Abstract
An unresolved issue in the task-switching literature is whether preparatory reconfiguration occurs before a change of task. In this study, we used event-related potentials (ERPs) to determine whether preparatory reconfiguration occurs during two different task-switching procedures: voluntary and cued task switching. We focused on two ERP components that index different cognitive operations. The contingent negative variation (CNV) is a sensitive measure of a participant’s preparedness to use a specific stimulus–response mapping. In contrast, the P3 indexes memory updating. We found a pronounced modulation of the CNV before voluntary task switches, but not before cued task switches. Instead, cued task switches were preceded by a larger P3, as compared with task repetitions. Our findings suggest that task set reconfiguration is carried out prior to voluntary task switches, whereas memory processes dominate cued task switches.
Similar content being viewed by others
When we switch from one task to another, we pay a price in time and mental effort. For example, in the office, we might switch from working on a spreadsheet to responding to a new e-mail signaled by an alert, and back to the spreadsheet. Indeed, technology increasingly allows or even demands rapid switching between tasks, such as driving and operating an onboard navigation device. This flexible, goal-directed behavior is studied in the laboratory with the aim of understanding executive control mechanisms that allow us to operate in our dynamic task environments. Typically, task switches result in longer reaction times (RTs) and higher error rates than do task repetitions, and these differences in RT and error rate are called task-switching costs (Karayanidis et al., 2010; Kiesel et al., 2010). Ultimately, if we can determine the cause of these task-switching costs, we will better understand the nature of executive control processes that allow flexible behavior and govern subordinate information-processing operations.
A common task-switching procedure uses an explicit cue instructing participants to switch tasks (Meiran, 1996). Two different types of theories have been offered to explain the task-switching costs during the cued task-switching paradigm. Perhaps the most frequent explanation is that active reconfiguration occurs following a cue to change tasks (Logan & Gordon, 2001; Mayr & Kliegl, 2000; Rogers & Monsell, 1995; Sohn & Anderson, 2001). However, others propose that task-switching costs result from memory retrieval processes such as priming (Allport & Wylie, 2000; Arrington & Logan, 2004b; Logan & Bundesen, 2003) or interference from the previous task set (Allport, Styles, & Hsieh, 1994). These memory-based explanations come in at least two varieties. One variety proposes that task switch costs are due to the need to use the cue and the target to retrieve the response from long-term memory (Logan & Bundesen, 2003; Schneider & Logan, 2005). Another variety proposes that automatic retrieval of memory representations of the previous trials contaminates performance on the current trial (Allport & Wylie, 2000).
In contrast to the cued task-switching procedure, the voluntary task-switching procedure requires participants to choose which task to perform. That voluntary choice seems more likely to require top-down task set reconfiguration (Arrington & Logan, 2004a, 2005). In voluntary task switching, there are no explicit cues that might be used to retrieve the new stimulus–response mapping (Arrington & Logan, 2004a). Even when experimenters present stimuli that could be used to trigger task switches externally, these stimuli appear to be ignored in favor of endogenous control of the current task set (Arrington & Logan, 2005).
In this study, we recorded event-related potentials (ERPs) from the same participants during voluntary and cued task switching. We used two different ERP components to diagnose whether reconfiguration or memory processes underlie task switching in the two procedures. The contingent negative variation (CNV) is believed to measure the preparation of stimulus–response mapping rules (Verleger, Wauschkuhn, van der Lubbe, Jaskowski, & Trillenberg, 2000; for a review, see Leuthold, Sommer, & Ulrich, 2004), which is a primary component of task set reconfiguration (Kiesel et al., 2010). The P3 is believed to measure memory updating (Donchin, 1981; Donchin & Coles, 1988; Luck, 2005; Woodman, 2010), which is an important component of the kind of memory retrieval that produces priming and interference.
The CNV and P3 components can be distinguished by their polarity and scalp distributions, as well as the mechanisms they appear to index. The CNV is a negativity that builds up as participants anticipate a target stimulus (Brunia, 2003; Walter, Cooper, Aldridge, McCallum, & Winter, 1964). The CNV is believed to measure preparation of stimulus–response mappings (Leuthold et al., 2004; Verleger et al., 2000).Footnote 1 The CNV is larger following a stimulus that indicates the specific speeded response that will be required for the upcoming target (Verleger et al., 2000). The scalp distribution of the CNV depends on both the nature of the stimuli and the nature of the required responses, suggesting that it indexes aspects of both stimulus and response (Brunia, van Boxtel, & Böcker, 2012; Lang, Lang, Kornhuber, Deecke, & Kornhuber, 1984; Ruchkin, Sutton, Mahaffey, & Glaser, 1986). The amplitude of the CNV increases with the complexity of the stimulus–response mapping rules (Leuthold et al. 1996).
The P3 is typically the third positive component after stimulus onset. It is maximal over the parietal lobe and is generally believed to measure the updating of memory or context (Donchin, 1981; Donchin & Coles, 1988; Luck, 2005; Woodman, 2010).Footnote 2 A similar potential is observed when information is successfully retrieved from long-term memory during a recognition task (Johnson, Pfefferbaum, & Kopell, 2007; Rugg et al., 1998). Several ERP studies of cued task switching found a P3 in the interval between the task cue and target presentation in the form of a larger positivity around 300 ms after the onset of a cue on task switch trials than on task repetition trials (Barceló, Periáñez, & Nyhus, 2008; Jost, Mayr, & Rosler, 2008; Nicholson, Karayanidis, Poboka, Heathcote, & Michie, 2005), and some of these studies refer to this as the switch-related positivity (Karayanidis et al., 2010).
We sought to determine the source of task-switching costs by measuring participants’ ERP waveforms during voluntary and cued task-switching procedures. Participants switched between making magnitude (higher or lower than 5) and parity (odd or even) judgments of single digits that followed one of the four colored cues. As is shown in Fig. 1, we presented exactly the same sequences of stimuli in the two procedures and had participants perform the same two tasks. In the voluntary condition, participants were told that the colored circle preceding each target simply served as a warning stimulus indicating that the target digit was imminent. They were instructed to perform each task on 50 % of trials and to switch between tasks as they chose (as described in Arrington & Logan, 2004a; Arrington & Logan, 2005). The responses for each task were assigned to a different hand and counterbalanced across participants so we could track which task was performed. The cued version of this task-switching paradigm was identical, except that the participants were told that the color of the cue indicated which task they were to perform on each trial. We used four cue colors, in which two cues were assigned to the odd/even task and the other two cues were assigned to the low/high task to remove a confound between cue repetitions and task repetitions when there was only one cue per task (Logan & Bundesen, 2003; Mayr & Kliegl, 2003; see also Schneider & Logan, 2011).
Stimuli presented during the voluntary and cued task-switching conditions. The stimulus sequence on each trial in both conditions consisted of the fixation cross, a colored cue (red, green, cyan, or purple), and a target digit (1, 2, 3, 4, 6, 7, 8, or 9). The different task responses were mapped to different hands for each participant
If task switching in either paradigm involves preparatory reconfiguration of the task set, the amplitude of the CNV should be larger before a task switch than before a repetition. If task switching in either paradigm relies upon bottom-up memory processes, the amplitude of the P3 should be larger before a task switch than before a repetition. If voluntary task switching requires preparatory reconfiguration but cued task switching does not, the CNV effects should occur in voluntary task switching but not in cued task switching. If cued task switches involve bottom-up memory processes but voluntary task switching does not, the P3 effects should occur in cued task switching but not in voluntary task switching. If the two procedures involve the same cognitive processes, we should see the same CNV and P3 effects in voluntary and cued task switching.
Method
Participants
Twenty-two volunteers provided informed consent of procedures approved by the Vanderbilt Institutional Review Board. Two participants’ data were discarded due to excessive artifacts in the electrophysiological data. Ten performed the cued task-switching condition first, and the other 10 performed the voluntary task-switching condition first.
Stimuli
We used identical stimulus sequences in both task-switching conditions, as shown in Fig. 1. The fixation point (0.5° × 0.5°; black, 2.39 cd/m2), cue (red, x = 0.548, y = 0.334; green, x = 0.314, y = 0.545; cyan, x = 0.200, y = 0.291; or purple, x = 0.191, y = 0.106; circle, 0.5° × 0.5°), and target digit (1, 2, 3, 4, 6, 7, 8, or 9; Courier, 0.7° × 0.7°; black, 2.39 cd/m2) were presented on a gray background (16.4 cd/m2).
Procedure
Each trial began with the presentation of the fixation point for 500 ms; then the cue was presented for either 800 or 300 ms with equal probability. The 800-ms temporal separation between the stimuli minimized the overlap in the neural responses to each event (Jost et al., 2008; Luck, 2005; Woodman, 2010). The shorter, 300-ms cue-to-target intervals (CTIs) of the remaining trials were included to increase the likelihood that we could observe participants’ behavioral task switch costs, particularly in the cued task-switching paradigm in which switch costs are known to be larger at short CTIs (Merian, 1996). But the 300-ms CTIs were excluded from ERP analyses to reduce overlapping electrophysiological responses from the cue and target. The target digit was then presented until the manual response was made. A brief 500-ms intertrial interval followed. Each task-switching condition consisted of 16 blocks of 64 trials.
Participants reported either the parity (odd or even) or the magnitude (larger or smaller than 5) of each target digit. The assignment of task to response hand was counterbalanced—that is, parity to the index and middle fingers of one hand and magnitude to the same fingers on the other hand. In cued task switching, the cue colors (red, green, cyan, and purple) indicated which task was to be performed, and the cue color and the task association were randomized across observers. In voluntary task switching, participants were instructed to choose the task to be performed themselves. The cues appeared but were not informative (Arrington & Logan, 2005).
ERP recording and analysis
The electroencephalogram (EEG) was recorded from tin electrodes in an elastic cap (Electrocap International, Eaton, OH). A subset of the International 10/20 System sites was used (Fz, Cz, Pz, F3, F4, C3, C4, P3, P4, PO3, PO4, T3, T4, T5, T6, O1, and O2), as well as the nonstandard sites OL (halfway between O1 and T5) and OR (halfway between O2 and T6). The right mastoid electrode served as the online reference, and the signals were rereferenced offline to the average of the left and the right mastoids (Nunez, 1981). The electrooculogram (EOG) was recorded by placing electrodes 1 cm lateral to the external canthi to measure horizontal eye movements and by placing an electrode above and beneath the left eye to measure vertical eye movements and blinks.
The EEG and EOG were amplified by an SA Instrumentation amplifier with a gain of 20,000 and a band-pass of 0.01–100 Hz. The amplified signals were digitized at 250 Hz by a PC-compatible computer and were averaged offline. Trials accompanied by artifacts such as ocular, myogenic, and signal saturation (9.8 % in the cued switching and 15.4 % in the voluntary switching) and incorrect trials (see details in the Result section) were excluded from the averages. The ERP waveforms were time-locked to the onset of the cue stimuli in the cued condition and to the warning stimuli in the voluntary condition and were baseline corrected to the interval −200–0 ms before cue onset. Waveforms were low-pass filtered (two-way least-squares finite impulse response filter with 0 and 35 Hz for low and high ends of the frequency band, respectively) for presentation in the figures only. The analyses were performed on the unfiltered mean voltages.
The CNV and P3 were measured as the mean amplitude of the voltage across midline frontal, central, and parietal electrode sites. On the basis of previous CNV and P3 experiments, we focused on the period 400–800 ms after the onset of the cue (or warning stimulus), before the target appeared (Brunia et al., 2012; Polich, 2012). Analyses of variance (ANOVAs) were used for all statistical tests, and p-values were adjusted using the Greenhouse–Geisser epsilon correction for nonsphericity (Jennings & Wood, 1976).
Several aspects of our analyses deserve comment to orient the reader. First, our analyses of the behavior included the factor of CTI (300- vs. 800-ms CTI). However, due to the overlap of the ERP components elicited by the cues and the targets at the 300-ms CTIs, our ERP analyses focused on the 800-ms CTI trials. Second, in the voluntary task-switching condition, the color of the cue presented prior to the targets significantly influenced neither the ERPs nor behavior (as noted below). As a result, our analyses of the voluntary and cued task-switching conditions focused on the trial types of task alternation versus task repetition, since these were common to both conditions. In voluntary task switching, these were trials on which participants chose to switch versus repeat tasks, respectively. In cued task switching, task alternation involves cue switches and task switches, task repetition involves cue switches and task repetitions, and cue repetition involves cue repetitions and task repetitions. Task switch costs, unconfounded with cue repetition effects, were assessed by comparing task alternations with task repetitions (Logan & Bundesen, 2003). Subsequently, we used the three trial types afforded by the use of multiple cues for each task to test hypotheses about the cognitive processes active during cued task switching (cue repetition, task repetition, and task alternation). Third, we focused on the midline electrodes (Fz, Cz, and Pz), since the P3 and the CNV are both maximal along the midline, but we performed subsequent analyses to confirm that our data showed this distribution by including electrodes from the left and right hemispheres across the scalp (F3/F4, C3/C4, P3/P4, PO3/PO4, O1/O2, OL/OR, T3/T4, and T5/T6).
Results
Behavior
The behavioral results are shown in Table 1. We found task-switching costs in RT and error rate that decreased as CTI increased in both procedures, replicating previous research. We analyzed the two procedures separately. In cued task switching, we performed 3 (trial type: cue repetition, task repetition, task alternation) × 2 (CTI: 300-vs. 800-ms CTI) ANOVAs on the RTs and error rates. In the RT data, there were significant main effects of trial type, F(2, 38) = 58.02, p < .001, and CTI, F(1, 19) = 44.83, p < .001, and a significant interaction between them, F(2, 38) = 22.16, p < .001. Cue repetitions were faster than task repetitions, task repetitions were faster than task alternations, and both effects decreased as CTI increased, replicating previous research. In the error data, the main effects of trial type, F(2, 38) = 8.81, p < .001, and CTI, F(1, 19) = 27.15, p < .001, were significant, but the interaction between them was not significant, F(2, 38) = 1.882, p = .17.
In voluntary task switching, we performed 2 (trial type: task repetition vs. task alternation) × 2 (CTI: 300- vs. 800-ms CTI) ANOVAs on the RTs and error rates. In the RT data, there were significant main effects of trial type, F(1, 19) = 20.49, p < .001, and CTI, F(1, 19) = 22.64, p < .001, and a significant interaction between them, F(1, 19) = 6.41, p < .05. In the error data, there were main effects of trial type, F(1, 19) = 7.05, p < .05, and CTI, F(1, 19) = 18.55, p < .001, but the interaction was not significant, F(1, 19) = 0.816, p = .39.
In the voluntary task-switching condition, participants’ choice of which task to perform was not affected by the noninformative cue. We showed this in two ways. First, participants performed the magnitude task as often as the parity task following each cue color [the percentages of times participants performed the magnitude task were 50.5 % for the red, 50.5 % for the green, 49.7 % for the cyan, and 48.9 % for the purple; F(3, 19) = 1.05, p = .38]. Second, participants’ task switching was not affected by cue changes: Task switches occurred on 39.9 % of the trials when the cue changed and 40.7 % of the trials when the cue repeated, t(19) = 0.80, p = .44. These two analyses confirmed that the participants did not use cue colors to decide which task to perform (Arrington & Logan, 2005). Note that in the voluntary condition, there was a weak tendency to repeat tasks, as opposed to switch tasks, on every trial, consistent with previous experiments showing that participants perseverate on the last task when short CTIs are used (Arrington & Logan, 2005).
In sum, these behavioral findings are consistent with the existing task-switching literature in showing task switch costs that decrease with CTI in both voluntary and cued task-switching conditions. Next, we ask whether these behavioral costs were associated with electrophysiological indices of preparatory reconfiguration or bottom-up memory processes.
ERPs
We found opposite patterns of ERPs in the two task-switching paradigms. In voluntary task switching, task alternations were preceded by a larger CNV (Fig. 2c), whereas in cued task switching, task alternations were preceded by a larger P3 (Fig. 2d). We first performed a 2 (condition: voluntary vs. cued) × 2 (trial type: task alternation vs. task repetition) × 3 (channel: Fz, Cz, Pz) ANOVA using a measurement window of 400–800 ms following cue onset. We found a significant interaction between condition and trial type, F(1, 19) = 28.57, p < .001, confirming our observation of qualitatively different patterns of ERPs.
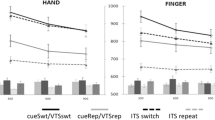
Event-related potential (ERP) findings. a, b Topographical distributions of the voluntary (panel a) and the cued (panel b) task-switching conditions. These plot the voltage difference between the task alternation and the task (cue) repetition trials within the measurement window (400–800 ms) following the warning (cue) onset. c, d ERP waveforms from the voluntary (panel c) and the cued (panel d) task-switching conditions for the three midline electrodes (Fz, Cz, and Pz). These ERP waveforms were obtained from the 800-ms CTI trials. ERPs are time locked to the onset of the warning (cue) stimulus, meaning that time zero represents its onset. The black lines show the average from the task alternation trials, gray lines show the average from the cue switch trials, and gray dotted lines the average from the repetition trials. Other details are identical to those in a. e, f Lateralized-readiness potential (LRP) waveforms from the voluntary (panel e) and the cued (panel f) task-switching conditions. These waveforms are calculated by subtracting the waveforms ipsilateral to the response hand from those contralateral to the response hand at electrodes C3/4. Other details are identical to those in c, d
We then performed a 2 (trial type: task alternation vs. task repetition) × 3 (channel: Fz, Cz, Pz) ANOVA to determine the nature of the CNV effects in voluntary task switching. We found significant main effects of trial type, F(1, 19) = 12.38, p < .01, and channel, F(2, 38) = 4.12, p < .05. The topographical distribution of the amplitude difference between alternation and repetition trials (Fig. 2a) shows that this CNV effect was maximal at the centroparietal electrodes, driving the main effect of channel. In a follow-up analysis to examine this distribution in greater detail, we ran an 8 (channel: F3/F4, C3/C4, P3/P4, PO3/PO4, O1/O2, OL/OR, T3/T4, and T5/T6) × 2 (left vs. right hemisphere) ANOVA. This analysis showed that the CNV effect was not lateralized, since there was no effect of hemisphere, F(1, 19) = 0.72, p > .41.
Next, we examined the cued task-switching ERPs in greater detail. We performed a 3 (trial type: cue repetition, task repetition, task alternation) × 3 (channel: Fz, Cz, Pz) ANOVA on the ERPs. We found significant main effects of trial type, F(2, 38) = 14.66, p < .001, and channel, F(2, 38) = 9.69, p < .001, and a significant interaction between them, F(4, 76) = 10.01, p < .001. In particular, task alternation trials elicited the P3, a more positive potential that was maximal at the parietal electrodes (Fig. 2b), driving the interaction between the trial type and the channel. There was no effect of hemisphere (left vs. right) in a separate 8 (channel: F3/F4, C3/C4, P3/P4, PO3/PO4, O1/O2, OL/OR, T3/T4, and T5/T6) × 2 (left vs. right hemisphere) ANOVA, F(1, 19) = 0, p > .5, indicating that the ERP effects had medial maxima and were not lateralized.
To determine the memory demands required to process the cues without task switching, we analyzed the P3 component in a 2 (trial type: cue repetition vs. task repetition) × 3 (channel: Fz, Cz, Pz) ANOVA. We found significant main effects of trial type, F(1, 19) = 16.04, p < .001, channel, F(2, 38) = 5.39, p < .01, and their interaction, F(2, 38) = 10.18, p < .001. In contrast, when we assessed the memory demands required to switch tasks while cues changed by analyzing the P3 component in a 2 (trial type: task repetition vs. task alternation) × 3 (channel: Fz, Cz, Pz) ANOVA, the main effect of trial type, F(1, 19) = 2.78, p = .11, was not significant, while the main effect of channel, F(2, 38) = 14.21, p < .001, and the interaction between trial type and channel, F(2, 38) = 3.61, p < .05, were significant. These findings indicate that processing the cues, even without the need to switch tasks, exerted similar memory demands as measured by the amplitude of the P3 component. Jost et al. (2008) observed similar effects using four cues, reinforcing the present finding that cued task switching is largely driven by memory processes associated with the cue processing, such as retrieval of information from long-term memory (Rugg & Curran, 2007) and updating of working memory (Donchin & Coles, 1988).
Finally, we analyzed the lateralized readiness potential (LRP) to determine whether the CNV effects we found during voluntary task switching reflected longer preparation of motor responses. The LRP measures response preparation and is characterized by an increasing negativity at contralateral electrodes. The LRPs were calculated by subtracting the waveforms ipsilateral to the response hand from those contralateral to the response hand at electrodes C3/4 prior to a finger movement. The parity and magnitude tasks required different hands in both task-switching procedures, so LRPs could reveal whether participants spent more time preparing responses in voluntary task switching. Figure 2e, f show that the LRP was increasingly negative on switch trials and increasingly positive on repetition trials following the color cue in both voluntary and cued task switches. A 2 (condition: voluntary vs. cued) × 2 (trial type: task switch vs. task repetition) yielded a significant main effect of trial type, F(1, 19) = 26.75, p < .001, but neither the main effect of condition, F(1, 19) = 1.109, p = .31, nor the interaction, F(1, 19) = .288, p > .5, was significant. We also performed a separate, one-way ANOVA with two levels of the trial type (cue repetition vs. task repetition), which showed that the LRPs were similar when participants repeated the same task regardless of whether there were cue switches or not, F(1, 19) = 1.50, p = .24. On the other hand, the LRPs on task repetition and task alternation trials were significantly different, F(1, 19) = 31.48, p < .001, when we performed a one-way ANOVA with two levels of the trial type (task repetition vs. task alternation). Our findings are consistent with previous studies of the LRP during task switching, which found that the LRP was increasingly negative before task switches but increasingly positive before task repetition responses in both voluntary (Vandamme et al., 2010) and cued (Galdwin et al., 2006) task-switching paradigms. We found the same pattern within participants in this study. These findings demonstrate that the CNV effects we observed cannot be explained by differences in motor preparation indexed by the LRP, because the LRP exhibited the same pattern of effects in the two task-switching procedures.
Discussion
Our behavioral findings replicated standard effects in cued and voluntary task switching: Switch costs decreased with CTI. Our ERP findings showed a robust modulation of the CNV component immediately prior to a voluntary task switch, suggesting that preparatory reconfiguration occurred prior to target presentation. In contrast, the same participants showed a larger P3 immediately prior to a cued task switch, suggesting that memory processing occurred prior to target presentation. The larger P3 may reflect storing the cue in working memory to combine it with the target (Logan, 2004; Logan & Bundesen, 2003). The modulation of the CNV prior to voluntary task switches and the modulation of the P3 prior to cued task switches are robust: We observed this same pattern in a between-subjects design excluded from this report for brevity. Despite the compelling dissociation we observed between voluntary and cued task switches in our participants’ ERPs, we acknowledge that the different processes that elicit the CNV or the P3 can occur simultaneously. Our cued task-switching paradigm with two cues per task and the voluntary task-switching paradigm with identical stimuli showed opposite polarity P3 and CNV effects, with no clear evidence that the same ERP components are elicited prior to task switches regardless of paradigm. Future research may derive better paradigms to reveal such overlapping activity than we did here.
Conclusions
Our ERP results suggest that top-down reconfiguration occurs prior to voluntary task switches, as indexed by a larger CNV. Despite the use of identical stimuli and tasks, we found no such CNV modulation during cued task switching. Instead, the results suggested that bottom-up memory retrieval occurs prior to cued task switches, as indexed by a larger P3. Together, these results converge on the interpretation of behavioral effects in voluntary and cued task switching, which attribute voluntary task switches to top-down reconfiguration (Arrington & Logan 2004a, 2005) and cued task switches to bottom-up memory (Allport & Wylie, 2000; Arrington & Logan, 2004b; Logan & Bundesen, 2003).
Our findings address a fundamental question in the literature on executive control: Are task switch costs due to active reconfiguration or bottom-up memory processes? Our findings show that both proposals may be valid, but in different task-switching paradigms. Voluntary task switching appears to rely on active reconfiguration (e.g., Arrington & Logan, 2004a, 2005), whereas cued task switching appears to rely more heavily on memory retrieval (Allport & Wylie, 2000; Arrington & Logan, 2004b; Logan & Bundesen, 2003).
Notes
When we refer to the P3 component here, we are referring to what is known as the P3b in the ERP literature. The P3b is a component with a parietal maximum and exhibits the largest amplitude when elicited by members of a low-probability category of task-relevant stimuli. This is distinguished from the P3a, which exhibits a large amplitude for any low-probability stimulus, even one that is task irrelevant (for a comprehensive discussion of the P3, see Polich, 2012).
References
Allport, A., Styles, E. A., & Hsieh, S. (1994). Shifting intentional set: Exploring the dynamic control of tasks. In C. Umiltà & M. Moscovitch (Eds.), Conscious and nonconscious information processing (pp. 421–452). Cambridge, MA: MIT Press.
Allport, A., & Wylie, G. (2000). Task switching, stimulus–response bindings, and negative priming. In S. Monsell & J. Driver (Eds.), Control of cognitive processes (pp. 35–70). Cambridge, MA: MIT Press.
Arrington, C. M., & Logan, G. D. (2004a). The cost of a voluntary task switch. Psychological Science, 15(9), 610–615.
Arrington, C. M., & Logan, G. D. (2004b). Episodic and semantic components of the compound-stimulus strategy in the explicit task-cuing procedure. Memory and Cognition, 32(6), 965–978.
Arrington, C. M., & Logan, G. D. (2005). Voluntary task switching: Chasing the elusive homunculus. Journal of Experimental Psychology: Learning Memory & Cognition, 31(4), 683–702.
Barceló, F., Periáñez, J. A., & Nyhus, E. (2008). An information theoretical approach to task-switching: Evidence from cognitive brain potentials in humans. Frontiers in Human Neuroscience, 1(13), 1–14.
Bocker, K. B., Brunia, C. H., & Cluitmans, P. J. (1994). A spatio-temporal dipole model of the readiness potential in humans. I. Finger movement. Electroencephalography and Clinical Neurophysiology, 91(4), 275–285.
Brunia, C. H. M. (2003). CNV and SPN: Indices of anticipatory behavior. In M. Jahanshahi & M. Hallett (Eds.), The bereitschaftspotential: Movement-related cortical potentials (pp. 207–227). New York, NY: Kluwer Academic/Plenum Publishers.
Brunia, C. H. M., van Boxtel, G. J. M., & Böcker, K. B. E. (2012). Negative slow waves as indices of anticipation: The bereitshaftspotential, the contingent negative variation, and the stimulus preceding negativity. In S. J. Luck & E. Kappenman (Eds.), Oxford handbook of event-related potential components (pp. 189–208). New York: Oxford University Press.
Donchin, E. (1981). Presidential address, 1980. Surprise!…Surprise? Psychophysiology, 18(5), 493–513.
Donchin, E., & Coles, M. G. H. (1988). Is the P300 component a manifestation of context updating. Behavioral & Brain Sciences, 11(3), 357–374.
Gladwin, T. E., Lindsen, J. P., & de Jong, R. (2006). Pre-stimulus EEG effects related to response speed, task switching and upcoming response hand. Biological Psychology, 72(1), 15–34.
Ikeda, A., Shibasaki, H., Kaji, R., Terada, K., Nagamine, T., Honda, M., & Kimura, J. (1997). Dissociation between contingent negative variation (CNV) and Bereitschaftspotential (BP) in patients with parkinsonism. Electroencephalography and Clinical Neurophysiology, 102(2), 142–151.
Jennings, J. R., & Wood, C. C. (1976). Letter: The epsilon-adjustment procedure for repeated-measures analyses of variance. Psychophysiology, 13(3), 277–278.
Johnson, R., Pfefferbaum, A., & Kopell, B. S. (2007). P300 and long-term memory: Latency predicts recognition performance. Psychophysiology, 22(5), 497–507.
Jost, K., Mayr, U., & Rosler, F. (2008). Is task switching nothing but cue priming? Evidence from ERPs. Cognitive, Affective, & Behavioral Neuroscience, 8(1), 74–84.
Karayanidis, F., Jamadar, S., Ruge, H., Phillips, N., Heathcote, A., & Forstmann, B. U. (2010). Advance preparation in task-switching: Converging evidence from behavioral, brain activation, and model-based approaches. Frontiers in Psychology, 1(25), 1–13.
Kiesel, A., Steinhauser, M., Wendt, M., Falkenstein, M., Jost, K., Philipp, A. M., & Koch, I. (2010). Control and interference in task switching–a review. Psychological Bulletin, 136(5), 849–874.
Lang, W., Lang, M., Kornhuber, A., Deecke, L., & Kornhuber, H. H. (1984). Brain potentials related to voluntary hand tracking: Motivation and attention. Human Neurobiology, 3, 235–240.
Leuthold, H., Sommer, W., & Ulrich, R. (1996). Partial advance information and response preparation: Inferences from the lateralized readiness potential. Journal of Experimental Psychology. General, 125(3), 307–323.
Leuthold, H., Sommer, W., & Ulrich, R. (2004). Preparing for action: Inferences from CNV and LRP. Journal of Psychophysiology, 18(2–3), 77–88.
Logan, G. D. (2004). Working memory, task switching, and executive control in the task span procedure. Journal of Experimental Psychology-General, 133(2), 218–236.
Logan, G. D., & Bundesen, C. (2003). Clever homunculus: Is there an endogenous act of control in the explicit task-cuing procedure? Journal of Experimental Psychology. Human Perception and Performance, 29, 575–599.
Logan, G. D., & Gordon, R. D. (2001). Executive control of visual attention in dual-task situations. Psychological Review, 108(2), 393–434.
Luck, S. J. (2005). An introduction to the event-related potential tech-nique. Cambridge, MA: MIT Press.
Mayr, U., & Kliegl, R. (2000). Task-set switching and long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory & Cognition, 26(5), 1124–1140.
Mayr, U., & Kliegl, R. (2003). Differential effects of cue changes and task changes on task-set selection costs. Journal of Experimental Psychology: Learning, Memory & Cognition, 29(3), 362–372.
Meiran, N. (1996). Reconfiguration of processing mode prior to task performance. Journal of Experimental Psycholog: Learning Memory and Cognition, 22(6), 1423–1442.
Nicholson, R., Karayanidis, F., Poboka, D., Heathcote, A., & Michie, P. T. (2005). Electrophysiological correlates of anticipatory task-switching processes. Psychophysiology, 42(5), 540–554.
Nunez, P. (1981). Electric fields of the brain. The neurophysics of EEG. New York, NY.: Oxford University PRess.
Polich, J. (2012). Neuropsychology of P300. In S. J. Luck & E. Kappenman (Eds.), Oxford handbook of event-related potential components (pp. 159–188). New York: Oxford University Press.
Rogers, R. D., & Monsell, S. (1995). Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology. General, 124(2), 207–231.
Ruchkin, D. S., Sutton, S., Mahaffey, D., & Glaser, J. (1986). Terminal CNV in the absence of motor response. Electroencephalography and Clinical Neurophysiology, 63, 445–463.
Rugg, M. D., & Curran, T. (2007). Event-related potentials and recognition memory. Trends in Cognitive Science, 11(6), 251–257.
Rugg, M. D., Mark, R. E., Walla, P., Schloerscheidt, A. M., Birch, C. S., & Allan, K. (1998). Dissociation of the neural correlates of implicit and explicit memory. Nature, 392(6676), 595–598.
Schneider, D. W., & Logan, G. D. (2005). Modeling task switching without switching tasks: A short-term priming account of explicitly cued performance. Journal of Experimental Psychology. General, 134, 343–367.
Schneider, D. W., & Logan, G. D. (2011). Task-switching performance with 1:1 and 2:1 cue-task mappings: Not so different after all. Journal of Experimental Psychology: Learning, Memory, and Cognition, 37, 405–415.
Sohn, M. H., & Anderson, J. R. (2001). Task preparation and task repetition: Two-component model of task switching. Journal of Experimental Psychology. General, 130(4), 764–778.
Vandamme, K., Szmalec, A., Liefooghe, B., & Vandierendonck, A. (2010). Are voluntary switches corrected repetitions? Psychophysiology, 47(6), 1176–1181.
Verleger, R., Wauschkuhn, B., van der Lubbe, R., Jaskowski, P., & Trillenberg, P. (2000). Posterior and anterior contribution of hand-movement preparation to late CNV. Journal of Psychophysiology, 14(2), 69–86.
Walter, W. G., Cooper, R., Aldridge, V. J., McCallum, W. C., & Winter, A. L. (1964). Contingent Negative Variation: An electric sign of sensori-motor association and expectancy in the human brain. Nature, 203, 380–384.
Woodman, G. F. (2010). A brief introduction to the use of event-related potentials in studies of perception and attention. Attention, Perception, & Psychophysics, 72(8), 2031–2046.
Acknowledgments
This research was supported by grants from the National Eye Institute (RO1-EY019882) and the National Science Foundation (BCS 09–57072 and BCS 09–57074).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kang, MS., DiRaddo, A., Logan, G.D. et al. Electrophysiological evidence for preparatory reconfiguration before voluntary task switches but not cued task switches. Psychon Bull Rev 21, 454–461 (2014). https://doi.org/10.3758/s13423-013-0499-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-013-0499-8