sexual dysfunction after colorectal cancer treatment they will also report a lower QoSL, since these concepts are related. However, patients may not be bothered with sexual dysfunction if they employ other ways to establish a satisfactory sexual relationship (2-4). For instance, couples coping with colorectal cancer stated that intimacy (e.g., hugging/kissing) is more important than being able to have sexual intercourse (6). On the other hand, patients may also report a low QoSL without an apparent sexual dysfunction (2-4). Secondly, the definition of sexuality shows that sexuality can be influenced by different factors, which warrants the need to evaluate sexual (dys)function and QoSL from a biopsychosocial perspective.
The current research on sexuality after colorectal cancer, however, is primarily focused on evaluating the levels of SF and treatment-related predictors of this SF. These studies show that multidisciplinary colorectal cancer treatment may influence patients’ SF (e.g., erectile or ejaculatory dysfunction in men and dyspareunia and lubrication problems in women). In addition, studies did found a significant relationship between SF and demographic factors [e.g., age (9,10), sex (11)], psychological issues [e.g., depressive or anxious symptoms (12), body image (13), fatigue (14)], and social aspects [e.g., social support (9)]. However, only one cross-sectional study examined SF using a biopsychosocial approach (i.e., incorporating all relevant variables in one study) (9). In this study, older age, having received an abdominoperineal resection, and poor social support were associated with low SF in men, while low SF in women was associated with higher age and poor global quality of life (9). Studies examining QoSL are scare. Currently, few studies reported that treatment-related factors were related to sexual satisfaction and/or sexual enjoyment, however, no study has yet evaluated biopsychosocial predictors of QoSL (10).
At present there is thus only limited information on biopsychosocial predictors of SF and QoSL in patients with colorectal cancer. Moreover, no study has yet evaluated to what extent SF and QoSL are related. Therefore, this study aimed to evaluate (I) relatedness between SF and QoSL, (II) the course of SF and QoSL, and (III) biopsychosocial predictors of SF and QoSL during the first year after colorectal cancer surgery.
Methods
Participants
Data were drawn from a larger study examining the (sexual) consequences of colorectal cancer for patients and their partners (NCT01234246). For this study, both patients and partners were invited to participate. In addition, partners were still invited to participate even if patients declined participation and vice versa, in order to prevent selection bias. Patients and partners were recruited from six Dutch hospitals: St. Elisabeth hospital (Tilburg), TweeSteden Hospital (Tilburg and Waalwijk), Catharina Hospital (Eindhoven), Jeroen Bosch Hospital (‘s Hertogenbosch), Amphia Hospital (Breda), and Maxima Medical Centre (Eindhoven and Veldhoven). To be eligible for participation, patients and partners had to be older than 18 years. Patients were excluded if one or more of the following criteria were applicable: (I) elderly age (>75 years), (II) non-curatively treated metastases at baseline, (III) poor expression of the Dutch language, (IV) dementia, and/or (V) a history of psychiatric illness. Partners with (I) insufficient knowledge of the Dutch language and (II) with dementia or a history of psychiatric illness were excluded. During a preoperative visit eligible patients and partners were asked, by their treating physician, if they gave permission to be approached by a member of the research team. Subsequently, this member contacted the potential participants by phone to explain the design and purpose of the study. If patients and/or partners agreed to participate they were asked to complete a set of questionnaires at home before surgery (Time-0) and 3 (Time-1), 6 (Time-2), and 12 months (Time-3) postoperative. However, the Dutch guidelines recommend that all patients with rectal cancer, except those with a clinical T1 stage without positive lymph nodes, receive neoadjuvant treatments [i.e., radio(chemo)therapy; www.oncoline.nl]. Therefore, a subset of patients and partners completed the first set of questionnaires prior to surgery, but potentially during or after the time patients received neoadjuvant therapy. Patients and partners returned the surveys in sealed postage-paid envelopes. Patients and partners who did not return the questionnaires within two weeks received reminders [phone call(s) and/or a reminder letter]. This study was approved by the institutional review board. All patients and partners gave written informed consent. As this specific study incorporated evaluating partner-related influences on the patients’ SF, only participating couples were included in the current sample.
Measures
The patient’s clinical information was retrieved from the Eindhoven Cancer Registry (ECR). The ECR routinely collects data on tumor characteristics and treatment. If needed, additional clinical information was retrieved from the patient’s medical records. Patients also completed questions regarding their age, sex, and length of the relationship with their partner.
Two aspects of the patients’ personality were assessed. Neuroticism was assessed with the Neuroticism facet of the Neuroticism-Extraversion-Openness-Five Factor Inventory (NEO-FFI) (15). This factor assesses six aspects belonging to neuroticism (i.e., anxiety, hostility, depression, self-consciousness, impulsiveness, vulnerability to stress). Trait anxiety was evaluated with the Dutch short form trait scale of the Spielberger State-Trait Anxiety Inventory (STAI) (16). The trait anxiety scale describes how persons generally feel and conceives anxiety as a personality disposition (16).
Patients’ psychological function was assessed with four constructs, specifically, body image, state anxiety, depressive symptoms, and fatigue. Body image was evaluated with the Body Image Scale (17,18). Depressive symptoms were evaluated with the 16-item version of the Center for Epidemiological Studies-Depression Scale (CES-D) (14). State anxiety was assessed with the short form (6-items) of the STAI state anxiety scale (19). State anxiety is a momentary emotional condition characterized by subjective feelings of apprehension and tension, and heightened autonomic nervous system activity and may thus vary in intensity and fluctuate over time (19). Fatigue was evaluated with the Fatigue Assessment Scale (FAS) (20). The FAS assessing perceived fatigue and exhaustion. NB: fatigue was in this study for clarity purposes seen as a psychological factor, even though we know that fatigue is a multidimensional construct encompassing both physical and psychological aspects.
Social characteristics (i.e., sexual activity, SF, non-sensuality, avoidance, non-communication, relationship adjustment) were completed by both patients and partners, except for sexual activity, which was only completed by patients. Patients’ sexual activity was assessed with the question ‘To what extent where you sexually active (with/without sexual intercourse)?’ from the European Organization for Research and Treatment of Cancer (EORTC) disease specific ColoRectal 38 (QLQ-CR38) (21). QoSL was evaluated with the Sexual Activity facet of the World Health Organization Quality of Life assessment (WHOQOL-100) (22,23). This facet contains the following items: ‘How would you rate your sex life?’, ‘How well are your sexual needs fulfilled?’, ‘How satisfied are you with your sex life?’, and ‘Are you bothered by any difficulties in your sex life?’ SF was evaluated with two sex-specific questionnaires. Men completed the Erectile Function, Orgasmic Function, and Sexual Desire domains of the International Index of Erectile Function (IIEF) (24,25). Women completed the Arousal, Lubrication, Orgasmic Function, Sexual Desire, and Sexual Pain domains of the Female SF Index (FSFI) (26,27). If needed, patients could indicate that an item was not applicable. For the IIEF the total score was computed as the sum of at least five items and, thus, up to five items were person-mean imputed. For the FSFI, domain scores were obtained following the standard scoring instruction. The IIEF and FSFI total scores were transformed into standardized z-scores. The z-scores were subsequently combined to obtain one SF score. Next, patients and partners completed the Avoidance, Non-Communication, and Non-Sensuality domains of the Golombok-Rust Inventory of Sexual Satisfaction (GRISS) (28). The GRISS has separate versions for men and women; however, the Avoidance, Non-Communication, and Non-Sensuality domains are comparable for both sexes. Finally, relationship (mal)adjustment was assessed with the Marital (Mal)adjustment scale of the Maudsley Marital Questionnaire (MMQ) (29-31). Sociodemographic factors and personality characteristics were assessed only at Time-0, while all other questionnaires were completed at each time point (Time-0-3). The psychometric properties of all questionnaires were satisfactory.
Statistical analyses
An independent t-test and Chi-square tests were used to examine potential differences in age, sex, and type of tumor for participants and non-participants. Bivariate correlations between SF and QoSL were at each time point assessed with the Pearson correlation coefficient. Correlations were grouped into small (r≤0.30), moderate (r=0.30-0.49), or high (r>0.49) (32). Linear mixed-effects models with an unstructured error covariance pattern model were used to examine (I) the course of SF and QoSL and (II) predictors for both constructs. Time was analysed as a categorical predictor with four levels (i.e., Time-0, Time-1, Time-2, and Time-3). The fixed-effects parameters of the models were estimated with maximum likelihood. Sociodemographic variables (i.e., age, sex), clinical variables (i.e., tumor type, type of surgery, radiotherapy (yes/no), chemoradiation (yes/no), chemoradiation (yes/no), stoma (yes/no)), and personality characteristics (i.e., trait anxiety and neuroticism) were analysed as time-invariant predictors as they were only assessed at baseline. Psychological variables and symptoms (i.e., anxiety, depressive symptoms, body image, and fatigue), patient-related social variables (i.e., sexual activity, relationship adjustment, SF, non-sensuality, avoidance, non-communication), and partner-related social variables (i.e., SF, relationship adjustment, QoSL, non-sensuality, avoidance, non-communication), were measured at each time point and analysed as time-varying predictors (33).
Analyses proceeded in three steps (method: forward). A basic set of predictors (i.e., age, sex, and type of tumor, Block 1) was formed to which, in separate analyses, a specific block of predictors was added. The following additional sets were formed: basic set + personality characteristics (Block 2), basic set + psychological variables (Block 3), basic set + patient-related social factors (Block 4), basic set + partner-related social factors (Block 5), and basic set + clinical characteristics (Block 6). These sets were formed based on content. To minimize data-driven choices and to identify the parsimonious model, a P<0.10 was used during the selection procedure. Second, the selected variables were analysed in one final model. If the effect of time-varying predictors was significant in the final model, then the effect was split into two effects in the second step of the analysis: between-subjects effects (e.g., the degree to which patients’ SF/QoSL is related to their average level on a predictor) and within-subjects effects (e.g., the degree to which variation in patients’ SF/QoSL over time is associated with a change in their levels on a predictor) (33). Finally, in the third step, the interaction between time and gender and time and type of tumor was evaluated in two separate modes, thus evaluating one interaction affect at a time, for both outcomes (i.e., SF and QoSL). In order to correctly interpret all model parameters, all time-varying variables have been grand-mean centered. Analyses were performed in IBM SPSS 19.0, using a significance level of P<0.05 (with exception of the selection procedure).
Results
Participants
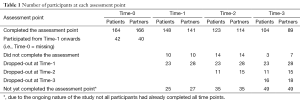
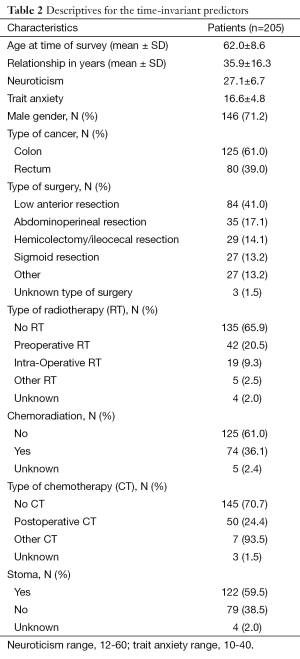
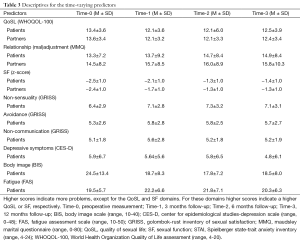
In total, 672 eligible patients agreed to be contacted by a member of the research group, who informed them about the study. Of them, 313 (47%) patients agreed to participate; 64% of patients with rectal cancer participated and 50% of the patients with colon cancer participated (P=0.001). Fewer women (38%) than men (62%) were approached for this study. In addition, women less often participated (43%) than men (66%). Of the 313 patients, 279 (89%) had a partner of which 206 (74%) participated. Since partner-related variables were taken into account, only couples were included in the analyses (n=206). An overview of the number of participants at each time point is provided in Table 1. In addition, patients’ time-invariant characteristics are presented in Table 2 while time-varying variables are presented in Table 3.
Full table
Full table
Full table
Relationship between SF and QoSL
At each time point a significant association was reported between SF and QoSL. At Time-0 a moderate correlation was reported (r=0.47, P=0.01), while at Time-1 a high association was noted (r=0.64, P=0.01). The correlation decreased at Time-2 (r=0.21, P=0.05), but subsequently increased to the baseline level at Time-3 (r=0.45, P=0.01).
Course and predictors of patients’ SF
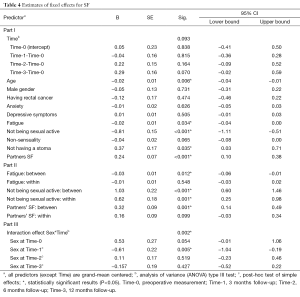
The selection procedure showed that a higher age (P=0.002, Block 1), having rectal cancer (P=0.001, Block 1), more anxiety (P=0.009, Block 3), more depressive symptoms (P=0.004, Block 3), more fatigue (P=0.001, Block 3), not being sexually active (P≤0.001, Block 4), more non-sensuality (P=.006, Block 4), higher levels of partners’ SF (P≤0.001, Block 5), and not having a stoma (P=0.011, Block 6) predicted the course and level of patients’ SF and were, therefore, included in the final model (Table 4, Part I).
Full table
In the final model for SF, the effect of time was not significant. A higher age (β=−0.02, P=0.006), more fatigue (β=−0.02, P=.034), no sexual activity (β=−0.81, P<0.001), higher partners’ SF (β=0.24, P<0.001), and having a stoma (β=0.24, P=0.035) contributed significantly to patients’ lower SF.
For the abovementioned significant time-varying predictors, the between- and within-subject analyses showed that patients who on average reported not to be sexually active had on average lower levels of SF (between-subjects effect, β=1.03, P<0.001, Table 4, Part II). Moreover, patients that showed a positive change in sexual activity on a time point also showed a positive change in SF (within-subjects effect, β=0.62, P=0.001). Patients that on average were more tired scored on average lower on SF (between-subjects effect, β=−0.03, P=0.012). Finally, if the partners reported on average higher SF this predicted on average better SF for the patients (between-subjects effect, β=0.32, P=0.001). The within-subject effects for fatigue and the partners’ SF were not significant.
Finally, there was a significant interaction effect between time and gender (P=0.002, see Figure 1A and Table 4, Part III). Overall, the scores on SF fluctuated across time, but the difference between men and women was only significant at Time-1. Women reported low SF at Time-0 and showed a large increase at Time-1. Men scored average on Time-0, Time-2 and Time-3 but showed a decrease at Time-1. Only at Time-1 women reported a significantly higher SF. At Time-2 and Time-3 the SF scores were very similar for women and men. The interaction between time and type of tumor was not significant (results not shown).
Course and predictors of patients’ QoSL
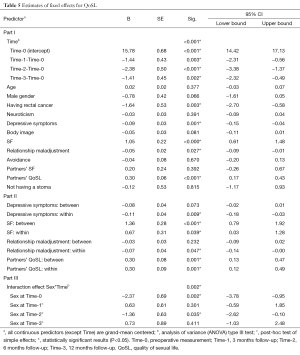
The selection procedure showed that having rectal cancer (P<0.001, Block 1), higher levels of neuroticism (P=0.037, Block 2), more depressive symptoms (P=0.003, Block 3), lower body image (P=0.019, Block 3), better SF (P<0.001, Block 4), better relationship adjustment (P=0.001, Block 4), more avoidance (P=0.009, Block 4), better partners’ SF (P=0.005, Block 5), higher partners’ QoSL (P<0.001, Block 5), and not having a stoma (P=0.020, Block 6) predicted the course and levels of QoSL.
The EMM of the final model showed that QoSL at Time-0 was 14.5 which decreased 1.4 points to 13.1 at Time-1, then decreased a little further to 12.1 at Time-2, and finally increased slightly to an EMM of 13.3 at Time-3 (P<0.001, Table 5, Part I). In this model, having rectal cancer (β=−1.64, P=0.003), more depressive symptoms (β=−0.09, P=0.001), lower SF (β=1.05, P<0.001), more relationship (mal)adjustment (β=-0.05, P=0.027), and lower partners’ QoSL (β=0.30, P<0.001) contributed significantly to a lower QoSL for the patients (P<0.05).
Full table
The between- and within-subjects analyses (Table 5, Part II), showed that patients with more depressive symptoms or higher relationship maladjustment on a time point also reported a negative change in QoSL on that time point [within-subject effects, β=−0.11 (P=0.009) and β=−0.07 (P=0.047), respectively]. Patients who on average reported a lower SF had on average lower levels of QoSL (between-subjects effect, β=1.36, P<0.001). Moreover, patients who showed change in SF on a time point also showed a change in the QoSL (within-subjects effect, β=0.67, P=0.039). If partners on average reported higher QoSL then patients, on average, also scored higher on QoSL (between-subjects effect, β=0.30, P=0.001). Also, if partners changed in their QoSL, then patients’ QoSL also changed (within-subjects effect, β=0.30, P=0.001).
The interaction between time and gender was significant (P=0.002, see Figure 1B and Table 5, Part III). Compared with Time-1, women showed lower but comparable QoSL scores at the subsequent time points. However, the pattern for men differed. Men’s QoSL decreased at Time-1 and continued to drop at Time-2, but finally increased somewhat at Time-3. At Time-0, women had a significantly higher QoSL then men (P=0.002, EMM=16.3 and 13.9, respectively). In addition, men scored significantly lower (EMM=11.7) than women (EMM=13.1) at Time-2 (P=0.035). Again, the interaction between time and type of tumor was not significant (results not shown).
Discussion
Compared to the preoperative scores, patients’ SF did not change significantly during the first year after surgery. However, the course of patients’ QoSL did change. Compared with the preoperative assessment, patients’ QoSL was significantly lower at 3, 6, and 12 months postoperative. Evaluating biopsychosocial predictors of patients’ SF and QoSL revealed that a higher age and having a stoma contributed to lower scores on SF, but did not contribute to QoSL. The association between sexual dysfunction and a higher age (9,10,34) and having a stoma (11,35-37) has been previously reported. Furthermore, patients who on average were more tired scored on average lower on SF, but this association was not found for the QoSL. This result is in line with a previous cross-sectional among colorectal cancer survivors (14). Moreover, anxiety and depressive symptoms did not predict SF, but depressive symptoms on a time point did predict a negative change in QoSL on that time point. In previous research, results are mixed. Milbury et al. 2013 (7) did not find a significant association between depressive symptoms and SF, while depressive symptoms were associated with both SF and sexual enjoyment in the study of Den Oudsten et al. 2012 (14). Therefore, more research examining the relationship between depressive symptoms and SF and QoSL is still needed.
Several observations regarding the relatedness between SF and QoSL can be made. Patients that on average reported a lower SF had on average lower levels of QoSL and a change in SF on a time point also predicted a change in QoSL at the that time point. This finding supports the idea that the constructs SF and QoSL are inter-related. However, a high correlation between SF and QoSL was only reported at Time-1. Thus, even though these concepts are related, they may be best viewed as separate constructs. The distinction between both constructs is further supported by the fact that different predictors were found for the QoSL and SF (see above). Finally, this study indicates that SF, relationship (mal)adjustment, and QoSL can be conceptualized as constructs ranging from narrow to broad: Being sexually active (with or without sexual intercourse) was both between- and within-subjects associated with SF, but not with the QoSL. Additionally, higher relationship maladjustment on a time point predicted a negative change in QoSL on that time point, but relationship maladjustment did not predict SF.
Another interesting finding is that partners’ SF and QoSL were predictive for patients’ SF and QoSL, respectively. Therefore, interdependence between patients and partners seems present. While previous research has already addressed the importance of evaluating and addressing the consequences of cancer from a couple-based perspective (38,39), this is the first study that incorporated partner-related variables as predictors for patients’ SF and QoSL.
Few clinical variables were significant predictors of patients’ SF and QoSL. Not having a stoma was predictive for better SF while having rectal cancer predicted a lower QoSL. The lack of other significant clinical predictors may seem remarkable as earlier studies have shown that radiotherapy (11,40,41), but especially surgical nerve damage (which can be roughly estimated based on type of surgery) seems to play an important role in the occurrence of sexual dysfunction (38). However, type and location of the tumor leads to a protocolled treatment schedule. The high correlation between the treatment related variables makes it difficult to find significant unique effects of each variable separately, especially since the number of patients included in the analyses was limited.
An important methodological consideration that needs to be acknowledged concerns the questionnaires used in the current study. While validated and reliable questionnaires were used, a difficulty lied in obtaining one SF score (regardless of gender). For men, aspects such as erectile and ejaculatory (dys)function were measured while women answered questions regarding problems with lubrication or pain during intercourse. Therefore, sex-specific sum scores were transformed into z-scores and combined in one SF score. This resulted in a loss of information. Unfortunately, it was not possible to evaluate the specific effects of each SF domain on the QoSL for men and women separately, due to a limited sample size. However, the significant interaction between time and gender showed that the SF and QoSL trajectories are different for men and women. Therefore, future more in-depth studies in this area are still warranted. Another limitation concerns the generalizability of the results. Since evaluating partner-related predictors of the patient’s SF and QoSL was part of the current study, only couples were included. However, sexuality is not only an issue for couples. Future research is, therefore, needed to evaluate biological and psychological predictors of SF and QoSL for single or widowed patients. The fact that all couples were in a heterosexual relationship is also unfortunate, as this was not a prerequisite for participation. Future studies are encouraged to include couples/patients with non-heterosexual orientations (e.g., gay, lesbian, or bisexual). Moreover, only information on the patient’s sexual activity (with or without sexual intercourse) was available. This is regrettable as the patient’s sexual activity status is not necessarily similar to the sexual activity status of the partner, due to possible sole or external sexual activities outside the nominated relationship. In addition, based on this question it was not possible to determine what type of sexual activities patients engaged in. For both patients and partners, more insight into what constitutes as sexual activity is still needed. Finally, this study did not evaluate biopsychosocial predictors of the partners’ SF and QoSL, nor did this study evaluate the dynamics between patients and partners in depth. Future studies are encouraged to address these topics.
Regardless of the abovementioned methodological considerations, the results show that not only clinical but also psychosocial factors play a role in patients’ SF and QoSL. Therefore, it is stimulated that clinicians address this issue during treatment. In addition, it is favored that clinicians focus not only on biological damage after treatment, but obtain a broader perspective in which they also pay attention to psychosocial factors that may impair SF and QoSL. Finally, the interdependence between patients and partners is imperative and entails that both in research and clinical practice should be attentive to the couple and may best perceive cancer as a ‘we-disease’ (42).
Regardless of the abovementioned methodological considerations, the results show that SF and QoSL are related but distinctive constructs, for which different biopsychosocial predictors were identified. In addition, SF did not change significantly over time, while the QoSL decreased from Time-0 to Time-1 and Time-2 and finally increased somewhat at Time-3. Discussing SF and QoSL has been found to be an important topic for both patients and partners (43,44). When discussing sexuality, it is important to realize that SF and QoSL are no interchangeable terms and they should, therefore, be discussed as two separate entities. In addition, it is favored that clinicians focus not only on biological predictors of SF and QoSL, but that they obtain a broader perspective in which they also pay attention to psychosocial factors that may impair SF and QoSL. The significant contribution of partners’ SF and QoSL to patients’ SF and QoSL suggests interdependence between patients and partners. However, future research is needed to entail more insight in the dynamic between patients and partners. In addition, studies focussing specifically on the partner and studies that include single of widowed patients are still warranted. Finally, the significant interaction between time and gender suggest that SF and QoSL trajectories differ for men and women. Future research is needed in order to evaluate gender effects and interdependence between patients and partners more in depth.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- van de Velde CJ, Boelens PG, Borras JM, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer 2014;50:1.e1-34.
- Orsini RG, Wiggers T, DeRuiter MC, et al. The modern anatomical surgical approach to localised rectal cancer. EJC Suppl 2013;11:60-71.
- Hamming JF, De Vries J. Measuring quality of life. Br J Surg 2007;94:923-4. [PubMed]
- Hassan I, Cima RR. Quality of life after rectal resection and multimodality therapy. J Surg Oncol 2007;96:684-92. [PubMed]
- Organization WH: Working definition for sexuality, 2006b
- Masters WJ, Johnson VE. eds. Human sexual response. Boston: Little, Brown and Company Boston, 1966.
- Verschuren JE, Enzlin P, Dijkstra PU, et al. Chronic disease and sexuality: a generic conceptual framework. J Sex Res 2010;47:153-70. [PubMed]
- American Psychiatric Association. eds. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association, 2011.
- Milbury K, Cohen L, Jenkins R, et al. The association between psychosocial and medical factors with long-term sexual dysfunction after treatment for colorectal cancer. Support Care Cancer 2013;21:793-802. [PubMed]
- Traa MJ, De Vries J, Roukema JA, et al. Sexual (dys)function and the quality of sexual life in patients with colorectal cancer: a systematic review. Ann Oncol 2012;23:19-27. [PubMed]
- Lange MM, Marijnen CA, Maas CP, et al. Risk factors for sexual dysfunction after rectal cancer treatment. Eur J Cancer 2009;45:1578-88. [PubMed]
- Punnen S, Cowan JE, Dunn LB, et al. A longitudinal study of anxiety, depression and distress as predictors of sexual and urinary quality of life in men with prostate cancer. BJU Int 2013;112:E67-75. [PubMed]
- Li CC, Rew L. A feminist perspective on sexuality and body image in females with colorectal cancer: an integrative review. J Wound Ostomy Continence Nurs 2010;37:519-25. [PubMed]
- Den Oudsten BL, Traa MJ, Thong MS, et al. Higher prevalence of sexual dysfunction in colon and rectal cancer survivors compared with the normative population: a population-based study. Eur J Cancer 2012;48:3161-70. [PubMed]
- Costa PT, McCrae RR. eds. NEO PI/FFI Manual Supplement for Use with the NEO Personality Inventory and the NEO Five-Factor Inventory. Odessa: Psychological Assessment Resources, 1989.
- De Vries J, Van Heck GL. Development of a short version of the Dutch version of the Spielberger STAI trait anxiety scale in women suspected of breast cancer and breast cancer survivors. J Clin Psychol Med Settings 2013;20:215-26. [PubMed]
- Hopwood P, Fletcher I, Lee A, et al. A body image scale for use with cancer patients. Eur J Cancer 2001;37:189-97. [PubMed]
- Whistance RN, Gilbert R, Fayers P, et al. Assessment of body image in patients undergoing surgery for colorectal cancer. Int J Colorectal Dis 2010;25:369-74. [PubMed]
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol 1992;31:301-6. [PubMed]
- Michielsen HJ, De Vries J, Van Heck GL. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J Psychosom Res 2003;54:345-52. [PubMed]
- Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer 1999;35:238-47. [PubMed]
- The World Health Organization Quality of Life Assessment (WHOQOL). development and general psychometric properties. Soc Sci Med 1998;46:1569-85. [PubMed]
- Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Qual Life Res 1993;2:153-9. [PubMed]
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res 2002;14:226-44. [PubMed]
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. [PubMed]
- Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther 2000;26:191-208. [PubMed]
- ter Kuile MM, Brauer M, Laan E. The Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale (FSDS): psychometric properties within a Dutch population. J Sex Marital Ther 2006;32:289-304. [PubMed]
- Rust J, Golombok S. The Golombok-Rust Inventory of Sexual Satisfaction (GRISS). Br J Clin Psychol 1985;24:63-4. [PubMed]
- Joseph O, Alfons V, Rob S. Further validation of the Maudsley Marital Questionnaire (MMQ). Psychol Health Med 2007;12:346-52. [PubMed]
- Arrindell WA, Boelens W, Lambert H. On the psychometric properties of the Maudsley Marital Questionnaire (MMQ): Evaluation of self-ratings in distressed and ‘normal’ volunteer couples based on the dutch version. Person Individ Diff 1983;4:293-306.
- Arrindell WA, Schaap C. The Maudsley Marital Questionnaire (MMQ): an extension of its construct validity. Br J Psychiatry 1985;147:295-9. [PubMed]
- Cohen J. eds. Statistical power analysis for the behavioral scienses. Hillsdalen NJ: Lawrence Erlbaum, 1988.
- Hedeker D, Gibbons RD. eds. Longitudinal data analysis. Hoboken NJ: Wiley, 2006.
- Bober SL, Varela VS. Sexuality in adult cancer survivors: challenges and intervention. J Clin Oncol 2012;30:3712-9. [PubMed]
- Ross L, Abild-Nielsen AG, Thomsen BL, et al. Quality of life of Danish colorectal cancer patients with and without a stoma. Support Care Cancer 2007;15:505-13. [PubMed]
- Mols F, Lemmens V, Bosscha K, et al. Living with the physical and mental consequences of an ostomy: a study among 1-10-year rectal cancer survivors from the population-based PROFILES registry. Psychooncology 2014;23:998-1004. [PubMed]
- Orsini RG, Thong MS, van de Poll-Franse LV, et al. Quality of life of older rectal cancer patients is not impaired by a permanent stoma. Eur J Surg Oncol 2013;39:164-70. [PubMed]
- Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psychooncology 2013;22:1688-704. [PubMed]
- Manne S, Badr H. Intimacy and relationship processes in couples' psychosocial adaptation to cancer. Cancer 2008;112:2541-55. [PubMed]
- Bruheim K, Tveit KM, Skovlund E, et al. Sexual function in females after radiotherapy for rectal cancer. Acta Oncol 2010;49:826-32. [PubMed]
- Nagpal K, Bennett N. Colorectal surgery and its impact on male sexual function. Curr Urol Rep 2013;14:279-84. [PubMed]
- Kayser K, Watson LE, Andrade JT. Cancer as a “We-disease”: Examining the Process of Coping From a Relational Perspective. Fam Syst Health 2007;25:404-18.
- Traa MJ, De Vries J, Roukema JA, et al. The sexual health care needs after colorectal cancer: the view of patients, partners, and health care professionals. Support Care Cancer 2014;22:763-72. [PubMed]
- Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74-7. [PubMed]



