Abstract
Trait anxiety is associated with an impaired ability to inhibit task-irrelevant distractions. However, distractions may cause conflict at multiple stages within the information-processing stream, which may be resolved by different subsystems, depending on the type of conflict. Here, we contrasted two forms of conflict that have been distinguished in the literature: stimulus–stimulus (SS) versus stimulus–response (SR) competition. Across two experiments, participants completed a Stroop-like task that included distractor color words that could be either a different color promoting the same response as the target (SS interference) or a different color promoting a different response (SR interference). In line with previous studies, anxiety increased overall task-irrelevant distraction, measured by incongruent versus congruent reaction times. Importantly, this increase was driven solely by SR conflict, with no evidence of group differences in SS interference, despite clear within-subjects costs. These results clarify that trait anxiety may modulate inhibitory responses primarily by disrupting the resolution of response competition, while having little effect on stimulus conflict. Additionally, the results highlight the utility of distinguishing forms of conflict resolution, particularly in individual-difference approaches in which inhibition is impaired in a variety of clinical and nonclinical populations.
Similar content being viewed by others
“Cognitive inhibition,” the process of “stopping or overriding a mental process” (MacLeod, 2007) is conceptualized as a broad mechanism helping us to maintain focus on task goals and ignore irrelevant distractions, which is necessary for successful performance outcomes.
However, defining inhibition in a broad manner may be misleading as distractions can arise from numerous types of interference, from failing to focus attention on task-relevant information, failing to prioritize relevant over irrelevant information, or failing to suppress reflexive responses. In all cases, performance outcome is impaired. Although cognitive inhibition could constitute a single process responding to all types of interference, it could also consist of domain-specific elements devoted to negating specific types of conflict. Thus, distinguishing the possible stages at which inhibitory control can fail may give a more precise understanding of how inhibition operates, especially in vulnerable populations suffering from inhibitory control deficits (e.g., from attention deficit-hyperactivity disorder, Bartley, 1997; autism, Ciesielski & Harris, 1997; depression, Joormann, 2010; posttraumatic stress disorder, Cloitre, 1998; or schizophrenia, Nestor & O’Donnell, 1998).
Here, we focus on impaired inhibition in trait anxiety. We examine evidence of impaired inhibitory control in anxiety, before considering what subtypes of inhibitory control could occur. Specifically, we review findings pointing to one major dichotomy: resolution of stimulus–stimulus (SS) and stimulus–response (SR) conflict. We then investigate whether anxiety modulates inhibition in response to these specific kinds of conflict.
Inhibitory control in trait anxiety
Impaired selective attention is a central characteristic of trait anxiety, with consistent findings that self-report levels of anxious emotion correspond positively with the magnitude of a threat-related bias in selective attention (see Bar-Haim et al., 2007, for review). However, the threat bias does not provide strong insight into the underlying cognitive processes; examining selective attention in paradigms in which no element of threat is included gives a stronger means of assessing the broader ability to inhibit irrelevant information. Accumulating evidence shows that anxious individuals have an impaired ability to ignore distractions, such as taking longer to avert gaze from stimuli in the antisaccade task (Derakshan, Ansari, Hansard, Shoker, & Eysenck, 2009) and being slower to ignore task-irrelevant distractors in the flanker task (Berggren & Derakshan, 2013; Bishop, 2009; Pacheco-Unguetti, Acosta, Callejas, & Lupiáñez, 2010). Neuroimaging results have also highlighted anxiety modulations to key brain regions implicated in inhibitory control, such as the dorsolateral prefrontal cortex (DLPFC), in the inhibition of distracting material (Basten, Stelzel, & Fiebach, 2011; Bishop, 2009).
Forms of conflict: Stimulus–stimulus and stimulus–response
On the basis of this evidence, cognitive theories have proposed a general impairment in top-down cognitive control in anxiety (e.g., Eysenck, Derakshan, Santos, & Calvo, 2007), which affects inhibition of irrelevant information, regardless of its type. This logic is similar to that of Miyake and colleagues (e.g., Friedman & Miyake, 2004), who, dividing inhibitory function into aspects such as behavioral versus oculomotor control, concluded that strong correlations in performance across tasks suggest a common inhibitory mechanism in the brain. However, inhibitory control may be compartmentalized into multiple facets. We focus on two facets proposed to involve distinct forms of inhibitory control; an interested reader should consult other accounts positing wider taxonomies (e.g., Nigg, 2000).
One of the most empirically examined dichotomies in inhibitory control arose from the dimensional-overlap model proposed by Kornblum and colleagues (e.g., Kornblum, 1994), in which common selective-attention paradigms such as the Stroop and flanker tasks were argued to fail in fully distinguishing the extents to which they weight SS or SR conflict. Kornblum proposed that the two forms of conflict operate additively, and although some tasks do test participants’ ability to ignore one type of conflict, many are confounded with both. For example, in the flanker task, participants respond to a target letter while ignoring irrelevant flanking letters that can denote the same or the opposite motor response. Here, conflict can arise due to the irrelevant letter promoting response competition (SR conflict), but could also arise due to the irrelevant letter simply being a letter that is not the same as the target, creating a stimulus overlap (SS conflict).
To test these ideas, variations of the Stroop task have been employed. For example, De Houwer (2003) used four possible target color words (blue, green, orange, purple). Two colors were assigned to one response each (e.g., “for blue and green press left, and orange and purple press right”), allowing comparisons between displays in which flanking distractor words were congruent (CO) to the middle word target (i.e., green–green–green), incongruent on the basis of stimulus identity but not response (stimulus interference, or SI; blue–green–blue), and incongruent on both stimulus identity and response (response interference, or RI; orange–green–orange). Reaction times (RTs) were significantly slower on SI than on CO trials, but slower still on RI trials. Using a subtractive logic, the difference between SI and CO trials is assumed to solely reflect SS conflict, whereas the difference between SI and RI trials is attributed to the incremental effect of SR conflict. Thus, both forms of interference additively impair performance in standard selective-attention tasks.
How are SS and SR conflict resolved?
If SS and SR interference are viewed as being independent types of conflict, does this necessarily mean that they are resolved through different, rather than a single global, mechanism of inhibitory control? Across neuroimaging paradigms, both SS and SR conflict increase activity in the DLPFC and anterior cingulate cortex (ACC; e.g., van Veen & Carter, 2005), with SS conflict typically increasing parietal response and SR affecting motor areas and wider activity in ACC (e.g., Liu, Banich, Jacobson, & Tanabe, 2004; Milham et al., 2001).
Although both forms of conflict are associated with increased frontal activity, crucially, there is no overlapping activation on the signal response (van Veen & Carter, 2005), suggesting that the resolution of SS and SR interference involves specific forms of inhibitory control. This highlights the idea that, whereas both types of conflict additively lead to the same result—increased interference—it is difficult to ascertain a precise locus of inhibitory failure without distinguishing the two. The implication for individual-difference approaches is that conclusions regarding impaired inhibition cannot be known without appreciating the types of interference that fostered it.
Poorer control of SS conflict, SR, or both in trait anxiety?
Although high levels of anxiety are believed to result in reduced top-down control (Eysenck et al., 2007), it is unclear whether this is underpinned by a relatively global deficit in control or is specific to certain forms of conflict.
Many demonstrations of anxiety affecting inhibition have come from tasks arguably combining SS and SR conflict. For example, flanker (e.g., Bishop, 2009; Derakshan et al., 2010) and Stroop-like (Fox, 1993; Hopko, Hunt, & Armento, 2005) tasks containing response incompatible versus compatible distractors are not differentiated on the basis of SS/SR conflict. Although neutral trials are sometimes included with response-ineligible distractors, behavioral floor effects for these items prevent examining the role of SS conflict on reaction times (e.g., Milham et al., 2001). Additionally, poorer antisaccade performance (e.g., Derakshan et al., 2009) may be problematic to class as solely SR conflict, as onsets is both a relevant stimulus to acknowledge so participants can look away, and an irrelevant stimulus to avoid looking at.
Evidence of increased SS conflict in anxiety has to date not been assessed, due to most standard selective attention paradigms containing both SS and SR conflict elements. Sole SR interference is argued to occur in Simon tasks, in which target items are placed in congruent or incongruent locations, creating a response conflict on the response mapping. No current evidence favors anxiety increasing Simon task distraction (Ng, Chan, & Schlaghecken, 2012). Another candidate paradigm is the go/no-go task, in which participants respond to a set stimulus but are sometimes asked to withhold a response. Righi, Mecacci, and Viggiano (2009) demonstrated in an event-related potential investigation that anxiety increased N2 amplitudes in the go/no-go task, but similarly for both go and no-go trials. Sehlmeyer et al. (2010) did find increased N2 amplitudes in anxiety primarily on no-go trials, but this was complemented by reduced behavioral errors, making interpretations of inefficient inhibitory control questionable.
In summary, it is unknown whether trait anxiety modulates inhibition in a global manner or selectively affects certain forms of inhibitory control. We investigated this question using the SS/SR conflict distinction highlighted in previous work. To disentangle the two forms of conflict, we utilized a Stroop-like task in which distracting items could have a different stimulus identity but promote the same or a different response from a target, allowing a comparison between SS and SR interference trials (De Houwer, 2003). We predicted that anxiety would modulate overall distractor interference when response-incongruent trials were compared to same-identity congruent trials, consistent with previous studies (e.g., Berggren & Derakshan, 2013). We then aimed to distinguish the contributions of SS and SR interference and to examine whether one or both forms would be modulated by anxiety.
Experiment 1
Method
Participants
A group of 60 naïve participants (17 male, 43 female; mean age = 23 years, SD = 4) were recruited from the University of London area via online advertisements. All were right-handed and had normal or corrected-to-normal vision. Three participants did not meet the requirement of English proficiency and so did not complete the experimental task, leaving 57 participants.
Apparatus and stimuli
The experiment was created and executed using the E-Prime software (Psychology Software Tools, Inc.) on an IBM-compatible PC attached to a Mitsubishi Diamondtron monitor. Viewing distance was kept at 60 cm using a chinrest, and keyboard buttonpresses were recorded as responses.
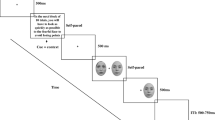
Stimuli were presented on a light gray background, with all items appearing in black font. The target words varied in size depending on the item (between 2.67º × 0.95º to 5.53º × 0.95º) and were equally likely to be BLUE, GREEN, RED, or YELLOW. Distractor words appeared either above or below the target word with 0.76º eccentricity spacing, and followed the same dimensions to target if fixated. For each experimental block, 24 possible trials were created and run through four times. Within each block, six trials were used for each target identity, with two being distractor-absent baseline trials and the remaining four covering each target–distractor combination. See Fig. 1 for example trials from the different conditions.
Examples of possible target–distractor combinations used in Experiment 1. From left to right are a baseline distractor-absent trial, a distractor that is the same stimulus (congruent), a different stimulus with the same associated response (stimulus interference), and a different stimulus with a different response (response interference)
Procedure
Participants first completed a self-report anxiety questionnaire (State–Trait Anxiety Inventory; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983) and rated their English language proficiency. On each trial, following a fixation cross (1,000 ms), a target color word appeared at fixation for 200 ms. Participants responded by pressing the “0” key on the numeric keypad if the target word was BLUE or GREEN, and the “2” key if the word was RED or YELLOW. In addition, participants were told that on some trials, an additional color word could appear above or below the target word. They were instructed that this word could be the same as the target word or different, and that they should ignore this and respond to the target word as quickly and accurately as possible. A response window of 1,500 ms was given for each trial, with a subsequent 1,500-ms intertrial interval. If participants failed to respond within the allotted time, or made an incorrect response, a short beep provided auditory feedback.
Participants completed 52 training trials in which only the target word was presented on each display, in order for the key mappings to be learned (each possible target appearing on 25% of the trials). Following this, participants completed a practice block of 24 trials in which the target words could appear alone or with additional distractor words, as in the main experiment. Finally, participants completed six blocks of 96 trials apiece as part of the main task.
Results and discussion
The data from three of the 57 participants were removed, two for mean reaction times over three SDs from grand mean and one for chance-level accuracy. The remaining 54 participants were divided into two anxiety groups using a median split method; participants with a score of 40 or below were designated low-anxious, and those with a score above 40 high-anxious. The two groups’ scores significantly differed [M = 33 vs. 50; t(52) = 9.90, p < .001].
Reaction times
Only accurate trials were used for the reaction time (RT) analysis. First, we calculated the distractor cost from the difference between RI and CO trials, the comparison typically made in standard compatibility tasks, in order to assess whether anxiety modulated general, task-irrelevant distraction. High-anxious participants had larger distractor costs (M = 64 ms, SD = 34) than did their low-anxious peers (M = 48 ms, SD = 27), [t(52) = 1.88, p < .05 one-tailed]. However, there was no correlation between RI–CO distractor costs and anxiety when we used anxiety score as a continuous variable (r < .1). The general result that anxiety increases task-irrelevant distraction was thus partly replicated.
Examining RTs more fully, we conducted a mixed analysis of variance (ANOVA) with the factors Condition (baseline, CO, SI, RI) and Group (low-anxious, high-anxious). This analysis showed a main effect of condition [F(3, 156) = 136.11, p < .001, η p 2 = .72], with RTs increasing at each level from baseline to RI (all ts > 4.93, ps < .001). We found no main effect of group (F < 1), but a significant Condition × Group interaction [F(3, 156) = 2.85, p = .04, η p 2 = .05; see Table 1]. A series of t tests were conducted, comparing baseline versus CO (reflecting a distractor presence-vs.-absence cost), CO versus SI (reflecting an SS interference cost), and SI versus RI (reflecting an SR interference cost). The low- and high-anxious groups did not differ for distractor presence costs (mean differences [M diff] = 9 vs. 15 ms, respectively; t < 1) nor SS interference costs (M diff = 33 vs. 34 ms) [t(52) = 1.06, p > .29], but did for SR interference, displaying greater distraction in the high-anxious group (M diff = 15 vs. 30 ms) [t(52) = 2.53, p < .02]. Additionally, we found no correlation between anxiety score and costs associated with distractor presence (r < 1) or SS interference (r = –.14, N = 54, p = .30), but a significant positive correlation with SR interference (r = .32, N = 54, p = .02; see Fig. 2). A Williams test for comparing two nonindependent r values (Williams, 1959) also showed that the correlation with anxiety was significantly stronger for SR than for SS interference [t(51) = 2.22, p < .05].
Error rates
Distractor effects from the difference between RI and CO trials between groups missed significance [t(52) = 1.67, p = .055, one-tailed]. We also found no correlation between anxiety score and general distractor costs (r < .1).
A mixed ANOVA similar to the one from the RT analysis showed a main effect of condition [F(3, 156) = 29.45, p < .001, η p 2 = .49], with no significant difference between baseline and CO trials (M = 6% vs. 6%; t < 1) or between CO and SI trials (M = 6% vs. 7%) [t(53) = 1.17, p > .24]; only RI trials appeared to increase error rates relative to SI trials (M = 11% vs. 7%) [t(53) = 6.06, p < .001]. No significant interaction emerged between condition and group (F < 1). The marginal effects of anxiety on general distractor costs were led by a trend for differences in SR interference costs [t(52) = 1.67, p = .10] rather than SS interference or distractor presence costs (ts < 1). We found no significant correlations between any of these forms of interference and anxiety score (all rs < .15, ps > .29).
The results from Experiment 1 suggest that anxiety impacts inhibition specifically in response to SR, rather than to both SS and SR, conflict. However, in this experiment CO and SI trials differed in their degrees of visual complexity; target and distractor items were visually identical on CO trials, but differed on SI trials. As a result, items may have been processed more quickly on CO trials (De Houwer, 2003), masking individual-difference effects when subtracting to create SS interference costs. In addition, RI trials occurred more often than did SI or CO trials, due to an additional possible combination of color items. The greater number of trials could explain why anxiety modulated SR interference exclusively, in that SS interference differences might have been observed with more experimental trials. In Experiment 2, we changed the target item to a color bar rather than a color word. As a result, the visual complexity of the displays was constant across conditions. Additionally, the proportions of CO, SI, and RI trials were also balanced.
Experiment 2
Method
Participants
A group of 44 new naïve participants (16 male, 28 female; mean age = 24 years, SD = 5) took part in Experiment 2. All were right-handed and had normal or corrected-to-normal vision. Participants were prescreened for English language proficiency.
Apparatus and stimuli
The methods matched those of Experiment 1, with the following exceptions. Rather than a target color word, the targets were now color bars at fixation (6.20º × 1.43º). Distractor words remained at the same eccentricity. For RI trials, distractor words were chosen at random rather than counterbalanced, to ensure that one third of trials apiece were CO, SI, and RI.
Procedure
The procedures were similar to those of Experiment 1. Participants were instructed to respond to the color bars as quickly and accurately as possible and to ignore the words appearing above or below. Assignments of colors to response keys were counterbalanced across participants. Following training and practice, participants completed four blocks of 96 trials.
Results and discussion
The data from two participants were removed, one for chance-level accuracy and one for RTs over three SDs from the grand mean. The remaining 42 participants were divided into two anxiety groups through a median split, with 21 participants scoring below 40 being designated low-anxious, and 21 scoring above 40 being designated high-anxious. The two groups’ scores differed significantly [M = 34 vs. 46; t(40) = 9.05, p < .001].
Reaction times
Correct RTs were used to measure overall distractor costs (i.e., RI minus CO trial RT), with groups being compared. This revealed a significant effect of group [t(40) = 1.91, p < .04 one-tailed], with higher distractor costs for the high-anxious (M = 38 ms, SD = 26) than for the low-anxious (M = 25 ms, SD = 16) group. Additionally, anxiety score correlated positively with the RI–CO distractor cost (r = .43, N = 42, p < .01).
A mixed ANOVA with the factors Condition (CO, SI, RI) and Group (low-anxious, high-anxious) showed a main effect of condition [F(2, 80) = 47.48, p < .001, η p 2 = .54], with RTs significantly increasing from CO to SI and from SI to RI trials (ts > 3.11, ps < .01); a main effect of group [F(1, 40) = 5.40, p < .03, η p 2 = .12], with high-anxious participants being significantly slower overall (M = 572.50, SD = 71.27) than low-anxious ones (M = 518.45, SD = 79.24); and a Condition × Group interaction [F(2, 80) = 3.36, p = .04, η p 2 = .08; see Table 2]. This reflected no difference between groups in SS interference (M diff = 11 vs. 8 ms, respectively, for low- vs. high-anxious participants; t < 1), but importantly, a significant effect on SR costs, with higher costs in the high-anxious group (M diff = 14 vs. 30 ms), [t(40) = 2.30, p < .03]. Correlations showed no association between anxiety score and SS interference (r < .1), but, replicating Experiment 1, a positive correlation with SR costs was observed (r = .42, N = 42, p < .01; see Fig. 3). These correlations differed significantly [t(39) = 1.70, p < .05, one-tailed].
Error rates
Overall distractor costs were not modulated by group (M diff = 1% vs. 1%; t < 1), and did not correlate with anxiety score (r < .1).
A mixed ANOVA showed a main effect of condition [F(2, 80) = 9.64, p < .01, η p 2 = .19], with significantly fewer errors being made on SI than on CO trials (M diff = 6% vs. 7%) [t(41) = 2.28, p < .03], but significantly greater errors on RI than on SI trials (M diff = 6% vs. 8%) [t(41) = 4.47, p < .01]. We observed no main effect of group and no Condition × Group interaction (Fs < 1). Finally, anxiety scores did not correlate with the derived SS or SR interference effects (rs < .1).
General discussion
We examined whether trait vulnerability to anxiety impairs inhibitory response to irrelevant information in general, or whether this is specific to only certain kinds of interference. Despite interference effects within subjects from both SS and SR types of conflict, trait anxiety solely influenced the ability to resolve SR interference, and was not associated with SS costs. One could argue that this was due to SS conflict occurring automatically, outside the influence of top-down control. Yet, variability in SS interference was strong across participants, and previous evidence has suggested that modulations to SS costs are found in other groups (e.g., aging; Prakash et al., 2009). Our findings provide important extensions to theoretical accounts that trait anxiety should generally disrupt aspects of top-down control such as inhibition (Eysenck et al., 2007); here anxiety influenced attentional control and its subfunction of inhibition in a more specific manner, primarily with impairment in rejecting a prepotent response.
Anxiety increasing SR costs extends previous evidence of inhibitory deficits from other paradigms, such as the flanker (e.g., Pacheco-Unguetti et al., 2010) and antisaccade (e.g., Derakshan et al., 2009) tasks, by suggesting that increased task-irrelevant processing in anxiety is primarily caused by distractors’ response-competing dimension. This contrasts with views of purely enhanced bottom-up processing in anxiety (Moser, Becker, & Moran, 2012)—according to which any irrelevant information should be more disruptive to task performance in the highly anxious—and more strongly emphasizes the impairment in top-down control.
Taken together, these results highlight that accounts of poorer executive control in trait anxiety (Bishop, 2009; Eysenck et al., 2007) may be too general in their predictions regarding impaired inhibition in response to distraction from all possible sources of interference. A refined account could weigh more strongly on the role of stimulus–response associations, with these associations specifically being more difficult to suppress in anxiety when they are irrelevant. Furthermore, the results may allow for a clearer understanding of the underlying neural mechanisms in trait anxiety. Indeed, in light of evidence that SR conflict exclusively associates with activity in the ventrolateral region of prefrontal cortex (i.e., inferior frontal gyrus; van Veen & Carter, 2005) and that this region is heavily involved in response inhibition (Aron, Robbins, & Poldrack, 2004), this presents a feasible locus of inhibitory failure in trait anxiety that has received less empirical attention than have other areas, such as DLPFC.
A number of other avenues for future research remain. For example, enhanced attentional capture by irrelevant singletons has been found in high-anxious individuals (Moser et al., 2012), whereas in Experiment 1 we found no difference in distractor-absent versus -present costs with anxiety. This implies that capture or filtering costs may be evident in anxiety when task-irrelevant stimuli increase in saliency. In addition, the present results suggest that anxiety would impact interference levels in pure SR paradigms such as the Simon or go/no-go tasks; further investigation will be warranted, considering that previous work has failed to find clear evidence of such an association (e.g., Ng et al., 2012; Righi et al., 2009). Finally, it has been proposed that state-induced anxiety may impact attention by enhancing bottom-up processing of task-irrelevant information, versus trait anxiety primarily reducing top-down cognitive control (Bishop, 2007). According to this account, state anxiety might be predicted to increase SS while having little effect on SR interference, directly opposite to the effect of trait anxiety. Indeed, there is evidence that different aspects of the attention network are impaired by state and trait anxiety exclusively (see Pacheco-Unguetti et al., 2010).
More generally, the extent to which differences in inhibitory control may be specific or global in other forms of individual differences is an important question. As we highlighted in our introduction, deficits are seen in a range of groups, and yet some theoretical accounts lack specificity and consider inhibition failure to be global. To date, an SS/SR conflict distinction has been studied in only two areas, to our knowledge: cognitive aging (e.g., Prakash et al., 2009) and, here, trait anxiety. How other individual differences impact on aspects of selective attention and distraction may aid in understanding the similarities and differences in cognitive inhibition across the population.
References
Aron, A. R., Robbins, T. W., & Poldrack, R. A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8, 170–177. doi:10.1016/j.tics.2004.02.010
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & van IJzendoorn, M. H. (2007). Threat-related attentional bias in anxious and non-anxious individuals: A meta- analytic study. Psychological Bulletin, 133, 1–24. doi:10.1037/0033-2909.133.1.1
Bartley, R. A. (1997). Behavioral inhibition, sustained attention, and executive function: Constructing a unified theory of ADHD. Psychological Bulletin, 121, 65–94.
Basten, U., Stelzel, C., & Fiebach, C. J. (2011). Trait anxiety modulates the neural efficiency of inhibitory control. Journal of Cognitive Neuroscience, 23, 3132–3145. doi:10.1162/jocn_a_00003
Berggren, N., & Derakshan, N. (2013). The role of consciousness in attentional control differences in trait anxiety. Cognition and Emotion, 27, 923–931.
Bishop, S. J. (2007). Neurocognitive mechanisms of anxiety: An integrative account. Trends in Cognitive Sciences, 11, 307–316. doi:10.1016/j.tics.2007.05.008
Bishop, S. J. (2009). Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience, 12, 92–98.
Ciesielski, K. T., & Harris, R. J. (1997). Factors related to performance failure on executive tasks in autism. Child Neuropsychology, 3, 1–12.
Cloitre, M. (1998). International forgetting and clinical disorders. In J. M. Golding & C. M. MacLeod (Eds.), Intentional forgetting: Interdisciplinary approaches (pp. 395–412). Mahwah: Erlbaum.
De Houwer, J. (2003). On the role of stimulus-stimulus and stimulus–response compatibility in the Stroop effect. Memory & Cognition, 31, 353–359. doi:10.3758/BF03194393
Derakshan, N., Ansari, T. L., Hansard, M., Shoker, L., & Eysenck, M. W. (2009). Anxiety, inhibition, efficiency, and effectiveness: An investigation using the antisaccade task. Experimental Psychology, 56, 48–55.
Eysenck, M. W., Derakshan, N., Santos, R., & Calvo, M. G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7, 336–353. doi:10.1037/1528-3542.7.2.336
Fox, E. (1993). Attentional bias in anxiety: Selective or not? Behaviour Research and Therapy, 31, 487–493.
Friedman, N. P., & Miyake, A. (2004). The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology: General, 133, 101–135. doi:10.1037/0096-3445.133.1.101
Hopko, D. R., Hunt, M. K., & Armento, M. E. A. (2005). Attentional task aptitude and performance anxiety. International Journal of Stress Management, 12, 389–408.
Joormann, J. (2010). Cognitive inhibition and emotion regulation in depression. Current Directions in Psychological Science, 19, 161–166.
Kornblum, S. (1994). The way irrelevant dimensions are processed depends on what they overlap with: The case of Stroop- and Simon-like stimuli. Psychological Research, 56, 130–135.
Liu, X., Banich, M. T., Jacobson, B. L., & Tanabe, J. L. (2004). Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event- related fMRI. NeuroImage, 22, 1097–1106. doi:10.1016/j.neuroimage.2004.02.033
MacLeod, C. M. (2007). The concept of inhibition in cognition. In D. S. Gorfein & C. M. MacLeod (Eds.), Inhibition in cognition (pp. 3–23). Washington, DC: American Psychological Association.
Milham, M. P., Banich, M. T., Webb, A., Barad, V., Cohen, N. J., Wszalek, T., & Kramer, A. F. (2001). The relative involvement of anterior cingulated and prefrontal cortex in attentional control depends on nature of conflict. Cognitive Brain Research, 12, 467–473.
Moser, J. S., Becker, M. W., & Moran, T. P. (2012). Enhanced attentional capture in trait anxiety. Emotion, 12, 213–216.
Nestor, P. G., & O’Donnell, B. F. (1998). The mind adrift: Attentional dysregulation in schizophrenia. In R. Parasuraman (Ed.), The attentional brain (pp. 527–546). Cambridge: MIT Press.
Ng, J., Chan, H. Y., & Schlaghecken, F. (2012). Dissociating effects of subclinical anxiety and depression on cognitive control. Advances in Cognitive Psychology, 8, 38–49.
Nigg, J. T. (2000). On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition hypothesis. Psychological Bulletin, 126, 220–246.
Pacheco-Unguetti, A. P., Acosta, A., Callejas, A., & Lupiáñez, J. (2010). Attention and anxiety: Different attentional functioning under state and trait anxiety. Psychological Science, 21, 298–304.
Prakash, R. S., Erickson, K. I., Colcombe, S. J., Kim, J. S., Voss, M. W., & Kramer, A. F. (2009). Age- related differences in the involvement of the prefrontal cortex in attentional control. Brain and Cognition, 71, 328–335. doi:10.1016/j.bandc.2009.07.005
Righi, S., Mecacci, L., & Viggiano, M. P. (2009). Anxiety, cognitive self-evaluation and performance: ERP correlates. Journal of Anxiety Disorders, 23, 1132–1138.
Sehlmeyer, C., Konrad, C., Zwitserlood, P., Arolt, V., Falkenstein, M., & Beste, C. (2010). ERP indices for response inhibition are related to anxiety-related personality traits. Neuropsychologia, 48, 2488–2495.
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., & Jacobs, G. A. (1983). Manual for the State–Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press.
van Veen, V., & Carter, C. S. (2005). Separating semantic conflict and response conflict in the Stroop task: A functional MRI study. NeuroImage, 27, 497–505. doi:10.1016/j.neuroimage.2005.04.042
Williams, E. J. (1959). The comparison of regression variables. Journal of the Royal Statistical Society, Series B, 21, 396–399.
Author note
The research reported in this article was supported by a 1+3 ESRC PhD studentship awarded to N.B. and was carried out under the supervision of N.D., who was also supported in part by a Visiting Research Associate fellowship from St John’s College Research Centre, Oxford. N.D. is currently a Visiting Professor at the Department of Experimental Psychology at the University of Oxford.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berggren, N., Derakshan, N. Inhibitory deficits in trait anxiety: Increased stimulus-based or response-based interference?. Psychon Bull Rev 21, 1339–1345 (2014). https://doi.org/10.3758/s13423-014-0611-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-014-0611-8