Abstract
We compared visual priming and comparison tasks to assess information processing of a stimulus during the first 2 s after its onset. In both tasks, a 13-ms prime was followed at varying SOAs by a 40-ms probe. In the priming task, observers identified the probe as rapidly and accurately as possible; in the comparison task, observers determined as rapidly and accurately as possible whether or not the probe and prime were identical. Priming effects attained a maximum at an SOA of 133 ms and then declined monotonically to zero by 700 ms, indicating reliance on relatively brief visuosensory (iconic) memory. In contrast, the comparison effects yielded a multiphasic function, showing a maximum at 0 ms followed by a minimum at 133 ms, followed in turn by a maximum at 240 ms and another minimum at 720 ms, and finally a third maximum at 1,200 ms before declining thereafter. The results indicate three stages of prime processing that we take to correspond to iconic visible persistence, iconic informational persistence, and visual working memory, with the first two used in the priming task and all three in the comparison task. These stages are related to stages presumed to underlie stimulus processing in other tasks, such as those giving rise to the attentional blink.
Similar content being viewed by others
Visual priming and comparison tasks are useful means of exploring poststimulus information processing (Breitmeyer, Ogmen, & Chen, 2004; Dehaene et al., 1998; Eimer & Schlaghecken, 2002; Farell, 1985; Posner & Mitchell, 1967). Conceptually, such processing has been compartmentalized into various stages—traditionally, in terms of various visual short-term memories (VSTMs). Early stages include registration of visual information in a high-capacity but brief visuosensory memory (Sperling, 1960), subsequently called iconic memory (Neisser, 1967). Later stages include readout of visual information from the icon and its encoding for subsequent processing in various posticonic STMs characterized by lower capacity but longer duration (Neisser, 1967; Sperling, 1963; Turvey, 1978). Depending on the experimental task, the posticonic stimulus information can be represented in an abstract visual format or recoded into a nonvisual—for example, phonological/verbal—format (Neisser, 1967; Sperling, 1960). Additionally, iconic memory can be parceled into visible persistence and nonvisible informational persistence (Coltheart, 1980).
In the present experiment, a brief stimulus, the prime, is followed at stimulus onset asynchronies (SOAs) varying from 0 to nearly 2,000 ms by another brief stimulus, the probe. In the priming task, by pressing one of two predesignated keys as rapidly and accurately as possible, the observers identified which of two probes was presented; similarly, in the comparison task, observers determined whether the probe matches or differs from the prime along a given feature dimension. The two tasks impose different decision criteria and different types of information processing. Following Vorberg, Mattler, Heinecke, Schmidt, and Schwarzbach (2003), we assume that priming relies on information accumulating in a sensory store or buffer akin to iconic memory. In contrast, the comparison task requires not only the early visuosensory stores, but also, especially at the longer prime–probe SOAs that well exceed the duration of the icon, the additional encoding of information into a posticonic format—for example, visual working memory (VWM).
To investigate such differences, we track the poststimulus processing of the prime by exploring how priming effects and comparison effects change with prime–probe SOA. In both tasks, the prime and probe features consist either of one of two colors, blue or green, or else of one of two forms, square or rhombus. The prime–probe pairing can be congruent (e.g., prime/probe color, blue; prime/probe form, rhombus) or else incongruent (e.g., prime/probe colors, green/blue; prime/probe forms, rhombus/square). A common finding in priming research is that the choice reaction time (RT) to an incongruent probe is significantly longer than the RT to a congruent probe. Here, the priming effect is the difference between the RT to an incongruent probe and the RT to a congruent probe. Similarly, past research on simple comparisons, such as those used in the present study, found that the correct “different” RTs to incongruent prime–probe pairings are longer than the correct “same” RTs to congruent pairings (Farell, 1985; Posner & Mitchell, 1967). Here, correspondingly, the comparison effect is the difference between “different” and “same” choice RTs.
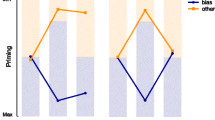
Although comparison effects have been obtained in prior studies, using SOAs ranging from 0 up to values of over 1,000 ms (e.g., Posner & Keele, 1967), priming studies have typically confined SOAs to within 200 ms (e.g., Mattler, 2003; Vorberg et al., 2003). One exception (Mattler, 2005), in which a longer range of SOAs was explored, reported priming effects at SOAs of about 800 ms. Whether they extend to longer SOAs remains an open question. Moreover, prior findings indicate that priming and comparison effects differ at short SOAs. Figure 1 is adapted from results reported by Vorberg et al.’s priming study and those reported by Decker’s (1974) comparison study. Note that comparison effects attain a maximum of 40 ms at a prime–probe SOA of 0 ms and decline to a minimum of nearly 0 ms at an SOA of 100 ms before increasing to values of 25–30 ms at SOAs of 150 and 200 ms. In contrast to the decrease of comparison effects, priming effects increase at a steady rate over SOAs ranging from 0 to 100 ms. Using an evidence accumulator model (for reviews of accumulator and related models, see Smith & Ratcliff, 2004), Vorberg et al. predicted and found that, at least up to SOAs of about 100 ms, priming effects increased linearly with SOA. Given that residual priming effects can be found in excess of 500 ms (Mattler, 2005), the model does not apply for such longer SOA ranges. By exploring both priming and comparison effects over a range of SOAs extending from 0 to roughly 2,000 ms, the present study allows us to investigate more fully how priming and comparison effects change with SOA. In view of the above-noted findings, we expect priming effects to attain a maximum somewhere between 100 and 500 ms and to decline thereafter. Moreover, given the assumptions that priming effects rely primarily on a visuosensory or iconic buffer and that comparison effects rely additionally on later posticonic stages of processing, we predict that there will be a significant interaction between the variations of task and of SOA.
Depictions of ΔRT (difference between RTs for congruent and incongruent prime–probe pairing) results from two previous studies using priming (Vorberg et al., 2003) and comparison (Decker, 1974) tasks. Priming effect increases linearly with SOA up to 100 ms, whereas comparison effects decline from 0 to 100 ms and then increase at later SOAs of 150 and 200 ms
Method
Apparatus and stimuli
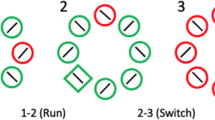
We followed methods previously detailed in Tapia, Breitmeyer, and Schooner (2010). Priming and comparison tasks were performed in a semi-lit room. The stimuli were displayed at a rate of 75 Hz on a Sony Trinitron, 1,024 × 768 color monitor. Stimulus presentation and response recording were controlled by a Macintosh II-ci computer. The viewing distance was roughly 60 cm. The prime and probe stimuli were desaturated green or blue (20 cd/m2) diamonds or squares (0.86° × 0.86°), presented on a gray (12.3 cd/m2) background. In one half of the trial blocks, the shape of the stimuli varied while their color was kept constant (form-response task). In the other half, the color of the stimuli varied while their shape was kept constant (color-response task) (see Fig. 2). Within each block, the trials were further divided so that on half of the trials, the prime and probe stimuli were congruent (e.g., diamond prime and diamond probe in the form-response task), and on the other half of the trials, the prime and probe stimuli were incongruent (e.g., green prime and blue probe in the color-response task). Figure 2 illustrates examples of congruent and incongruent target–probe pairs for form and color response tasks. The prime was centered 1° below fixation, and the probe was centered 1° above fixation.
Depiction of the sequence of presentation of prime and probe stimuli in priming and comparison tasks. A fixation cross appears at the center of the screen for 600 ms, followed by the 13-ms prime stimulus; the 40-ms probe appeared at variable SOAs ranging from 0 to 1,920 ms after the onset of the prime. Observers were given up to 5 s after probe presentation to respond. As is indicated, on form-response trials, the prime and probe maintained the same color but could vary in shape; on color-response trials, the prime and probe maintained the same shape but could vary in color. Incongruent prime and probe pairs are depicted in a form-response trial (left) and a color-response (right) trial
Participants
Thirty-one individuals from University of Houston, most of them undergraduate students, were recruited as experimental observers. Observers ran in both the comparison and priming tasks. Informed consent was obtained from all observers. All had normal or corrected-to-normal vision.
Procedure
The two tasks assessed the temporal dynamics of poststimulus processing for form and, separately, for color features. In both tasks, a prime was followed by a probe at one of ten SOAs: 0, 53, 133, 240, 480, 720, 960, 1,200, 1,400, or 1,920 ms. The durations of the prime and probe were 13 and 40 ms, respectively. While stimulus presentations in both tasks were identical, the instructions varied. In the priming task, observers were instructed to report, as quickly and accurately as possible, the shape of the probe during the form trials or the color of the probe during the color trials. Similarly, in the comparison task, observers were instructed to report whether the shapes (during the form-response task) or the colors (during the color-response task) of the probe and prime were the same or different. Separate trial blocks were assigned to each of the form- and color-response tasks. Additionally, within each of the feature-response blocks, SOA also was varied across separate blocks of trials. Each SOA block consisted of 64 trials, with 32 trials devoted to each of the congruent and incongruent prime–probe pairings. The orders of stimulus feature and SOA within a feature block were counterbalanced across observers.
Results
Twelve participants did not finish the experiment. Statistical analyses were performed on the results of the 19 observers who completed the experiment. A 2 (task: priming, comparison) × 2 (feature: form, color) × 10 (SOA) repeated measures mixed ANOVA was performed on ΔRTs (difference between RTs for congruent and incongruent prime–probe pairing). The significant main effect of task, F(1, 18) = 7.250, p = .015, ŋ2 p = . 287, reflects overall smaller ΔRTs in the priming (13.98 ms) than in the comparison (26.93 ms) task. The overall smaller ΔRTs in the priming task are attributable to the near-zero and, in some cases, slightly negative priming effects at the 0-ms SOA and at SOAs ≥500 ms (see overall priming effects in Fig. 3), whereas the ΔRTs in the comparison task were greater than zero at all SOAs. Moreover, the expected main effect of SOA, F(9, 162) = 7.696, p < .001, ŋ2 p = . 300, was also significant, reflecting the overall decline of ΔRTs in both tasks as SOAs increase (see black solid lines in Fig. 3). A significant interaction between task and SOA was also observed, F(9, 162) = 5.262, p < .001, ŋ2 p = . 226, due to (1) the opposing trends of priming and comparison effects at the lower SOAs (replicating the results of the prior studies shown in Fig. 1), followed by (2) a decline in priming effect at SOAs beyond 133 ms, while comparison ΔRTs remained above zero. All other, two-way and three-way, interactions were nonsignificant.
Discussion
We used simple priming and comparison tasks to investigate poststimulus processing of a prime followed at varying SOAs by a probe. The reason for using, in particular, comparisons along simple stimulus dimensions such as color or shape is that they tend to show a clear “same” RT advantage (Farell, 1985) and, thus, clear comparison effects. Our findings of priming and comparison effects go beyond related findings reported in prior studies, allowing for several interpretations that can serve as fruitful ideas for further research. One clear and expected upshot of our findings is that, similar to the results reported by Mattler (2005), our priming effects extend over prime–probe SOAs ranging up to about 700 ms, whereas comparison effects extend up to nearly 2,000 ms. These results confirm Vorberg et al.’s (2003) proposal that priming effects rely primarily on a brief visuosensory buffer and the hypothesis that comparison effects rely additionally on a more durable VWM. Moreover, consistent with the results of prior studies, the results (see Figs. 3 and 4) show that whereas priming effects increase over SOAs ranging from 0 to 133 ms, replicating Vorberg et al.’s findings, comparison effects decrease over the same SOA range, replicating Decker’s (1974) findings.
As has been noted, Vorberg et al. (2003) proposed that priming effects like those reported here rely on information accumulated and stored in a visuosensory buffer. Over SOAs ranging from 0 to 100+ ms, the accumulator model predicts that priming effects increase linearly as SOA increases. However, such increase of priming effects cannot continue indefinitely, since Mattler’s (2005) and the present study found only residual priming effects at SOAs exceeding 500 ms. Hence, it is reasonably expected that priming effects attain a maximum at some SOA between 0 and 700 ms—in our case, an SOA of 133 ms. Assuming that the priming effect is directly proportional to the strength of prime evidence, our results show that the evidence accumulates to a maximum over a limited time interval and thereafter decays. Sensori-motor priming effects appear to reflect the dynamics of the vision-for-action system (Milner & Goodale, 2008), which uses visual information over intervals of, at most, several hundred milliseconds.
They additionally indicate that priming effects can be used to track the dynamics of poststimulus information processing in the iconic buffer. Since the interactions between SOA and feature were nonsignificant for both tasks, Fig. 4 shows only the overall priming (and comparison) effects averaged across color and form dimensions. We take the increasing priming effects over SOAs ranging up to 133 ms to indicate evidence accumulation in the initial visible-persistence phase of the icon. According to Di Lollo (1977), the visible icon, rather than merely reflecting persistence after stimulus offset, reflects stimulus processing over an interval, lasting 100–200 ms, that begins with the onset of the stimulus (Di Lollo, 1977). We agree with this view and furthermore claim that visibly available stimulus information accumulates over this brief interval, before it begins to decay. In our study, the accumulation lasts roughly 133 ms, a value near the limit of visible iconic persistence (Di Lollo, 1980). Thereafter, up to about 700 ms, priming effects are governed by the evidence in a nonvisible, more abstract iconic informational persistence.
In contrast to priming effects, the overall comparison effects shown in Fig. 4 reveal an SOA-dependent variation that is more complicated. Comparison effects show a maximum at the 0-ms SOA, a minimum at the 133-ms SOA, followed by another maximum at the 240-ms SOA and a minimum at a 700-ms SOA, in turn followed by a third maximum at a 1,200-ms SOA before a gradual decline at still larger SOAs. Again, here, we take (1) the decline of the comparison effects over the SOAs ranging from 0 to 133 ms and (2) the increase and subsequent decline of the comparison effect over the SOAs ranging from 133 to 700 msFootnote 1 as indicators of two processing stages akin but not necessarily identical to those found for priming effects. A possible reason for their nonidentity is that they rely on task-dependent differences, not only between the prime’s poststimulus processing, but also between rules that map the prime’s information onto decision processes. As has been noted, the decisions required of observers in the two tasks clearly differ. In the priming task, observers are required simply to identify the probe without need to actively encode and store information about the prime. In contrast, the comparison task requires active encoding and maintenance of the prime’s information over longer durations; consequently, instead of being lost, prime information can be accessed at longer prime–probe SOAs. Besides these task-dependent differences between poststimulus processing of the prime, task-dependent differences between (1) encodings of visual choices and (2) processes of response selection can contribute to the differences between the dynamics of priming and comparison effects. Recent fMRI findings indeed indicate (1) that visual processing and choices based on that processing are dissociable (White, Mumford, & Poldrack, 2012) and (2) that, in turn, the encoding of visual choices can proceed independently from response programming (Hebart, Donner, & Haynes, 2012).
An adequate theoretical explanation of comparison effects would be more complicated than the corresponding explanation of our priming effects. Beginning with Vorberg et al.’s (2003) model, one could limit evidence accumulation to a short SOA range and add a process of evidence decay at longer SOAs. Such an extended model of evidence accumulation and decay could account for priming effects like the current ones. Moreover, priming effects are known to depend on allocation of feature-based and space-based attention (Tapia, Breitmeyer, Jacob, & Broyles, in press; Tapia et al., 2010). Besides incorporating similar attentional components, explanations of the comparison effects would require additional processes, including those supporting (1) active maintenance of the encoded prime information in VWM and (2) comparison of the prime information available at any given SOA to the encoded probe information. Particularly in the SOA range extending over the first 100–200 ms, the active processing of prime and probe information could compete with each other for limited attentional resources. In this SOA range, one would expect temporal aspects of attention, such as its dwell time (Duncan, Ward, & Shapiro, 1994) and switching time (Logan, 2005) to play significant roles. These factors are important because observers may have adopted different task-dependent attentional strategies.Footnote 2 In the present study, trials were blocked by task and SOA, and the primes and probes always appeared in their respective predictable locations. These methodological constraints may be conducive to adopting SOA-dependent strategic control of maintenance of attention on both stimuli in the comparison task and to switching of attention between prime and probe in the priming task.
Relation to other paradigms
The fact that, in the comparison task, the prime information, besides registering in the sensory icon, must be actively encoded and stored in a more durable VSTM or VWM suggests that the comparison effects might also relate to the attentional blink (AB), in which active encoding of visual stimuli in VWM also plays a prime role (Chun & Potter, 1995; Nieuwenstein, Van der Burg, Theeuwes, Wyble, & Potter, 2009). On the basis of our tripartite parsing of poststimulus processing in the comparison task (see Fig. 4), we take the first phase, defined by prime–probe SOAs ranging from 0 to 133 ms, to define the duration of temporal integration within the visible icon. Moreover, we take this integration also to apply to the AB paradigm—in particular, to the lag-1 sparing commonly found in studies of the AB. This interpretation finds support in a recent study by Akyürek et al. (2012), showing that temporal integration underlies lag-1 sparing. The AB is typically maximal at lag-2 or lag-3, corresponding to SOAs ranging from 100 to 300 ms, and then declines progressively at larger lags. We suggest that the second phase, defined by prime–probe SOAs ranging from 133 to 700 ms, corresponds to the duration over which the AB is obtained. During this interval, the active encoding of the first target, T1, is accompanied by a suppression of processing resources (Wyble, Potter, Bowman, & Nieuwenstein, 2011) that would otherwise be available to actively encode the second target, T2. Finally, we take the third phase, defined by prime–probe SOAs ranging from 700 to 1,920 ms, to correspond to the interval during which the prime’s fully encoded information is consolidated and maintained in VWM. In relation to the AB paradigm, having been fully encoded, T1 no longer interferes with the active encoding of T2—hence, the absence of AB at the larger lags that correspond to these longer T1–T2 SOAs.
Priming effects like ours may find their counterpart in flanker compatibility effects obtained in the flanker paradigm introduced by Eriksen and Eriksen (1974). In that paradigm, the probe stimulus to which a discriminative response must be made is spatially flanked by stimuli that map either onto the same response as the probe or onto a different response. Akin to the priming effects obtained in the present study, response-compatible flankers produce shorter RTs than do response-incompatible flankers. Mattler (2003; see his Fig. 7) found that, averaged across his two flanker presentation conditions, flanker compatibility effects were approximately 45 ms at a 0-ms SOA, 70 ms at the 100-ms SOA, and 35 ms at the 400-ms SOA. This nonmonotonic relation of flanker incompatibility effects reflects the similar SOA-dependent trend observed in the present priming effects. At what SOA flanker compatibility effects vanish is yet to be determined. However, on the basis of Taylor’s (1977) related study of flanker compatibility effects, one would expect them, like our priming effects, to be small, if not absent, at SOAs exceeding 500 ms. Results based on studies of the well-known Simon and the Stroop phenomena (Eimer, Hommel, & Prinz, 1995; Hommel, 1997) suggest that similar temporal dynamics may apply also to stimulus–response compatibility effects.
Notes
The nonmonotonicity of comparison effects for SOAs ranging from 133 to 700 ms was replicated twice (Treviño, Jacob, & Breitmeyer, 2012; and unreported observations). Hence, we take it to be a reliable trend.
We thank Dirk Vorberg for suggesting the importance of task- and SOA-dependent attentional strategies.
References
Akyürek, E. G., Eshuis, S. A. H., Nieuwenstein, M. R., Saija, J. D., Başkent, D., & Hommel, B. (2012). Temporal target integration underlies performance at lag 1 in the attentional blink. Journal of Experimental Psychology. Human Perception and Performance, 38, 1448–1464.
Breitmeyer, B. G., Ogmen, H., & Chen, J. (2004). Unconscious priming by color and form: Different processes and levels. Consciousness and Cognition, 13, 138–157.
Chun, M. M., & Potter, M. C. (1995). A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology. Human Perception and Performance, 21, 109–127.
Coltheart, M. (1980). Iconic memory and visible persistence. Perception & Psychophysics, 27, 183–228.
Decker, L. R. (1974). The effect of method of presentation, set, and stimulus dimensions on “same” -“different” reaction times. Perception & Psychophysics, 16, 271–275.
Dehaene, S., Naccache, L., Le Clec’H, G., Koechlin, E., Mueller, M., Dehaene-Lambertz, G., ... Le Bihan, D. (1998). Imaging unconscious semantic priming. Nature, 395, 597–600.
Di Lollo, V. (1977). Temporal characteristics of iconic memory. Nature, 267, 241–243.
Di Lollo, V. (1980). Temporal integration in vision. Journal of Experimental Psychology. General, 109, 75–97.
Duncan, J., Ward, R., & Shapiro, K. (1994). Direct measurement of attentional dwell time in humans. Nature, 369, 313–315.
Eimer, M., Hommel, B., & Prinz, W. (1995). S-R compatibility and response selection. Acta Neuropsychologica, 90, 301–313.
Eimer, M., & Schlaghecken, F. (2002). Link between conscious awareness and response inhibition: Evidence from masked priming. Psychonomic Bulletin & Review, 9, 514–520.
Eriksen, B. A., & Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16, 143–149.
Farell, B. (1985). “Same”-“different” judgments: A review of current controversies in perceptual comparisons. Psychological Bulletin, 98, 419–456.
Hebart, M. N., Donner, T. H., & Haynes, J.-D. (2012). Human visual and parietal cortex encode visual choices independent of motor plans. NeuroImage, 63, 1393–1403.
Hommel, B. (1997). Interactions between stimulus-stimulus congruence and stimulus–response compatibility. Psychological Research, 59, 248–260.
Logan, G. D. (2005). The time it takes to switch attention. Psychonomic Bulletin & Review, 12, 647–653.
Mattler, U. (2003). Delayed flanker effects on lateralized readiness potentials. Experimental Brain Research, 151, 272–288.
Mattler, U. (2005). Inhibition and decay of motor and nonmotor priming. Perception & Psychophysics, 67, 285–300.
Milner, A. D., & Goodale, M. A. (2008). Two visual systems re-viewed. Neuropsychologia, 46, 774–785.
Neisser, U. (1967). Cognitive psychology. New York: Appleton-Century-Crofts.
Nieuwenstein, M., Van der Burg, E., Theeuwes, J., Wyble, B., & Potter, M. (2009). Temporal constraints on conscious vision: On the ubiquitous nature of the attentional blink. Journal of Vision, 9, 1–14.
Posner, M. I., & Keele, S. W. (1967). Decay of visual information from a single letter. Science, 158, 137–139.
Posner, M. I., & Mitchell, R. F. (1967). Chronometric analysis of classification. Psychological Review, 74, 392–409.
Smith, P. L., & Ratcliff, R. (2004). Psychology and neurobiology of simple decisions. Trends in Neurosciences, 27, 161–168.
Sperling, G. (1960). The information available in a brief visual presentation. Psychological Monographs, 74(whole No. 498), 1–29.
Sperling, G. (1963). A model for visual memory tasks. Human Factors, 5, 19–31.
Tapia, E., Breitmeyer, B. G., Jacob, J., & Broyles, E. C. (in press). Properties of spatial attention during conscious and nonconscious processing of visual features and objects. Journal of Experimental Psychology: Human Perception and Performance.
Tapia, E., Breitmeyer, B. G., & Schooner, C. R. (2010). Role of task-directed attention in nonconscious and conscious response priming by form and color. Journal of Experimental Psychology. Human Perception and Performance, 36, 74–87.
Taylor, D. A. (1977). Time course of context effects. Journal of Experimental Psychology. General, 106, 404–426.
Treviño, M., Jacob, J., & Breitmeyer, B. (2012). Assessment of object processing in visual short-term memories. Poster presented at the annual Object Perception, Attention and Memory (OPAM) conference. Minneapolis, MN, 15 November.
Turvey, M. (1978). Visual processing and short-term memory. In W. K. Estes (Ed.), Handbook of learning and cognitive processes. Vol. 5, Human information processing (pp. 91–142). Hillsdale, NJ: Erlbaum.
Vorberg, D., Mattler, U., Heinecke, A., Schmidt, T., & Schwarzbach, J. (2003). Different time courses for visual perception and action priming. Proceedings of the National Academy of Science U. S. A., 100, 6275–6280.
White, C. N., Mumford, J. A., & Poldrack, R. A. (2012). Perceptual criteria in the human brain. Journal of Neuroscience, 32, 16716–16724.
Wyble, B., Potter, M. C., Bowman, H., & Nieuwenstein, M. (2011). Attentional episodes in visual perception. Journal of Experimental Psychology. General, 140, 488–505.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacob, J., Breitmeyer, B.G. & Treviño, M. Tracking the first two seconds: three stages of visual information processing?. Psychon Bull Rev 20, 1114–1119 (2013). https://doi.org/10.3758/s13423-013-0482-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-013-0482-4