Abstract
In daily life, we often need to inhibit a certain behavior or thought; however, sometimes we need to remove inhibition (deinhibition). Numerous studies have examined inhibition control, but it is unclear how deinhibition functions. In Experiment 1, we adopted a modified stop-signal task in which participants were instructed to immediately stop the prepared response to a stimulus appended by an accidental signal. The results showed that when the preceding trial was a stop-signal trial and participants successfully inhibited the action to the stimulus, the reaction time (RT) for the repeated stimuli in the current trial was significantly longer than that of the switched stimuli, reflecting the cost of deinhibition. Deinhibition ability is correlated with inhibitory control and cognitive flexibility. In Experiment 2, we manipulated stimulus onset asynchrony (SOA) between presentation of the stimuli and the stopping signals to exclude the interference of the signal preparation effect on the deinhibition cost. These findings suggest that an individual’s deinhibition ability, as a previously ignored subcomponent of cognitive control, may play an important role in human adaptive behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive control, also known as executive function, enables us to focus on goal-directed behavior in the face of conflicting events or stimuli, allowing us to flexibly adapt to changing tasks (Y. Chen et al., 2019; Diamond, 2013; Friedman et al., 2006; Hsu & Jaeggi, 2013; Miyake et al., 2000) and help us to focus on and effectively complete the task at hand in a complex environment (Ferguson et al., 2021). It is widely accepted that cognitive control consists of three subcomponents: shifting between tasks or mental sets, updating and monitoring of working memory, and inhibition of prepotent responses or interference information (Hendricks & Buchanan, 2016; Miyake et al., 2000; Quigley et al., 2020). Miyake et al. (2000), who examined individual differences in cognitive control, demonstrated that the three subcomponents of cognitive control are separable but also work together to maintain the functioning of cognitive control. However, as emphasized by Miyake et al. (2000), the aftereffects of inhibition, such as backward inhibition (BI), were not included in their study.
In the BI experiment, participants switched between three different tasks (e.g., Task A, B, and C). When switching from Task A to Task B, the preceding Task A would be inhibited, and if the next trial was Task A, which was previously inhibited (A→B→A), the previous inhibition would need to be overcome, resulting in worse performance (Arbuthnott & Frank, 2000; Mayr & Keele, 2000). The difference in RT between CBA and ABA was defined as the BI effect. The size of the BI effect reflects the extent to which previous inhibition is overcome. In the current study, we focused on the process of overcoming residual inhibition caused in the preceding trial, which is related to the “inhibition” and “shifting” subcomponents of cognitive control.
Inhibitory control is the ability to inhibit a dominant automatic response when necessary (Aron, 2007; Miyake et al., 2000; Mostofsky & Simmonds, 2008; Verbruggen & Logan, 2009a). This is of great importance to human adaptive behavior and health (Cai et al., 2021; Sahib et al., 2020; Venables et al., 2018). People with higher inhibitory control have a higher ability to control emotions and have good interpersonal communication (Van den Bussche et al., 2020). Inhibitory control includes the inhibition of attention and response (or action). Attention inhibitory control is interference control at the perceptual level, allowing us to selectively focus on what we choose and inhibit attention to other stimuli (Barras & Kerzel, 2016; Folk et al., 1992; Folk & Remington, 2008; Lien et al., 2008; Lien et al., 2010). Response inhibition refers to the ability to inhibit inappropriate behaviors or responses that do not meet current needs. In the laboratory, stop-signal, Simon, and go/no-go tasks are often used in research on response inhibition (Brand et al., 2019; Peterson et al., 2002; Schmidt et al., 2020; Sylvester et al., 2003; Wegmann et al., 2020). For example, in the stop-signal task, go stimuli (e.g., a white colored letter ) are presented in the most of trials. However, in a few trials, when the participant is about to respond, a stop signal (e.g., a sound or a change in the stimulus color) appears, indicating that no response should be made. When a stop signal appears, participants must immediately inhibit the impending reaction (Band et al., 2003; Band & van Boxtel, 1999; Lee & Kang, 2020; Logan, 1994; Logan & Cowan, 1984; Verbruggen & Logan, 2008a).
In most tasks involving inhibitory control, such as go/no-go and stop-signal tasks, participants must shift flexibly between different types of trials (e.g., from a no-go trial to a go trial). Cognitive flexibility in shifting is a critical aspect of cognitive control (Norman & Shallice, 1986). Flexible shifting between multiple tasks enables individuals to free themselves from previous tasks and begin new tasks quickly and effectively (Grange & Houghton, 2014; Kiesel et al., 2010; Monsell, 2003; Tona et al., 2020; Vandierendonck et al., 2010). Higher flexibility beneficially affects various aspects of human behavior, such as speech and reading skills in childhood (Cartwright et al., 2017; Cartwright et al., 2019; Cartwright et al., 2020), resilience in coping with stressful events (Genet & Siemer, 2011), level of creativity (C. Q. Chen et al., 2014), and mental health in older adults (Davis et al., 2010). Researchers typically measure cognitive flexibility using the task-switching paradigm, in which participants are prompted to switch between two tasks (Monsell, 2003; Tona et al., 2020). In the process of task switching, switch costs indexed by longer RT and higher error rates for task-switching trials than for task-repeat trials can be observed (Philipp & Koch, 2005; von Bastian & Druey, 2017). In the present study, we used the task-switching paradigm and stop-signal task to examine the relationship between individuals’ cognitive flexibility and their ability to overcome inhibition.
Overcoming inhibition is also referred to as deinhibition (J. Chen et al., 2022). This term is borrowed from biophysical literature, where it refers to the removal of cholinesterase production, thereby activating acetylcholine and then allowing impulses to be transmitted across synapses (Emmelin & Muren, 1950). When an inhibitory process occurs in a trial or event of a given task, it may last for a long time and affect subsequent tasks, regardless of whether it is explicit response inhibition or implicit attentional inhibition (Schuch & Koch, 2003; Verbruggen, Liefooghe, Szmalec, & Vandierendonck, 2005a). Once the subsequent task requires the reactivation of information related to the previously inhibited task, the previous inhibition needs to be removed.
Several related studies indicate that deinhibition, such as BI, is conceptually related to, but different from, inhibition evaluated using stop signal tasks or Stroop tasks (Dreher & Berman, 2002; Miyake et al., 2000; Sdoia et al., 2020). First, as deinhibition is a cognitive process that follows inhibition, the two processes occur at entirely different points in time. Second, inhibition mainly entails inhibiting interference from irrelevant factors and avoiding inappropriate behaviors, whereas deinhibition entails removing or overcoming residual inhibition to better respond to the previously inhibited stimulus or event.
Some researchers have termed deinhibition sequential cognitive flexibility. However, substantial evidence suggests that the deinhibition process has neural underpinnings different from those of cognitive flexibility. For example, Whitmer and Banich (2012) divided participants into high and low groups based on the size of BI effect and analyzed their brain activation under task-switching conditions. The results showed that brain activation related to deinhibition differed from that related to cognitive flexibility. Similarly, some ERP studies have shown that cognitive flexibility in task switching is mainly reflected in a late positive wave (400–600 ms), whereas deinhibition is reflected in the early components such as N1/P1 (Giller et al., 2019; Giller et al., 2020; Giller & Beste, 2019).
Recently, a few studies addressed the deinhibition process using various BI paradigms (Dreher & Berman, 2002; Giller et al., 2019; Mayr & Keele, 2000; Scheil & Kleinsorge, 2019; Sdoia et al., 2020). For example, Scheil and Kleinsorge (2022) asked participants to determine the size, shape, and color of stimuli. These three tasks were denoted as A, B, and C. A stop signal was likely (50%) to occur 100 ms after the stimulus presentation in Task A, rather than in the other two tasks, B and C. Moreover, when trial N − 2 was Task A, the RT of the ABA sequence was significantly longer than that of CBA, regardless of whether participants responded to Task A at trial N − 2. This result suggests that the no-go signal not only triggers the inhibition of task-specific responses but also facilitates the inhibition of task sets (Regev & Meiran, 2016, 2017). Moreover, the probability of inhibiting the response to Task A results in the observation that the standard condition (i.e., the go tasks) yields either no costs or slight N − 2 repetition benefits (Scheil & Kleinsorge, 2022). The reversal of the N − 2 repetition cost is mainly attributable to the presence of no-go-related tasks in the base condition (e.g., ACB). Whether Task A is located in trial N − 2 or N − 1, it induces a global inhibition of response, which consequently affects the other two subsequent tasks, resulting in a slower response in these sequences and ultimately generating a slight reversal of the N − 2 repetition cost. Studies on motor imagery have also observed global inhibition (Bart et al., 2021). We assumed that both the effect of inhibiting a specific task and global inhibition in the existing studies can be explained in terms of deinhibition. In other words, when Task A in trials N − 2 or N − 1 is required to be actually or imaginatively inhibited, the subsequent reactivation of either Task A or other tasks (e.g., B and C) requires the previous inhibition to be released. The longer reaction time required to release this inhibition is the cost of deinhibition (Table 1).
Deinhibition always occurs in daily life. For instance, we are often asked to explicitly stop (inhibit) a certain behavior and then repeat the recently inhibited behavior. For example, when we drive and see a red light, we step on the brake and stop for a short time, and then we need to release the brake and move on when the red light turns green. In the workplace, we may inhibit a warm and friendly attitude toward a colleague because of certain unhappy events, but after a period of time, we may remove the inhibition and cease our hostility, showing friendly cooperation with them in some emergent situations. How can we examine the ability to overcome explicit inhibition in the laboratory and analyze individual differences in deinhibition? How is the deinhibition ability related to inhibitory control and cognitive flexibility?
To answer the above questions, we combined the classic stop-signal task with a stimulus-switching paradigm. It was expected that after successful inhibition of the prepared response to a specific stimulus (e.g., letter X) in trial N − 1, there would be a cost of deinhibition when responding to the same stimulus again in the current trial; that is, the RT and error rate of the response under the stimulus-repeat condition would be larger than under the stimulus-switch condition. As deinhibition is conceptually and functionally related to “inhibition,” we also predicted that participants’ deinhibition ability might be correlated to inhibitory control; that is, an individual with a stronger deinhibition ability might have a higher ability of inhibitory control. In addition, deinhibition has been suggested to reflect another type of cognitive flexibility (Giller et al., 2019; Giller et al., 2020; Giller & Beste, 2019; Wolff et al., 2018): the ability to flexibly implement new behaviors when participants need to respond to the stimuli that were previously inhibited. Therefore, we expected that deinhibition might also be correlated with cognitive flexibility in the switching task.
Experiment 1
We used a modified stop-signal task paradigm (i.e., a hybrid version of the SST and go/no-go tasks) to explore the deinhibition process. Additionally, a digit classification task was adopted to further explore the relationship between deinhibition ability and cognitive control (cognitive flexibility and inhibition).
Method
Participants
The required sample size was calculated using G*Power 3.1 (Faul et al., 2007). We applied regular statistical analyses and error probabilities (α = 0.01, power = 95%, f = 0.25). The sample size analyses led to a total sample size of 48 required for a repeated measure. A total of 83 college students (24 males, with an average age of 19.26 years) participated in this study. None of the participants were excluded from the data analysis. All participants had normal or corrected-to-normal vision, and none had color blindness or weakness in color vision. Each participant provided verbal and written consent to participate in this study. This study was approved by the Ethics Committee of Jiangxi Normal University.
Materials, task, and design
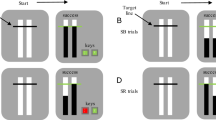
Stimuli were presented on a 17-inch monitor at a distance of 50 cm in front of the participants. Each participant completed two experimental tasks, including a stop-signal task (Fig. 1) and a digit classification task. The order of the two experimental tasks was balanced among the participants. After completing a task, the participants were allowed to rest for 3–5 minutes, and then the next task was performed. It took approximately 25 min to complete both tasks. In the stop-signal task, participants were required to judge whether to respond to the stimulus according to the color of the stimulus. Different button responses were made for different stimuli (“X,” “O”). In the digit classification task, participants needed to make a parity/magnitude judgment on the digits (0–9, except for 5) based on the color of the stimulus. The stimulus was presented at the center of the screen. Participants were instructed to press buttons (“F,” “J”) on the keyboard using the corresponding fingers. The presentation of the stimuli and response recordings (RT and Accuracy) were implemented using presentation software (E-Prime 2.0).
Experimental conditions and the procedure of the stop-signal task. When a stop signal (red color) appears, participants should inhibit the reaction, and this trial is an N − 1 inhibition trial; when the trial N − 1 is not a stop signal trial, it is defined as N − 1 noninhibition. When the stimulus in the current trial is different from that of trial N − 1, it is a switch trial (the proportion of switch trials is 50%). (Color figure online)
Stop-signal task
A modified stop-signal task was used, with a 2 (transition type: repeat vs. switch) × 2 (N − 1 inhibition: inhibition vs. noninhibition) experimental design. When trial N − 1 was not a stopping trial, trial N was a trial after noninhibition (Fig. 1, Table 2). When trial N − 1 was a stopping trial, participants were instructed to inhibit the reaction in that trial, and trial N was defined as a trial after inhibition. When the stimulus in trial N − 1 was different from that in trial N, it was defined as a switch trial (the proportion of switch trials was 50%); when the stimulus of trial N − 1 was the same as in trial N, it was a repeat trial.
On each trial, participants viewed a white fixation cross on a black background for 800 ms, at which point the fixation cross was replaced by “X” or “O.” An intertrial interval of 600 ms followed stimulus presentation. Participants were to respond by pressing “F” (if “X” appeared) or “J” (if “O” appeared) on the keyboard during the stimulus presentation. They were told to respond as quickly as possible, unless a stop signal occurred. Stop signals followed approximately one-third of the stimuli. The stop signal occurred when the stimulus (X/O) changed to a red color 200 ms after the stimulus onset, in which participants were instructed to withhold their response. Half of the participants used the left index finger to make a response to “X” by pressing “F” and right index finger to make a response to “O” by pressing “J”; the other half used the opposite stimulus–response (S–R) mapping rules. The total number of formal experimental trials for the stop-signal task was 138, the number of stop trials was 50, and the remaining trials totaled 22 trials per condition.
The tasks were divided into practice and experimental sessions. In the practice session, feedback was provided for each trial. When the accuracy rate reached 85% or more, the participants were allowed to enter the experimental session.
Digit classification task
Arabic digits (1–9, excluding 5) were presented in different colors (red/green). In each trial, a fixation point was first presented at the center of the screen for 800 ms, and then a digit was presented for 3,000 ms. Participants were required to perform different tasks according to the color of the stimulus. The trial order was arranged pseudo-randomly. The total number of trials for the digit classification task was 96, with switch trials accounting for 50%. For a given color, half of the participants were instructed to judge the magnitude of the number (compared with 5), and the other half were asked to judge the parity of the number. They were instructed to press “F” with the left index finger for the numbers smaller than 5, and press “J” with the right index finger for numbers larger than 5. If the cue was green, participants made a parity judgment by pressing “F” for odd numbers and pressing “J” for even numbers. The stimulus disappeared after pressing a key (or after 3,000 ms), and a random blank screen appeared for 500–800 ms before the next trial. Stimulus–response mappings were counterbalanced across participants. Each participant was allowed to practice before the formal experiment.
Data processing
RTs and accuracy were analyzed as dependent measures in the two experimental tasks. In the RT analysis, the trials with RTs higher/lower than three standard deviations of the average RT, the error response trials and the first following trials were not included. In the stop-signal task, behavior was evaluated by measuring RT and accuracy in go trials and the ratio of successful stopping for stop-signal trials. According to the horse-race model proposed by Logan and Cowan (1984), the delay in either reaction or inhibition causes the other party to reach the threshold first and dominate the behavior, and the incubation period of the inhibition reaction is the basis for measuring the efficiency of the inhibition process. The error stop rate (p = response| signal) and the RT of the go trial can be calculated according to the interval time (stop-signal delay, SSD) between the stimulus and the stop signal. We used the integration method to estimate stop-signal reaction time (SSRT; Verbruggen et al., 2019), so that the RTs in go correct trials in each session were rank ordered and a percentile of the distribution, corresponding to the percentage of failed inhibitions in that condition, was selected (estimated response time). SSRT was calculated by subtracting the corresponding mean SSD from the estimated RT, which was only computed if the assumptions of the racehorse model were met (i.e., mean go RT > mean unsuccessful stop RT; Verbruggen et al., 2019). We defined SSRT as the participant’s inhibitory control ability (Aron et al., 2003; Logan & Cowan, 1984).
When trial N − 1 is a stopping trial (i.e., a red X), the inhibitory effect may persist for a period of time and continue into trial N, which is similar to the carryover of lateral inhibition in task switching (Dreher & Berman, 2002; Giller et al., 2019; Giller & Beste, 2019). In this case, if the stimulus of the current trial is the same as that in the previous trial (repeat) and participants need to respond to it (i.e., a black X), the inhibition of the stimulus (i.e., X) in the previous trial should be removed first, and then a correct response must be made to this stimulus. In contrast, if the stimulus of the current trial is different from that of the previous trial, there is no process of deinhibition. Comparing the difference in reaction times between these two types of trials allows us to calculate a participant’s deinhibition cost. The lower the cost of deinhibition, the stronger the deinhibition ability.
Another way to measure the deinhibition ability of participants is to calculate the duration of the effect, that is, the number of trials with longer RT for repeat trials than for switch trials under the N − 1 inhibition condition. Assuming that trial N − 1 is a stop signal that requires inhibition, and if the deinhibition cost (i.e., longer RT for the repeat trial than for the switch trial under the N − 1 inhibition condition) is absent in trial N, then the time duration (trial length) of deinhibition is 0; if trial N has a deinhibition cost and trial N + 1 does not, then the trial length of deinhibition is 1; if trials N and N + 1 have a deinhibition cost and trial N + 2 does not, then the length is 2; if trials N, N + 1, and N + 2 have a deinhibition cost, but trial N + 3 does not, then the length is 3. When analyzing the number of trials for persistent inhibition, there were no stop trials between the current trial and inhibited trial being analyzed. Participants with a length of 0 were defined as individuals who had a fast deinhibition speed, whereas those with a length of 3 were individuals with a slow deinhibition speed.
In the digit classification task, the RTs of the error trial and the first following trial were excluded from the reaction time statistics. Moreover, trials with RTs that were higher/lower than the average RT by three standard deviations were also excluded. The difference in RT or accuracy between repeat and switch trials is defined as the switch cost, which reflects cognitive flexibility.
Results
Deinhibition cost in the stop-signal task
For the RTs, a 2 N − 1 inhibition (inhibition vs. noninhibition) × 2 transition type (repeat vs. switch) repeated-measures ANOVA revealed a main effect of N − 1 inhibition, F(1, 82) = 73.08, p < .001, ηp2 = .471, and a main effect of transition type, F(1, 82) = 14.14, p < .001, ηp2 = .147. The interaction between transition type and N − 1 inhibition was significant, F(1, 82) = 26.51, p < .001, ηp2 = .244. Further analysis showed that there was no significant difference between repeat trials and switch trials under the noninhibition condition (p = .23; see Fig. 2), while under the inhibition condition, the RTs were significantly longer in repeat trials than in switch trials (p < .001).
For accuracy, there was no significant main effect of transition type, F(1, 82) = .186, p = .668, or main effect of N − 1 inhibition, F(1, 82) = .265, p = .608. The interaction between transition type and N − 1 inhibition was significant, F(1, 82) = 12.926, p = .001, ηp2 = .141. Further analysis found that, under the noninhibition condition, there was a significant switching effect (p = .019), with lower accuracy for switch trials than for repeat trials. Under the inhibition condition, the difference in accuracy between the repeat and switch trials was marginally significant (p = .054), with lower accuracy for repeat trials than for switching trials (Fig. 2).
The above analysis shows that under the N − 1 inhibition condition, the RT of stimulus repetition was significantly longer, and the accuracy was marginally significantly lower than that of stimulus switching, indicating that when required to respond to a recently inhibited stimulus, participants exhibited a cost of overcoming residual inhibition (longer RT and more error). To further explore the temporal length of residual inhibition, paired-sample t tests were used to analyze the difference in RTs between repeat and switch trials in trial N + 1 or trial N + 2, when trials N − 1 were stop-signal trials. We observed a significant effect of transition type, t(73) = 4.303, p < .001, for trial N + 1, with slower responses for repeat trials (659.23 ms) than for switching trials (633.25 ms). Moreover, for trial N + 2, the RTs between the repeat (630.43 ms) and switch trials did not differ (631.35 ms), t(73) = .109, p = .913. We counted the number of trials in which a single participant had residual inhibition and found that after a stop signal trial, the average number of trials showing residual inhibition was 1.5 (±1.16).
Interestingly, we found that 21 participants did not show any effect of transition type in trial N when trial N − 1 was the inhibition condition. These participants seemed to successfully overcome the inhibition of trial N − 1 immediately before the presentation of the stimulus in trial N, indicating that these participants had a faster speed of deinhibition than others.
Performance on the digit classification task
The mean RT in the switching condition (1083.16 ± 17.58 ms) was significantly longer than in the repeat condition (939.75 ± 14.88 ms), t(82) = −13.948, p < .001, indicating that the switch process has a great time cost. The switch cost was also found in accuracy, with significantly lower accuracy in the switching condition (0.89 ± 0.01) than in the repeat condition (0.92 ± 0.01), t(82) = 4.433, p < .001, Table 3.
Correlations between deinhibition, inhibition, and flexibility
Similar to the definition of BI (Giller et al., 2019; Sinai et al., 2007; Zhang, Stock, Fischer, & Beste, 2016), we defined the cost of overcoming the residual inhibition caused in trial N − 1 as deinhibition ability. When N − 1 is a stop trial, the difference in RT between the repeat and switch stimuli is defined as the deinhibition cost. In addition, we used deinhibition speed as another indicator of deinhibition ability. The SSRT in the stop-signal task is defined as response inhibition ability (Logan & Cowan, 1984). The switch cost of RTs or accuracy in the digit classification task is defined as the flexibility in task switching (Cepeda et al., 2001; J. H Han et al., 2019; Rogers & Monsell, 1995; Zhuo et al., 2021a, b).
The results of the correlation analysis show that (Fig. 3) the deinhibition ability is moderately correlated with the ability to inhibit responses (r = .378, p = .001) and the flexibility indexed by the switch cost in accuracy (r = .256, p = .020). There was no significant correlation between deinhibition and switch cost in RTs (p = .250). We also calculated the correlation between the speed of deinhibition (i.e., the length of deinhibition time) and flexibility and inhibition control. The results showed that the deinhibition speed was marginally significantly correlated with the switch cost in accuracy (r = .207, p = .060), but it was not significantly correlated with response inhibition (r = .139, p = .211) or switch cost in RTs (r = .126, p = .258).
The absence of a correlation between deinhibition speed and response inhibition might be due to the nondiscrete data of deinhibition speed. To explore whether there was a significant difference in response inhibition or cognitive flexibility between the fast and slow deinhibition groups, we classified all the participants according to the number of trials in which each participant showed the deinhibition cost. Participants with a length of 0 were defined as individuals with a fast deinhibition speed (21 participants), whereas those with a length of 3 were defined as individuals with a slow deinhibition speed (24 participants). The results are shown in Fig. 4. In the stop-signal task, the successful stop rate was significantly higher in the fast group (0.97) than in the slower group (0.91), t(43) = 2.92, p = .003; the SSRT of the faster group (205.62) was significantly lower than that of the slower group (229.88), t(43) = −2.425, p = .011. In the digit classification task, the switch cost of accuracy in the faster group (−0.007) was also significantly lower than that in the slower group (0.029), t(43) = −2.66, p = .005 (Fig. 4). The difference in switch cost in RT was marginally significant between the groups, with a smaller switch cost for the faster group (123.26 ms) than for the slower group (161.79 ms), t(43) = −1.37, p = .089.
Discussion
The results of Experiment 1 indicate that when the participants were required to respond to the stimulus that had just been successfully inhibited in the preceding trial, there was a greater cost to overcome the previous inhibition, which is consistent with the BI effect (J. Chen et al., 2022; Dreher & Berman, 2002; Giller et al., 2019; Mayr & Keele, 2000; Scheil & Kleinsorge, 2019; Sdoia et al., 2020). In other words, if participants successfully exerted inhibition when the stop signal appeared, the inhibition effect lasted until the next trial or even longer. The average duration of the inhibition effect of all participants was 1.5 trials, which was approximately 4 seconds. This finding implies that in the hybrid version of the SST and go/no-go task, the effect of successful inhibition of a stop signal lasts approximately 4 s. Within this time period, if participants are required to respond to the stimulus that has been previously inhibited, they will show a higher error rate and a longer reaction time, which is called the deinhibition cost and is the same as the process of removing residual inhibition in BI studies (Dreher & Berman, 2002; Fales et al., 2006; Giller & Beste, 2019; Mayr & Keele, 2000; Picazio et al., 2020).
The finding that after a trial with response inhibition, the RT in the current trial was longer for the repeat than for the switch stimulus is also in line with the findings that the post-stop-signal slowing is greater when the primary-task stimulus is repeated than when it is switched (Anguera et al., 2013; Enticott et al., 2009; Verbruggen, Liefooghe, Szmalec, & Vandierendonck, 2005a; Verbruggen, Liefooghe, & Vandierendonck, 2005b; Verbruggen & Logan, 2008a). The episodic retrieval account was used to interpret these findings. In the stop signal trial, the task stimulus is associated with the “inhibition” tag. The “inhibition” tags formed for a specific stimulus will stay in the memory for a period, and the performance will be affected by re-reacting to the stimulus during this time. When a stimulus repeats, the stop goal associated with it in the preceding trial is retrieved, and the stop goal activates the stop process, thereby slowing the response to the repeated stimulus (Verbruggen & Logan, 2008a, 2008b). The episodic retrieval account seems to explain the deinhibition cost in our study, but it cannot explain the individual differences found. According to the episodic retrieval hypothesis, individuals with low deinhibition costs may have poor episodic memory, causing them to fail to remember (or retrieve) the response pattern corresponding to an inhibited stimulus when they see it again. Currently, no data are available to prove this inference.
It should be noted that we found significant switch costs in the digit classification task that tested participants’ cognitive flexibility, replicating the findings of previous studies (Allport et al., 1994; Baniqued et al., 2017; Braem et al., 2019; J. Han et al., 2018; J. H., Han et al., 2019; Jurado & Rosselli, 2007; Meiran et al., 2000; Monsell, 2003; Rogers & Monsell, 1995; Zhuo, Chen, et al., 2021a; Zhuo, Zhu, et al., 2021b); however, there was no significant RT difference between switch and repeat trials in the SST. The switching RT tended to be larger than the repetition RT, and the accuracy of the response repetition was significantly higher than that of response switching. Taken together, these findings indicate that participants in the SST demonstrated the cost of accuracy without the cost of RT when switching responses, which is possibly due to the response strategy participants adopted to respond as quickly as they could for both switch and repeat trials. Moreover, the pattern of RT results is in line with some studies that used visual stop signals (Anguera et al., 2013; Enticott et al., 2009).
In addition, our results revealed an interesting pattern that the RT in N − 1 noninhibition trials was longer than that in N − 1 inhibition trials. It is necessary to optimize the relative precision of empirical priors and sensory evidence using a perceptual hierarchical model from a brain-learning perspective (Friston, 2009; Kass & Steffey, 1989). This optimization is crucial for the inference. Mehta (2001) indicated that learning causal relationships between stimuli is a critical task for the nervous system to anticipate future events. Expectations are generated when the brain learns to inhibit a specific stimulus. Although the participants in this study were not informed that consecutive stop trials would not be presented in the experiment, they may have perceived this pattern spontaneously and adopted a cognitive strategy or formed an expectation. Participants may be aware that if trial N − 1 is a stop trial, then the following trial must be a go trial. In this case, participants may not have waited but responded quickly as soon as they saw the stimulus in trial N. Conversely, if trial N − 1 was a nonstop trial, a stop signal may have occurred in trial N (approximately 30%). Accordingly, participants may have considered waiting for a short time (approximately 200 ms) to see if the stop signal appeared before responding. This strategy may have resulted in significantly longer RTs in the N − 1 noninhibited condition than in the N − 1 inhibited condition. Additionally, several studies using the oddball paradigm have found that the frontal and occipital lobes are highly activated when unpredicted and rare stimuli are present (Brazdil et al., 2007; Hooi et al., 2018), which overlaps with brain activation during the deinhibition process (Dreher & Berman, 2002; Picazio et al., 2020; Sdoia et al., 2020).
Experiment 2
The results of Experiment 1 showed that when participants were asked to respond to stimuli that had been inhibited, there was indeed a deinhibition cost. Specifically, the accuracy decreased and the reaction time increased. Furthermore, deinhibition ability was significantly correlated with the typical components of cognitive control. These results suggest that deinhibition may be a previously overlooked component of cognitive control. However, in Experiment 1, the SOA before the stop signal appeared had a fixed duration (200 ms), which might have enabled the participants to prepare for the stop signal. In Experiment 2, we aimed to demonstrate that the deinhibition cost is still obtained when the stop signal is presented at variable SOA.
Method
Participants
The required sample size was calculated using G*Power 3.1 (Faul et al., 2007). We applied regular statistical analyses and error probabilities (α = 0.05, power = 80%, f = 0.25). The sample size analyses led to a total sample size of 24 required for repeated measurements. Thirty college students (10 males, with and an average age of 23.43 years) participated in this experiment. None of the participants were excluded from the data analysis. All participants had normal or corrected-to-normal vision, and none had color0blindness or weakness in color vision. Each participant provided verbal and written informed consent. This study was approved by the Ethics Committee of Jiangxi Normal University.
Materials, task, and design
The same materials used in Experiment 1 were used in Experiment 2. Stimuli were presented on a 17-inch monitor 50 cm in front of the participants. It took approximately 15 min to complete the stop-signal task. The procedure for Experiment 2 was similar to that of Experiment 1, except that the SOA changed from a fixed 200 ms to five different SOAs (i.e., 80, 160, 240, 320, or 400 ms) and each SOA occurred with equal probability (Verbruggen & Logan, 2008b).
Results
For the RTs (Fig. 5), a 2 N − 1 inhibition (inhibition vs. noninhibition) × 2 transition type (repeat vs. switch) repeated-measures ANOVA revealed a main effect of N − 1 inhibition, F(1, 29) = 47.96, p < .001, ηp2 = .623, and a main effect of transition type, F(1, 29) = 5.79, p = .023, ηp2 = .167. The interaction between transition type and N − 1 inhibition was significant, F(1, 29) = 33.95, p < .001, ηp2 = .539. Further analysis showed that there was no significant difference between repeat trials and switch trials under the noninhibition condition (p = .23), whereas under the inhibition condition, the RTs were significantly longer in repeat trials than in switch trials (p < .001).
For accuracy, there was a significant main effect of transition type, F(1, 29) = 7.90, p = .009, and no significant main effect of N − 1 inhibition, F(1, 29) = .465, p = .501. The interaction between transition type and N − 1 inhibition was marginally significant, F(1, 29) = 2.31, p = .139, ηp2 = .074.
To further explore the temporal length of residual inhibition, paired-sample t tests were used to analyze the difference in RTs between repeat and switch trials in trial N + 1 or trial N + 2, when trials N − 1 were stop-signal trials. We observed a significant effect of transition type, t(29) = 3.545, p < .001, for trial N + 1, with slower responses for repeat trials (753.02 ms) than for switching trials (721.58 ms). Moreover, for trial N + 2, the RTs between the repeat (722.42 ms) and switch trials did not differ (725.58 ms), t(29) = .199, p = .844. We counted the number of trials in which a single participant had residual inhibition and found that after a stop signal trial, the average number of trials showing residual inhibition was 1.7 (±1.09).
Interestingly, we found that five participants did not show any effect of transition type in trial N when trial N − 1 was the inhibition condition. That is, these participants seemed to successfully overcome the inhibition of trial N − 1 immediately before the presentation of the stimulus in trial N, reflecting that they had a faster deinhibition speed than the others. In addition, we analyzed the RT difference between repeat trials and switch trials after failed inhibition and found that even if stopping was not successful in stop signal trials, the deinhibition effect still existed, t(20) = 2.684, p = .014. That is, the repeat trials were also slower than the switch trials after an inhibition trial, when participants failed to stop.
Discussion
Consistent with the main results of Experiment 1, the results of Experiment 2 show that under the N − 1 inhibition condition, the RTs were longer, and the accuracies were lower for stimulus repetition than for stimulus switching, indicating that when a recently inhibited stimulus needs to be responded to again, participants showed a marked cost to overcome the effect of the residual inhibition in the current trial. It was also found that the rate of successful stopping decreased from 94% in Experiment 1 to 90% in Experiment 2, implying that the task difficulty increased when the SOAs were not fixed. However, the main results, such as the deinhibition cost and speed, were almost the same between the two experiments.
In brief, by using five varied SOAs (80, 160, ms, 320, and 400 ms) designed by Verbruggen and Logan (2008b), Experiment 2 successfully replicated the main results of Experiment 1, indicating that although the use of a fixed SOA in Experiment 1 may lead to the participants being prepared for inhibition, response inhibition still exists. The hybrid pattern of inhibition of no-go-like stimuli and the initiated response induced by the stop signal were required when participants detected a stop or no-go signal (Huster et al., 2010, 2011). The residual effect of inhibition lasts for a certain period, leading to a cost to overcome this inhibition when the previously inhibited stimuli should be responded to again (Dreher & Berman, 2002; Giller et al., 2019; Mayr & Keele, 2000; Scheil & Kleinsorge, 2019; Sdoia et al., 2020).
General discussion
This study used a stop-signal task to explore the process of overcoming inhibition, which is termed deinhibition. We compared the relationship between deinhibition and the “inhibition” and “shifting” components of cognitive control. The following findings were obtained. First, deinhibition is reflected in the cost of accuracy and reaction time when participants respond again to the stimulus that has just been successfully inhibited, which is consistent with the results of studies on the BI effect in task switching (Dreher & Berman, 2002; Giller et al., 2019). Second, deinhibition ability is moderately correlated with inhibitory control and cognitive flexibility.
Response inhibition and the cost of overcoming its carryover
This study found that when the preceding trial was not a stop signal (noninhibition), there was no significant RT difference between stimulus switching and repetition in the current trial, but the response accuracy was obviously lower for stimulus switching trials than for stimulus repetition trials, reflecting the typical cost of attention shifting and response changes to different stimuli (Berti, 2008; Ding et al., 2022; Janczyk et al., 2008; Janczyk & Grabowski, 2011). However, when the preceding trial was a stop signal, the RT was longer and the accuracy was lower for the stimulus repetition than for the stimulus switching. This suggests that when participants in the current trial encounter and execute a response to a stimulus that has recently been inhibited, they must overcome the interference produced by the previous inhibition. That is, if participants successfully exerted inhibition when the stop signal appeared, the inhibition effect lasted until the next trial, or even longer. We found that the average duration of the inhibition effect of all participants was 1.5-1.7 trials, which was approximately 4 seconds. This finding implies that in the stop-signal task, the effect of successful inhibition of a stop signal lasts approximately 4 s. Within this time duration, if participants were required to respond to a stimulus that had been previously inhibited, they would show a higher error rate and a longer reaction time, which is called the deinhibition cost.
The average time of the deinhibition cost in the stop-signal task was 30 ms. This is close to the deinhibition costs reported in BI studies on task switching (Giller et al., 2019; Giller & Beste, 2019). However, it is smaller than the cost in Schuch and Koch (2003, Experiment 1A), who found that the time cost is approximately 100 ms in overcoming the effect of inhibition of the preceding task and response, when the preceding trial is a no-go trial. This suggests that, compared with only inhibiting the task (in BI studies) or only inhibiting the response (in the present study), the inhibition of the task and the response (Schuch & Koch, 2003) requires stronger inhibitory control, and the cost of deinhibition is greater.
Deinhibition costs in the BI paradigm have been explained by the inertia theory and episodic retrieval account. Inertia theory in task switching assumes that old tasks need to be inhibited when switching to new tasks. The tasks that have just been executed need even stronger inhibition, and this inhibition will continue until a period after the completion of the new task. In this period, if participants performed the previously inhibited task again, they need to overcome the residual inhibition, resulting in the deinhibition costs (Allport et al., 1994; Koch et al., 2010; Mayr & Keele, 2000; see J. Chen et al., 2022, for review). The episodic retrieval account has been adopted to explain several findings in the task switching literature, such as the typical task switch cost (Altmann & Gray, 2008; Schmidt & Liefooghe, 2016), the reduction of the task switch cost with increasing response-cue interval (Grange, 2016; Horoufchin et al., 2011), and the BI effect (Grange, 2018; Grange et al., 2017). For example, Grange et al. (2017) used a modified version of the movement direction switching task to explore the interaction effect of task repetition and response repetition (i.e., button press) on BI effect (Mayr, 2002). They found that the N − 2 task repetition costs were reduced in the N − 2 response repetition condition, implying that episodic retrieval of task-related responses can modulate N − 2 task repetition costs. Grange et al. (2017) claimed that although the BI paradigm is mainly used to measure inhibition in task switching, episodic retrieval also has an important role in BI. In the SST paradigm used in the present study, mismatch costs occurred when the task parameters in the current trial were different from the retrieved episodic memory (Grange et al., 2017; Mayr, 2002). However, as discussed in Experiment 1, the current study did not manipulate these parameters and did not provide data to support the episodic retrieval account. Future studies are needed to explore the role of episodic memory retrieval in the deinhibition cost using the SST-like paradigms.
Most importantly, we found that about a quarter of the participants had no significant difference in RT between the stimulus switching and repetition trials under the N−1 inhibition condition; that is, these participants seemly did not show the cost of deinhibition. The most likely reason is that these participants were able to quickly remove the effect of inhibition. After they successfully completed one inhibition, before the stimulus appeared in the next trial, or before they responded, they had overcome the residual effect of inhibition. Is the higher ability to quickly remove inhibition due to these participants’ stronger inhibition and cognitive flexibility? The relationship between deinhibition and the other two components of cognitive control is discussed in detail in the following sections.
Relation between deinhibition and response inhibition
Deinhibition seems to be closely related to inhibitory control from the perspective of concepts and cognitive function. Our results showed that the cost of deinhibition was positively correlated with the SSRT. Individuals with a lower cost of deinhibition had a shorter SSRT. Compared to those with slower deinhibition speed, individuals with faster deinhibition speed have a higher probability of successfully stopping and responding faster to stop signals. The moderate correlation between deinhibition and response inhibition may be due to the fact that both inhibition and deinhibition are associated with conflict monitoring and resolution. On the one hand, inhibitory control is mostly used to reflect the process of conflict or interference regulation in cognitive control. When irrelevant information conflicts with the current task, it needs to be inhibited. Therefore, inhibitory control embodies the process of conflict resolution (Schmidt et al., 2020; Yu et al., 2012). On the other hand, deinhibition can be regarded as a conflict between inhibiting the response to a specific stimulus and executing the response to stimuli (Sexton & Cooper, 2017). According to conflict monitoring theory in cognitive control, recognizing when a conflict occurs can provide a key signal indicating when control needs to be strengthened (Botvinick et al., 2001; Carter et al., 1998).
Another reason why deinhibition is moderately related to inhibition control may be that these two processes are closely linked in time dimension. In other words, deinhibition always occurs after inhibition. When participants successfully inhibit a response on the occurrence of a stop signal with a shorter SSRT, they will have more time to prepare for the following tasks (including deinhibition), which leads to a reduction in the cost of deinhibition. This is consistent with the conclusion that the BI effect is modulated by CSI (Astle et al., 2012; Grange & Houghton, 2011; Koch et al., 2010; Mayr & Keele, 2000). When CSI increases, the BI effect decreases; that is, if the individual has a longer time to prepare for the next task, the cost of deinhibition is smaller, although this cost cannot be completely eliminated (Astle et al., 2012; Grange & Houghton, 2011).
However, in the present study, when trial N − 1 was a stop signal, inhibition occurred in the trial and deinhibition occurred in trial N. Therefore, deinhibition and inhibition occurred at different points in time dimension, and their corresponding cognitive functions were also different. The cognitive function of inhibition is to inhibit irrelevant factors and eliminate interference in the current task. In the stop-signal task, inhibition functions to suppress a certain action that is about to be performed and to avoid inappropriate behavior. In contrast, the cognitive function of deinhibition is to remove or overcome existing inhibition so as to give an immediate response to a certain stimulus or event and avoid missing or delaying a response. Therefore, in terms of time point and main cognitive function, deinhibition and inhibition are not the same processes. This conclusion is consistent with the views of some studies on the BI effect, which suggest that deinhibition is essentially different from backward inhibition or inhibition in task switching (Dreher & Berman, 2002; Sdoia et al., 2020).
Relation between deinhibition and flexibility
We found that the deinhibition cost was moderately correlated with the switch accuracy costs. The worse the deinhibition performance of an individual, the lower the accuracy of their task switching (Wolff et al., 2018). This suggests that deinhibition and cognitive flexibility may share certain cognitive processes. Brain imaging studies have shown a partial overlap between brain regions involved in BI and cognitive flexibility (Dreher & Berman, 2002; Miller & Cohen, 2001; Sohn et al., 2000).
In addition, when switching between dual tasks, participants easily fall into an established behavior pattern; therefore, it is difficult to switch flexibly when proceeding to the next task. Similarly, after inhibiting a specific stimulus/task, the participant can easily fall into the established behavior pattern (inhibiting the response to a specific stimulus); therefore, when responding to the next task that is previously inhibited), it is difficult for the participant to flexibly release the inhibition, which makes it difficult to respond well and quickly. Therefore, from this perspective, the transient process of shifting from response inhibition to deinhibition is correlated with cognitive flexibility (Giller et al., 2019; Giller & Beste, 2019; Wolff et al., 2018).
However, we believe that an individual’s deinhibition ability and cognitive flexibility are relatively independent components. First, we found that the deinhibition ability shown by individuals in stop-signal tasks was not correlated with the RT cost in task switching, and the speed of deinhibition was also not correlated with task-switching performance. Second, cognitive flexibility mainly manifests as the ability to flexibly switch between multiple tasks, that is, the ability to switch from Task A to Task B. However, deinhibition manifests as the ability to quickly and completely remove or overcome the residual inhibition of a given stimulus or event that needs to be responded to in the same task. Third, several brain imaging and ERP studies on the BI effect have also shown that the brain regions and ERP components activated by the BI effect differ from those activated by cognitive flexibility (Dreher & Berman, 2002; Giller et al., 2020; Sdoia et al., 2020; Sinai et al., 2007).
Conclusion
This study used a stop-signal task to explore the deinhibition ability and found that when participants were required to respond to a stimulus that had been recently inhibited, the response was slower and the error rate was higher, reflecting the deinhibition cost. Deinhibition ability is moderately correlated with the “inhibition” and “shifting” components of cognitive control. These findings suggest that deinhibition ability is an important component of cognitive control that plays a critical role in human adaptive behavior.
References
Allport, A., Styles, E. A., & Hsieh, S. (1994). Shifting intentional set: Exploring the dynamic control of tasks. In C. Umiltá & M. Moscovitch (Eds.), Attention and performance (pp. 421–452). MIT Press.
Altmann, E. M., & Gray, W. D. (2008). An integrated model of cognitive control in task switching. Psychological Review, 115(3), 602–639.
Anguera, J. A., Lyman, K., Zanto, T. P., Bollinger, J., & Gazzaley, A. (2013). Reconciling the influence of task-set switching and motor inhibition processes on stop signal after-effects. Frontiers in Psychology, 4, 649. https://doi.org/10.3389/fpsyg.2013.00649
Arbuthnott, K., & Frank, J. (2000). Executive control in set switching: Residual switch cost and task-set inhibition. Canadian Journal of Experimental Psychology/Revue Canadienne de Psychologie Expérimentale, 54(1), 33–41. https://doi.org/10.1037/h0087328
Aron, A. R. (2007). The neural basis of inhibition in cognitive control. Neuroscientist, 13(3), 214–228. https://doi.org/10.1177/1073858407299288
Aron, A. R., Fletcher, P. C., Bullmore, E. T., Sahakian, B. J., & Robbins, T. W. (2003). Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience, 6(2), 115–116. https://doi.org/10.1038/nn1003
Astle, D. E., Jackson, G. M., & Swainson, R. (2012). Two measures of task-specific inhibition. Quarterly Journal of Experimental Psychology, 65(2), 233–251. https://doi.org/10.1080/17470210903431732
Band, G. P. H., & van Boxtel, G. J. M. (1999). Inhibitory motor control in stop paradigms: Review and reinterpretation of neural mechanisms. Acta Psychologica, 101(2), 179–211. https://doi.org/10.1016/S0001-6918(99)00005-0
Band, G. P. H., van der Molen, M. W., & Logan, G. D. (2003). Horse-race model simulations of the stop-signal procedure. Acta Psychologica, 112(2), 105–142. https://doi.org/10.1016/s0001-6918(02)00079-3
Baniqued, P. L., Low, K. A., Fletcher, M. A., Gratton, G., & Fabiani, M. (2017). Shedding light on gray(ing) areas: Connectivity and task switching dynamics in aging. Psychophysiology, 55(3), Article e12818. https://doi.org/10.1111/psyp.12818
Barras, C., & Kerzel, D. (2016). Nogo stimuli do not receive more attentional suppression or response inhibition than neutral stimuli: Evidence from the N2pc, PD, and N2 components in a spatial cueing paradigm. Frontiers in Psychology, 7, 630. https://doi.org/10.3389/fpsyg.2016.00630
Bart, V. K. E., Koch, I., & Rieger, M. (2021). Inhibitory mechanisms in motor imagery: disentangling different forms of inhibition using action mode switching. Psychological Research, 85, 1418–1438. https://doi.org/10.1007/s00426-020-01327-y
Berti, S. (2008). Object switching within working, memory is reflected in the human event-related brain potential. Neuroscience Letters, 434(2), 200–205. https://doi.org/10.1016/j.neulet.2008.01.055
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. https://doi.org/10.1037/0033-295X.108.3.624
Braem, S., Bugg, J. M., Schmidt, J. R., Crump, M., & Egner, T. (2019). Measuring adaptive control in conflict tasks. Trends in Cognitive Sciences, 23(9), 769–783. https://doi.org/10.1016/j.tics.2019.07.002
Brand, M., Wegmann, E., Stark, R., Muller, A., Wolfling, K., Robbins, T. W., & Potenza, M. N. (2019). The Interaction of Person-Affect-Cognition-Execution (I-PACE) model for addictive behaviors: Update, generalization to addictive behaviors beyond internet-use disorders, and specification of the process character of addictive behaviors. Neuroscience and Biobehavioral Reviews, 104, 1–10. https://doi.org/10.1016/j.neubiorev.2019.06.032
Brazdil, M., Mikl, M., Marecek, R., Krupa, P., & Rektor, I. (2007). Effective connectivity in target stimulus processing: A dynamic causal modeling study of visual oddball. Neuroimage, 35(2), 827–835. https://doi.org/10.1016/j.neuroimage.2006.12.020
Cai, W., Griffiths, K., Korgaonkar, M. S., Williams, L. M., & Menon, V. (2021). Inhibition-related modulation of salience and frontoparietal networks predicts cognitive control ability and inattention symptoms in children with ADHD. Molecular Psychiatry, 26(8), 4016–4025. https://doi.org/10.1038/s41380-019-0564-4
Carter, C. S., Braver, T. S., Barch, D. M., Botvinick, M. M., Noll, D., & Cohen, J. D. (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science, 280(5364), 747–749. https://doi.org/10.1126/science.280.5364.747
Cartwright, K. B., Coppage, E. A., Lane, A. B., Singleton, T., Marshall, T. R., & Bentivegna, C. (2017). Cognitive flexibility deficits in children with specific reading comprehension difficulties. Contemporary Educational Psychology, 50, 33–44. https://doi.org/10.1016/j.cedpsych.2016.01.003
Cartwright, K. B., Marshall, T. R., Huemer, C. M., & Payne, J. B. (2019). Executive function in the classroom: Cognitive flexibility supports reading fluency for typical readers and teacher-identified low-achieving readers. Research in Developmental Disabilities, 88, 42–52. https://doi.org/10.1016/j.ridd.2019.01.011
Cartwright, K. B., Marshall, T. R., & Hatfield, N. A. (2020). Concurrent and Longitudinal Contributions of a Brief Assessment of Reading-Specific Executive Function to Reading Comprehension in First and Second Grade Students. Mind, Brain, and Education, 14(2), 114–123. https://doi.org/10.1111/mbe.12236
Cepeda, N. J., Kramer, A. F., & de Sather, J. (2001). Changes in executive control across the life span: Examination of task-switching performance. Developmental Psychology, 37(5), 715–730. https://doi.org/10.1037/0012-1649.37.5.715
Chen, C. Q., Yang, W. J., Li, W. F., Wei, D. T., Li, H. J., Lei, Q., Zhang, Q. L., & Qiu, J. (2014). Association of creative achievement with cognitive flexibility by a combined voxel-based morphometry and resting-state functional connectivity study. NeuroImage, 102, 474–483. https://doi.org/10.1016/j.neuroimage.2014.08.008
Chen, Y., Spagna, A., Wu, T. T., Kim, T. H., Wu, Q., Chen, C. Q., Wu, Y. H., & Fan, J. (2019). Testing a cognitive control model of human intelligence. Scientific Reports, 9, Article 2898. https://doi.org/10.1038/s41598-019-39685-2
Chen, J., Wu, S., & Li, F. (2022). Cognitive neural mechanism of backward inhibition and deinhibition: A review. Frontiers in Behavioral Neuroscience, 16, Article 846369. https://doi.org/10.3389/fnbeh.2022.846369
Davis, J. C., Marra, C. A., Najafzadeh, M., & Liu-Ambrose, T. (2010). The independent contribution of executive functions to health related quality of life in older women. Bmc Geriatrics, 10, Article 16. https://doi.org/10.1186/1471-2318-10-16
Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
Ding, G., Ye, W., Cao, B., & Li, F. (2022). Electrophysiological correlates of the effect of set size on object switching in working memory. Psychophysiology, 1–11. https://doi.org/10.1111/psyp.14135
Dreher, J. C., & Berman, K. F. (2002). Fractionating the neural substrate of cognitive control processes. Proceedings of the National Academy of Sciences of the United States of America, 99(22), 14595–14600. https://doi.org/10.1073/pnas.222193299
Emmelin, N., & Muren, A. (1950). Acetylcholine release at parasympathetic synapses. Acta Physiologica Scandinavica, 20, 13–32. https://doi.org/10.1111/j.1748-1716.1950.tb00677.x
Enticott, P. G., Bradshaw, J. L., Bellgrove, M. A., Upton, D. J., & Ogloff, J. (2009). Stop task after-effects. Experimental Psychology, 56(4), 247–251.
Fales, C. L., Vanek, Z. F., & Knowlton, B. J. (2006). Backward inhibition in Parkinson's disease. Neuropsychologia, 44, 1041–1049. https://doi.org/10.1016/j.neuropsychologia.2005.11.002
Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. G. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146
Ferguson, H. J., Brunsdon, V. E. A., & Bradford, E. E. F. (2021). The developmental trajectories of executive function from adolescence to old age. Scientific Reports, 11(1), Article 1382. https://doi.org/10.1038/s41598-020-80866-1
Folk, C. L., & Remington, R. W. (2008). Bottom-up priming of top-down attentional control settings. Visual Cognition, 16(2/3), 215–231. https://doi.org/10.1080/13506280701458804
Folk, C. L., Remington, R. W., & Johnston, J. C. (1992). Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance, 18(4), 1030–1044. https://doi.org/10.1037/0096-1523.18.4.1030
Friedman, N. P., Miyake, A., Corley, R. P., Young, S. E., DeFries, J. C., & Hewitt, J. K. (2006). Not all executive functions are related to intelligence. Psychological Science, 17(2), 172–179. https://doi.org/10.1111/j.1467-9280.2006.01681.x
Friston, K. (2009). The free-energy principle: a rough guide to the brain? Trends in Cognitive Sciences, 13(7), 293–301. https://doi.org/10.1016/j.tics.2009.04.005
Genet, J. J., & Siemer, M. (2011). Flexible control in processing affective and nonaffective material predicts individual differences in trait resilience. Cognition & Emotion, 25(2), 380–388. https://doi.org/10.1080/02699931.2010.491647
Giller, F., & Beste, C. (2019). Effects of aging on sequential cognitive flexibility are associated with fronto-parietal processing deficits. Brain Structure & Function, 224(7), 2343–2355. https://doi.org/10.1007/s00429-019-01910-z
Giller, F., Zhang, R., Roessner, V., & Beste, C. (2019). The neurophysiological basis of developmental changes during sequential cognitive flexibility between adolescents and adults. Human Brain Mapping, 40(2), 552–565. https://doi.org/10.1002/hbm.24394
Giller, F., Muckschel, M., Ziemssen, T., & Beste, C. (2020). A possible role of the norepinephrine system during sequential cognitive flexibility-Evidence from EEG and pupil diameter data. Cortex, 128, 22–34. https://doi.org/10.1016/j.cortex.2020.03.008
Grange, J. A. (2016). Temporal distinctiveness in task switching: Assessing the mixture-distribution assumption. Frontiers in Cognition, 7, 251.
Grange, J. A. (2018). Does task activation in task switching influence inhibition or episodic interference? Experimental Psychology, 65(6), 393–404.
Grange, J. A., & Houghton, G. (2011). Task preparation and task inhibition: a comment on Koch, Gade, Schuch, & Philipp (2010). Psychonomic Bulletin & Review, 18(1), 211–216. https://doi.org/10.3758/s13423-010-0023-3
Grange, J. A., & Houghton, G. (2014). Task switching and cognitive control. Oxford University Press. https://doi.org/10.1093/acprof:osobl/9780199921959.001.0001
Grange, J. A., Kowalczyk, A. W., & O'Loughlin, R. (2017). The Effect of Episodic Retrieval on Inhibition in Task Switching. Journal of Experimental Psychology: Human Perception and Performance, 43(8), 1568–1583.
Han, J., Dai, Y., Xie, L., & Li, F. (2018). Brain responses associated with different hierarchical effects on cues and targets during rule shifting. Biological Psychology, 134, 52–63. https://doi.org/10.1016/j.biopsycho.2018.02.010
Han, J. H., Xie, L. F., Cao, B. H., Li, J. C., Chen, Y., & Li, F. H. (2019). More abstract, more difficult to shift: Behavior and electrophysiological evidence. Behavioural Brain Research, 362, 273–278. https://doi.org/10.1016/j.bbr.2019.01.001
Hendricks, M. A., & Buchanan, T. W. (2016). Individual differences in cognitive control processes and their relationship to emotion regulation. Cognition & Emotion, 30(5), 912–924. https://doi.org/10.1080/02699931.2015.1032893
Hooi, L., S., Nisar, H., Thee, K. W., & Yap, V. V. (2018). A novel method for tracking and analysis of EEG activation across brain lobes. Biomedical Signal Processing and Control, 40, 488–504. https://doi.org/10.1016/j.bspc.2017.06.017
Horoufchin, H., Philipp, A. M., & Koch, I. (2011). The dissipating task-repetition benefit in cued task switching: Task-set decay or temporal distinctiveness? Journal of Experimental Psychology: Human Perception & Performance, 37, 455–472.
Huster, R. J., Westerhausen, R., Pantev, C., & Konrad, C. (2010). The role of the cingulate cortex as neural generator of the N200 and P300 in a tactile response inhibition task. Human Brain Mapping, 31(8), 1260–1271. https://doi.org/10.1002/hbm.20933
Huster, R. J., Westerhausen, R., & Herrmann, C. S. (2011). Sex differences in cognitive control are associated with midcingulate and callosal morphology. Brain Structure & Function, 215(3–4), 225–235. https://doi.org/10.1007/s00429-010-0289-2
Hsu, N. S., & Jaeggi, S. M. (2013). The emergence of cognitive control abilities in childhood. Current Topics in Behavioral Neurosciences, 16, 149–166. https://doi.org/10.1007/7854_2013_241
Janczyk, M., & Grabowski, J. (2011). The focus of attention in working memory: Evidence from a word updating task. Memory, 19(2), 211–225. https://doi.org/10.1080/09658211.2010.546803
Janczyk, M., Wienrich, C., & Kunde, W. (2008). On the costs of refocusing items in working memory: A matter of inhibition or decay? Memory, 16(4), 374–385. https://doi.org/10.1080/09658210801941742
Jurado, M. B., & Rosselli, M. (2007). The elusive nature of executive functions: A review of our current understanding. Neuropsychology Review, 17(3), 213–233. https://doi.org/10.1007/s11065-007-9040-z
Kass, R. E., & Steffey, D. (1989). Approximate Bayesian inference in conditionally independent hierarchical models (parametric empirical Bayes models). Journal of the American Statistical Association, 84(407), 717–726. https://doi.org/10.1080/01621459.1989.10478825
Kiesel, A., Steinhauser, M., Wendt, M., Falkenstein, M., Jost, K., Philipp, A. M., & Koch, I. (2010). Control and interference in task switching—A review. Psychological Bulletin, 136(5), 849–874. https://doi.org/10.1037/a0019842
Koch, I., Gade, M., Schuch, S., & Philipp, A. M. (2010). The role of inhibition in task switching: A review. Psychonomic Bulletin & Review, 17(1), 1–14. https://doi.org/10.3758/PBR.17.1.1
Lee, W. T., & Kang, M. S. (2020). Electrophysiological evidence for distinct proactive control mechanisms in a stop-signal task: An individual differences approach. Frontiers in Psychology, 11, Article 1105. https://doi.org/10.3389/fpsyg.2020.01105
Lien, M. C., Ruthruff, E., Goodin, Z., & Remington, R. W. (2008). Contingent attentional capture by top-down control settings: Converging evidence from event-related potentials. Journal of Experimental Psychology–Human Perception and Performance, 34(3), 509–530. https://doi.org/10.1037/0096-1523.34.3.509
Lien, M. C., Ruthruff, E., & Cornett, L. (2010). Attentional capture by singletons is contingent on top-down control settings: Evidence from electrophysiological measures. Visual Cognition, 18(5), 682–727. https://doi.org/10.1080/13506280903000040
Logan, G. D. (1994). On the ability to inhibit thought and action: A users' guide to the stop signal paradigm. In Inhibitory processes in attention, memory, and language (pp. 189–239). Academic Press.
Logan, G. D., & Cowan, W. B. (1984). On the ability to inhibit thought and action: A theory of an act of control. Psychological Review, 91(3), 295–327. https://doi.org/10.1037/0033-295X.91.3.295
Mayr, U. (2002). Inhibition of action rules. Psychonomic Bulletin & Review, 9, 93–99.
Mayr, U., & Keele, S. W. (2000). Changing internal constraints on action: The role of backward inhibition. Journal of Experimental Psychology: General, 129(1), 4–26. https://doi.org/10.1037/0096-3445.129.1.4
Mehta, M. R. (2001). Neuronal dynamics of predictive coding. The Neuroscientist, 7(6), 490–495. https://doi.org/10.1177/107385840100700605
Meiran, N., Chorev, Z., & Sapir, A. (2000). Component processes in task switching. Cognitive Psychology, 41(3), 211–253. https://doi.org/10.1006/cogp.2000.0736
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. https://doi.org/10.1146/annurev.neuro.24.1.167
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. https://doi.org/10.1006/cogp.1999.0734
Monsell, S. (2003). Task switching. Trends in Cognitive Sciences, 7(3), 134–140. https://doi.org/10.1016/S1364-6613(03)00028-7
Mostofsky, S. H., & Simmonds, D. J. (2008). Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience, 20(5), 751–761. https://doi.org/10.1162/jocn.2008.20500
Norman, D., & Shallice, T. (1986). Attention to action: Willed and automatic control of behavior. Consciousness and Self-Regulation, 4. https://doi.org/10.1177/014662168300700407
Peterson, B. S., Kane, M. J., Alexander, G. M., Lacadie, C., Skudlarski, P., Leung, H. C., May, J., & Gore, J. C. (2002). An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Cognitive Brain Research, 13(3), 427–440. https://doi.org/10.1016/s0926-6410(02)00054-x
Philipp, A. M., & Koch, I. (2005). Switching of response modalities. Quarterly Journal of Experimental Psychology Section A–Human Experimental Psychology, 58(7), 1325–1338. https://doi.org/10.1080/02724980443000656
Picazio, S., Foti, F., Oliveri, M., Koch, G., Petrosini, L., Ferlazzo, F., & Sdoia, S. (2020). Out with the old and in with the new: The contribution of prefrontal and cerebellar areas to backward inhibition. Cerebellum, 19, 426–436. https://doi.org/10.1007/s12311-020-01115-9
Quigley, L., Wen, A., & Dobson, K. S. (2020). Cognitive control over emotional information in current and remitted depression. Behaviour Research and Therapy, 132, Article 103658. https://doi.org/10.1016/j.brat.2020.103658
Regev, S., & Meiran, N. (2016). Cue-type manipulation dissociates two types of task set inhibition: Backward inhibition and competitor rule suppression. Psychological Research Psychologische Forschung, 80, 625–639. https://doi.org/10.1007/s00426-015-0663-z
Regev, S., & Meiran, N. (2017). Cue response dissociates inhibitory processes: Task identity information is related to backward inhibition but not to competitor rule suppression. Psychological Research Psychologische Forschung, 81, 168–181. https://doi.org/10.1007/s00426-015-0742-1
Rogers, R. D., & Monsell, S. (1995). Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology, 124(2), 207–207. https://doi.org/10.1037/0096-3445.124.2.207
Sahib, A., Loureiro, J., Vasavada, M., Kubicki, A., Wade, B., Joshi, S., Woods, R., Congdon, E., Espinoza, R., & Narr, K. (2020). Modulation of inhibitory control networks relate to clinical response following ketamine therapy in major depression. Biological Psychiatry, 87(9), S309–S309. https://doi.org/10.1016/j.biopsych.2020.02.796
Scheil, J., & Kleinsorge, T. (2019). Effects of global and local task repetition proportion on N − 2 repetition costs. Quarterly Journal of Experimental Psychology, 72(3), 579–588. https://doi.org/10.1177/1747021818762087
Scheil, J., & Kleinsorge, T. (2022). No-go trials in task switching: effects on the task-set and task-space level. Psychological Research, 86, 1097–1107. https://doi.org/10.1007/s00426-021-01566-7
Schmidt, J. R., & Liefooghe, B. (2016). Feature integration and task switching: Diminished switch costs after controlling for stimulus, response, and cue repetitions. PLOS ONE, 11(3), Article e0151188.
Schmidt, C. C., Timpert, D. C., Arend, I., Vossel, S., Fink, G. R., Henik, A., & Weiss, P. H. (2020). Control of response interference: caudate nucleus contributes to selective inhibition. Scientific Reports, 10(1), Article 20977. https://doi.org/10.1038/s41598-020-77744-1
Schuch, S., & Koch, I. (2003). The role of response selection for inhibition of task sets in task shifting. Journal of Experimental Psychology–Human Perception and Performance, 29(1), 92–105. https://doi.org/10.1037/0096-1523.29.1.92
Sdoia, S., Zivi, P., & Ferlazzo, F. (2020). Anodal tDCS over the right parietal but not frontal cortex enhances the ability to overcome task set inhibition during task switching. PLOS ONE, 15(2), Article e0228541. https://doi.org/10.1371/journal.pone.0228541
Sexton, N. J., & Cooper, R. P. (2017). Task inhibition, conflict, and the N − 2 repetition cost: A combined computational and empirical approach. Cognitive Psychology, 94, 1–25. https://doi.org/10.1016/j.cogpsych.2017.01.003
Sinai, M., Goffaux, P., & Phillips, N. A. (2007). Cue-locked versus response-locked processes in backward inhibition: Evidence from ERPs. Psychophysiology, 44(4), 596–609. https://doi.org/10.1111/j.1469-8986.2007.00527.x
Sohn, M. H., Ursu, S., Anderson, J. R., Stenger, V. A., & Carter, C. S. (2000). The role of prefrontal cortex and posterior parietal cortex in task switching. Proceedings of the National Academy of Sciences of the United States of America, 97(24), 13448–13453. https://doi.org/10.1073/pnas.240460497
Sylvester, C. Y. C., Wager, T. D., Lacey, S. C., Hernandez, L., Nichols, T. E., Smith, E. E., & Jonides, J. (2003). Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia, 41(3), 357–370. https://doi.org/10.1016/s0028-3932(02)00167-7
Tona, K.-D., Revers, H., Verkuil, B., & Nieuwenhuis, S. (2020). Noradrenergic regulation of cognitive flexibility: No effects of stress, transcutaneous vagus nerve stimulation, and atomoxetine on task-switching in humans. Journal of Cognitive Neuroscience, 32, 1–15. https://doi.org/10.1162/jocn_a_01603
Van den Bussche, E., Vanmeert, K., Aben, B., & Sasanguie, D. (2020). Too anxious to control: The relation between math anxiety and inhibitory control processes. Scientific Reports, 10(1), Article 19922. https://doi.org/10.1038/s41598-020-76920-7
Vandierendonck, A., Liefooghe, B., & Verbruggen, F. (2010). Task switching: Interplay of reconfiguration and interference control. Psychological Bulletin, 136(4), 601–626. https://doi.org/10.1037/a0019791
Venables, N. C., Foell, J., Yancey, J. R., Kane, M. J., Engle, R. W., & Patrick, C. J. (2018). Quantifying inhibitory control as externalizing proneness: A cross-domain model. Clinical Psychological Science, 6(4), 561–580. https://doi.org/10.1177/2167702618757690
Verbruggen, F., & Logan, G. D. (2008a). Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences, 12(11), 418–424. https://doi.org/10.1016/j.tics.2008.07.005
Verbruggen, F., & Logan, G. D. (2008b). After-effects of goal shifting and response inhibition: A comparison of the stop-change and dual-task paradigms. Quarterly Journal of Experimental Psychology, 61(8), 1151–1159. https://doi.org/10.1080/17470210801994971
Verbruggen, F., & Logan, G. D. (2008c). Long-term aftereffects of response inhibition: Memory retrieval, task goals, and cognitive control. Journal of Experimental Psychology: Human Perception and Performance, 34(5), 1229–1235. https://doi.org/10.1037/0096-1523.34.5.1229
Verbruggen, F., & Logan, G. D. (2009a). Automaticity of cognitive control: Goal priming in response-inhibition paradigms. Journal of Experimental Psychology–Learning Memory and Cognition, 35(5), 1381–1388. https://doi.org/10.1037/a0016645
Verbruggen, F., Liefooghe, B., Szmalec, A., & Vandierendonck, A. (2005a). Inhibiting responses when switching: Does it matter? Experimental Psychology, 52(2), 125–130. https://doi.org/10.1027/1618-3169.52.2.125
Verbruggen, F., Liefooghe, B., & Vandierendonck, A. (2005b). On the difference between response inhibition and negative priming: Evidence from simple and selective stopping. Psychological Research Psychologische Forschung, 69(4), 262–271. https://doi.org/10.1007/s00426-004-0177-6
Verbruggen, F., Aron, A. R., Band, G. P. H., Beste, C., Bissett, P. G., Brockett, A. T., Brown, J. W., Chamberlain, S. R., Chambers, C. D., Colonius, H., Colzato, L. S., Corneil, B. D., Coxon, J. P., Dupuis, A., Eagle, D. M., Garavan, H., Greenhouse, I., Heathcote, A., Huster, R. J., Jahfari, S., Kenemans, J. L., …, Boehler, C. N. (2019). A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. Elife, 8, e46323. https://doi.org/10.7554/eLife.46323
von Bastian, C. C., & Druey, M. D. (2017). Shifting Between Mental Sets: An Individual Differences Approach to Commonalities and Differences of Task Switching Components. Journal of Experimental Psychology-General, 146(9), 1266–1285. https://doi.org/10.1037/xge0000333
Wegmann, E., Muller, S. M., Turel, O., & Brand, M. (2020). Interactions of impulsivity, general executive functions, and specific inhibitory control explain symptoms of social-networks-use disorder: An experimental study. Scientific Reports, 10(1), Article 3866. https://doi.org/10.1038/s41598-020-60819-4
Whitmer, A. J., & Banich, M. T. (2012). Brain activity related to the ability to inhibit previous task sets: An fMRI study. Cognitive Affective, & Behavioral Neuroscience, 12(4), 661–670. https://doi.org/10.3758/s13415-012-0118-6
Wolff, N., Giller, F., Buse, J., Roessner, V., & Beste, C. (2018). When repetitive mental sets increase cognitive flexibility in adolescent obsessive-compulsive disorder. Journal of Child Psychology and Psychiatry, 59(9), 1024–1032. https://doi.org/10.1111/jcpp.12901
Yu, J. X., Hung, D. L., Tseng, P., Tzeng, O. J. L., Muggleton, N. G., & Juan, C. H. (2012). Sex differences in how erotic and painful stimuli impair inhibitory control. Cognition, 124(2), 251–255. https://doi.org/10.1016/j.cognition.2012.04.007
Zhang, R., Stock, A. K., Fischer, R., & Beste, C. (2016). The system neurophysiological basis of backward inhibition. Brain Structure & Function, 221(9), 4575–4587. https://doi.org/10.1007/s00429-016-1186-0
Zhuo, B., Chen, Y., Zhu, M., Cao, B., & Li, F. (2021a). Response variations can promote the efficiency of task switching: Electrophysiological evidence. Neuropsychologia, 156, Article 107828.
Zhuo, B. X., Zhu, M. Q., Cao, B. H., & Li, F. H. (2021b). More change in task repetition, less cost in task switching: Behavioral and event-related potential evidence. European Journal of Neuroscience, 53(8), 2553–2566. https://doi.org/10.1111/ejn.15113
Funding
National Natural Science Foundation of China: 31760285, 31860278. Jiangxi Postgraduate Innovation Fund: YC2021-S288
Author information
Authors and Affiliations
Corresponding author
Additional information
Open practices statement
The data used in the manuscript are posted at Open Science Framework (https://osf.io/d2ztk/?view_only=b92344966239408ab375d43c53bedaf8). Access to the data is limited to qualified researchers. The materials used in this study are widely available.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Cao, B. & Li, F. Do after “not to do”: Deinhibition in cognitive control. Mem Cogn 51, 1388–1403 (2023). https://doi.org/10.3758/s13421-023-01403-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13421-023-01403-9