Abstract
Ruminative thinking is related to an increased risk for major depressive disorder (MDD) and perpetuates negative mood states. Rumination, uncontrollable negative thoughts about the self, may comprise both reflective and brooding components. However, only brooding rumination is consistently associated with increased negativity bias and negative coping styles, while reflective rumination has a less clear relationship with negative outcomes in healthy and depressed participants. The current study examined seed-to-voxel (S2.V) resting-state functional connectivity (FC) in a sample of healthy (HC) and depressed (MDD) adult women (HC: n=50, MDD: n=33). The S2V FC of six key brain regions, including the left and right amygdala, anterior and posterior cingulate cortex (ACC, PCC), and medial and dorsolateral prefrontal cortices (mPFC, dlPFC), was correlated with self-reported reflective and brooding rumination. Results indicate that HC and MDD participants had increased brooding rumination associated with decreased FC between the left amygdala and the right temporal pole. Moreover, reflective rumination was associated with distinct FC of the mPFC, PCC, and ACC with parietal, occipital, and cingulate regions. Depressed participants, compared with HC, exhibited decreased FC between the PCC and a region in the right middle frontal gyrus. The results of the current study add to the understanding of the neural underpinnings of different forms of self-related cognition—brooding and reflective rumination—in healthy and depressed women.
Similar content being viewed by others
Introduction
Rumination, characterized as recurrent and persistent negative thoughts about the self, is a common cognitive activity that increases an individual’s risk of developing major depressive disorder (MDD) (Abela & Hankin, 2011; Burwell & Shirk, 2007). More ruminative thoughts are related to increased bias towards negative information, decreased problem-solving ability, and sustained negative mood states in dysphoric individuals (Lyubomirsky, Caldwell, & Nolen-Hoeksema, 1998; Lyubomirsky & Nolen-Hoeksema, 1995). Women report higher levels of rumination than men, indicating that rumination may play a role in the gender difference found in diagnoses of depression (Johnson & Whisman, 2013; Jones, Siegle, & Thase, 2008). Also, more frequent ruminative thoughts are related to increased depression severity and worse outcomes from psychotherapy, suggesting that assessing rumination in depressed individuals may inform the development of effective treatments (Jones et al., 2008; Michalak, Holz, & Teismann, 2011; Nolen-Hoeksema, 2000). Although rumination has been primarily examined as it relates to MDD, research indicates that levels of rumination can vary in both healthy and depressed individuals (Junkins & Haeffel, 2017; Siegle, Moore, & Thase, 2004). Indeed, one study found dichotomous brain differences in the same region that were associated with rumination in healthy and depressed participants (Wang et al., 2015). Thus, understanding rumination in women with and without depression may be invaluable for identifying biomarkers for adults at-risk for developing MDD.
Researchers conceptualize rumination differently based on the proposed motivation, content, and outcome of these thoughts. In Nolen-Hoeksema’s model of rumination, the passive, negative, self-reflective nature of rumination is employed with the goal of alleviating low mood; however, it actually maintains, even exacerbates, negative mood. Although this theory emphasizes the negative consequences of rumination, both adaptive and maladaptive components have been proposed (Treynor, Gonzalez, & Nolen-Hoeksema, 2003). Rumination, as measured by the self-reported Ruminative Response Scale (RRS), was found to have three distinct factors: depression-related, brooding, and reflective (Treynor et al., 2003). Brooding rumination is conceptualized as “a passive comparison of one’s current situation with some unachieved standard,” whereas reflective rumination is “a purposeful turning inwards to engage in cognitive problem-solving to alleviate one’s depressive symptoms” (Treynor et al., 2003, p. 256). Brooding rumination examines current distress from a self-critical evaluative viewpoint (Why do I feel this way?). Reflective rumination examines current distress from a more neutral and distanced perspective (What do I feel?) (Kross, Gard, Deldin, Clifton, & Ayduk, 2012). In sum, reflective, as compared with brooding rumination, is a purposeful, self-distanced cognitive activity aimed at understanding distressing thoughts and emotions in preparation for active coping (see Table 1 for a comparison of types of rumination).
Overall, brooding rumination is consistently associated with negative outcomes, but there is discrepancy in the literature about the effects of reflective rumination. Brooding rumination is distinctly associated with a negative attentional bias in both healthy and depressed individuals (Duque, Sanchez, & Vazquez, 2014; Joormann, Dkane, & Gotlib, 2006; Lo, Ho, & Hollon, 2008; Owens & Gibb, 2017). However, a recent study found that reflective rumination, not brooding, was associated with memory interference when processing emotional stimuli in depressed participants (Joormann, Nee, Berman, Jonides, & Gotlib, 2010). Reflective rumination is associated with increased mindfulness—a purposeful, non-judgmental, present-moment awareness—in healthy participants (Alleva, Roelofs, Voncken, Meevissen, & Alberts, 2014). Moreover, some studies indicate that, when reflective rumination is implemented in combination with non-judgmental awareness, it is no longer associated with negative mood or depressive symptoms in healthy and depressed participants (Brennan, Barnhofer, Crane, Duggan, & Williams, 2015; Rude, Maestas, & Neff, 2007; Watkins & Teasdale, 2004). Indeed, reflective rumination appears to be associated with a more self-distanced perspective of negative emotions that allows for effective emotional processing in participants with varying levels of depressive symptoms (Kross, Ayduk, & Mischel, 2005; Mori & Tanno, 2015). Additionally, reflective rumination is found to be linked with active coping strategies whereas brooding rumination is associated with passive coping across participants with varying levels of depressive symptoms (Burwell & Shirk, 2007; Marroquin, Fontes, Scilletta, & Miranda, 2010).
Brooding rumination is consistently found to be associated with current and future depressive symptoms, but the relationship between reflective rumination and depressive symptoms is unclear. Increased brooding rumination in children was associated with a mother’s past depression status and thus may be linked with a specific genetic heritability factor (Gibb, Grassia, Stone, Uhrlass, & McGeary, 2012; Woody et al., 2016). In adolescents, brooding rumination is predictive of future depressive episodes, especially in girls (Burwell & Shirk, 2007; Cox, Funasaki, Smith, & Mezulis, 2011; Padilla Paredes & Calvete Zumalde, 2015). Also, levels of brooding rumination mediate the relationship between childhood trauma and depressive symptoms (Raes & Hermans, 2008). Reflective rumination has predominantly been found to have either a protective effect or no relationship with future depressive symptoms (Burwell & Shirk, 2007; Cox, Funasaki, Smith, & Mezulis, 2012; Grossmann & Kross, 2010; Hasegawa, Koda, Hattori, Kondo, & Kawaguchi, 2013; Siegle et al., 2004; Treynor et al., 2003). One study found that reflective rumination is predictive of brooding rumination but is not associated with depressive symptoms (Takano & Tanno, 2009).
However, it may be difficult to examine brooding and reflective rumination as distinct processes. A recent study determined profiles of rumination subtypes and found that participants largely had comparable levels of brooding and reflective rumination—only about 25% of the participants had a mismatch in levels of brooding and reflective rumination (e.g., high brooding, low reflective or low brooding, high reflective). Moreover, participants with high levels of both reflective and brooding rumination had the highest severity of depressive symptoms under high stress conditions. However, those participants who reported high reflective rumination and low brooding rumination had the lowest severity of depressive symptoms under high stress conditions (Junkins & Haeffel, 2017). Thus, it is important to consider the combined, as well as distinct, associations of brooding and reflective rumination and depressive symptoms.
Levels of brooding and reflective rumination vary between depressed and healthy participants when measured both behaviorally and neurally using functional magnetic resonance imaging (fMRI). Overall, participants diagnosed with MDD have distinctly higher levels of brooding rumination compared to healthy controls and individuals suffering from other mental disorders. Additionally, although MDD participants have higher reflective rumination, there is less variation in levels of reflective rumination among individuals with MDD, individuals with other mental disorders, and individuals that are healthy (Joormann et al., 2006; Siegle et al., 2004). Recent studies have uncovered intrinsic functional brain differences associated with higher levels of brooding, but not reflective, rumination in depressed and healthy participants (Berman et al., 2011; Vanderhasselt et al., 2013; Vanderhasselt, Kuhn, & De Raedt, 2011; Whiteman & Mangels, 2016). Thus, reflective and brooding rumination may be uniquely related to depressive symptoms and have distinct neural mechanisms regardless of diagnostic status.
An examination of the neural substrates of rumination suggests that two major nodes of the default mode network (DMN), the posterior cingulate cortex (PCC) and medial prefrontal cortex (mPFC), are implicated in rumination (Berman et al., 2011). Both the PCC and mPFC are found to reliably activate during self-referential and negative autobiographical memory tasks in healthy participants (Kross, Davidson, Weber, & Ochsner, 2009; Nejad, Fossati, & Lemogne, 2013; Northoff et al., 2006). Although the mPFC is related to self-referential thinking and making judgments about others, the PCC is associated with episodic memory and first-person perspective-taking processes in healthy and depressed participants (Amodio & Frith, 2006; Cavanna & Trimble, 2006; Uddin, Kelly, Biswal, Castellanos, & Milham, 2009). Compared to HCs, MDDs had increased activation in the mPFC when asked to make negative judgments about themselves (Yoshimura et al., 2010). Additionally, activation of these two regions during an emotional memory task was found to be predictive of changes in depressive symptoms in currently depressed participants (Foland-Ross et al., 2014). However, a meta-analysis of regional cerebral blood flow studies of the DMN in MDD did not indicate any baseline increase in activity in these regions (Hamilton, Farmer, Fogelman, & Gotlib, 2015). Results from resting-state neuroimaging studies of DMN connectivity in depressed participants, measuring intrinsic functional connectivity (FC), indicated increased connectivity of this self-related network (Berman et al., 2011). Additionally, Zhu et al. (2012) identified a link between DMN FC and increased rumination in depressed participants (Zhu et al., 2012). Thus, more research is needed in order to understand the specific regional connections of the major nodes of the DMN that may underlie reflective and brooding rumination in both healthy and depressed individuals.
One possibility suggested by the literature is that the increased FC of the DMN found in rumination in depressed participants may be linked to aberrant FC involving the anterior cingulate cortex (ACC). The ACC, specifically the rostral portion (rACC), is thought to play a role in integrating emotional information from limbic areas with self-referential information in the DMN and, thus, may be important for understanding rumination (Hamilton et al., 2015). Therefore, increased FC between the rACC and DMN in depression could provide a putative neural mechanism for the negative affective content and self-focus of rumination.
Evidence suggests the rACC is involved in both cognitive-affective processes such as negative self-judgments and emotional conflict resolution (de la Vega, Chang, Banich, Wager, & Yarkoni, 2016; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006; Yoshimura et al., 2010). Decreased grey matter volume and FC of the rACC is associated with increased ruminative thinking in healthy participants (Kuhn, Vanderhasselt, De Raedt, & Gallinat, 2012). Specifically, increased activity in the subgenual ACC (sgACC), a portion of the rACC, is related to poor emotion regulation and rumination induction in both healthy and depressed participants (Berman et al., 2011; Drevets, Savitz, & Trimble, 2008). Two recent articles have reported increased FC between the sgACC and the DMN which is thought to be related to feelings of sadness as well as cognitive bias towards negative information in depressed participants (Greicius et al., 2007; Zhu et al., 2012). Other studies have found an increased connectivity between the ACC and the DMN that specifically correlates with increased rumination in depressed participants (Berman et al., 2011; Hamilton, Furman, et al., 2011). Thus, connections between the rACC and nodes of the DMN appear to be vital to understanding ruminative cognition that occurs in depressed individuals, however without consistent findings for healthy individuals.
The amygdala may also be an important node associated with bottom-up maintenance of ruminative processing. The amygdala is a set of nuclei involved in many processes, such as fear learning and memory, and is connected with subcortical (e.g., hippocampus, thalamus) as well as cortical regions (e.g. mPFC) (Davis & Whalen, 2000; Kim et al., 2011). Increased activity in the amygdala in depressed participants is associated with a cognitive bias and memory for negative stimuli (Hamilton & Gotlib, 2008). Also, sustained activity in the amygdala is correlated with rumination in both healthy and depressed individuals, which indicates an overactive emotional response (Mandell, Siegle, Shutt, Feldmiller, & Thase, 2014; Ray et al., 2005; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002). Moreover, when rumination is induced in depressed participants, increased activation is found in the amygdala (Cooney, Joormann, Eugene, Dennis, & Gotlib, 2010). Few studies, however, have examined the specific FC of the amygdala and other regions in rumination in both depressed and healthy participants. We suggest that increased FC between the amygdala and affective integration regions, such as the ACC, may underlie the persistent and uncontrollable nature of rumination and give rise to findings of increased amygdala activation during rumination. The current study aims to investigate this possibility.
Another mechanism of rumination may be decreased inhibition from emotional regulatory regions such as the dorsolateral prefrontal cortex (dlPFC), allowing for dysregulated activity in emotional brain regions. The dlPFC is located in the lateral surface of the left and right frontal lobes and has been associated with executive functioning as well as regulation of negative emotions (Banks, Eddy, Angstadt, Nathan, & Phan, 2007; Erk et al., 2010; Koenigs & Grafman, 2009). For this reason, it has been suggested that reduced inhibition from the dlPFC may be associated with rumination about negative affective states (Ochsner, Silvers, & Buhle, 2012). Grey matter volume in the dlPFC has been linked with rumination in healthy and depressed participants (Wang et al., 2015). Aberrant activity in the dlPFC is proposed to be antecedent to abnormal activity in nodes of the DMN in depressed individuals (Marchetti, Koster, Sonuga-Barke, & De Raedt, 2012). On an emotional Go/No-Go task, healthy individuals who successfully disengaged from negative stimuli had increased activity in the dlPFC, indicating that this region is involved in inhibiting a prepotent response to negative information, which allows for the regulation of negative emotions (Vanderhasselt et al., 2011). However, in healthy and depressed participants, increased FC among prefrontal regions (e.g., mPFC and dlPFC) and among prefrontal and sgACC was discovered during resting-state and thought to relate to increased effortful cognitive control of emotional information (Davey, Harrison, Yucel, & Allen, 2012; Lemogne et al., 2009; Rosenbaum et al., 2016). Hence, there is some discrepancy in the literature about the FC between the dlPFC and emotion brain regions and how this connectivity may be related to rumination in depressed and healthy participants. In resting-state studies, participants are allowed to let their minds wander without focusing on specific tasks; therefore, this difference in brain-state may be contributing to the discrepancy in the literature. Accordingly, more research is needed in order to understand the relationship between FC of the dlPFC and ruminative cognition in individuals with varying levels of depressive symptoms.
Top-down (dlPFC) and bottom-up (amygdala) connections to medial cortical regions of the DMN (mPFC and PCC) and the ACC may play a central role in the functional network that underlies depressive rumination. Indeed, some aspects of FC between the dlPFC, anterior cortical midline regions, and the amygdala are already known to be disrupted in patients with MDD (Nejad et al., 2013; Siegle, Thompson, Carter, Steinhauer, & Thase, 2007). However, findings implicating the involvement of these regions gleaned from rumination studies have not yet been fully integrated in a sample of depressed and healthy participants. Such integration is paramount to understanding the complete picture of reflective and brooding components of rumination across the spectrum of depressive symptoms.
The current study aims to characterize how brain FC in limbic, cingulate, and prefrontal regions is associated with subtypes of rumination in healthy and depressed women. We hope to understand the FC differences associated with rumination in women with and without MDD. Furthermore, we seek to explore FC differences between MDD and HC participants. The literature provides support for increased FC between the rACC and the DMN in rumination in MDD, but the consensus on the prefrontal and limbic FC with these medial regions is less clear. Additionally, few studies have examined FC associated with reflective and brooding rumination in these regions independent of clinical status. In an attempt to integrate some of these diverse findings, we sought to investigate the voxel-wise FC of regions previously found to be related to rumination in either HC or MDD participants. For these analyses, the left and right amygdala, posterior and anterior cingulate, and dorsolateral and medial prefrontal regions served as FC “seeds,” and the average signal of each seed was correlated with every other brain voxel—seed to voxel analyses (S2V). Previous work has examined how the FC between a finite set of a priori seeds relates to rumination, but none have investigated, in a data-driven fashion, all possible functional connections that may underlie the associations previously observed. As rumination, especially brooding rumination, may be a risk-factor for development of future depressive symptoms and episodes in women, focusing on a sample of only women will allow specific examination of the neural underpinnings of the risk factor in this population (Burwell & Shirk, 2007; Lopez, Driscoll, & Kistner, 2009). We hypothesize that increased S2V FC of these selected regions of interest with other regions in the brain will be correlated with increased brooding, but not reflective, rumination in healthy and depressed women. Furthermore, we hypothesize that depressed participants, compared to healthy participants, will show aberrant S2V FC in these same regions of interest.
Methods
Participants
Participants were English-speaking women (age range: 17–65 years) who were recruited from the Chicago area using online and posted flyers. Interested women, with and without depression, were asked to participate in a study approved by the Institutional Review Board (IRB) at Northwestern University Feinberg School of Medicine in the Department of Psychiatry and Behavioral Sciences. All women were initially screened via telephone interviews. Those who were eligible were invited to an on-site visit at the Northwestern University campus in Chicago, IL. During the first visit, eligible participants provided written informed consent, completed clinician-administered and self-reported questionnaires, and participated in two computer-based tasks assessing affect and attention processes. Participants who passed an additional neuroimaging safety screening were invited back for a second visit to complete functional and anatomical neuroimaging scans within one week of the first visit. Participants who completed one or both of the visits were compensated for their time.
Inclusion and exclusion criteria
Included participants were English-speaking, right-handed, females with normal or corrected-to-normal vision. Depressed participants met criteria for current and primary MDD as diagnosed by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) and by a score of at least 24 on the Inventory of Depressive Symptomatology—Clinician Rated (IDS-C) (moderate depression severity) (First, Spitzer, Gibbon, & Williams, 2002; Rush, Carmody, & Reimitz, 2000). Women who were diagnosed with a co-morbid anxiety disorder were included only if MDD was the primary diagnosis. HCs did not meet criteria for any lifetime or current DSM-IV diagnosis and scored less than 11 on the IDS-C (no depression).
Participants were excluded if they met criteria for current or history of another DSM-IV Axis I disorder (bipolar I and II, schizophrenia or delusional disorder, post-traumatic stress disorder, obsessive-compulsive disorder, or substance dependence/abuse) or any Axis II disorder. We did not include individuals with co-morbid Axis II disorders because we hoped to examine the clearest symptomatic presentation of healthy and depressed women. Although this limits the generalizability of this study, functional connectivity differences in key regions, including the DMN, have been found in individuals with Axis II disorders (Chechko et al., 2016; Cullen et al., 2011; Tang et al., 2016; Wolf et al., 2011). Depressed participants were excluded if they reported current treatment or psychotropic medication use in the past two weeks for MDD symptoms or demonstrated current suicide risk. Other exclusion criteria were tobacco use (> 1 pack/week), catecholaminergic antihypertensive mediation use, vasovagal syncope, and a history of head trauma or a neurological disorder. For scanning safety purposes, participants were also excluded if they had metal implants, were claustrophobic, or were currently pregnant or planning on becoming pregnant. Finally, participants were excluded if they did not complete the neuroimaging scan or were found to have unusable neuroimaging data: no resting-state scan, excessive motion (> 2 mm), vision difficulties, and irregular brain findings. The loss of participants with no or usable neuroimaging data (N=49) is a limitation of the current study. However, no demographic or clinical differences between the participants with usable resting-state data and those with no or unusable resting-state data were found (all p’s > .05).
A final sample of participants (N=83; MDD: n=33, HC: n=50) was included in the analyses. The self-reported rumination measure was added to the study after running several participants through the protocol. Thus, a subset of this sample who completed the behavioral rumination measure (N=63; MDD: n=26, HC: n=37) was included in rumination-specific analyses. Two women who completed the self-reported rumination measure were excluded for missing data and a subscale score greater than two standard deviations from the mean (see Fig. 1 for flow of participants included through each phase of the study). Additional clinician-administered and self-report assessments were given as a part of a larger study, but only those relevant to the current study are detailed below.
Measures
Structured clinical interview for DSM-IV-TR Axis I disorders (SCID)
The SCID is a semi-structured clinician-administered interview that assesses for lifetime and current American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders—4th edition (DSM-IV) diagnoses (First et al., 2002). The SCID is an established clinical interview and has moderate to strong inter-rater reliability (κ=0.70–1.00) (First et al., 1995). Research assistants who were graduate students in clinical psychology at Northwestern University were trained in administration of the SCID by reviewing training videos and through supervised practice administration. Fleiss kappa was calculated and the inter-rater reliability for the graduate students was found to be strong (κ=0.82–0.88) (Fleiss, 1971; Fleiss & Cohen, 1973; Randolph, 2016). The SCID was used to diagnose current primary major depressive disorder for MDD and lack of diagnoses for HC.
Inventory of Depressive Symptomatology—Clinician Rated (IDS-C)
The Inventory of Depressive Symptomatology—Clinician Rated (IDS-C) is a 30-item clinician-rated questionnaire that assesses symptoms associated with MDD as detailed by the DSM-IV (American Psychiatric Association, 1994; Rush et al., 2000). Clinicians scored participants’ responses using a 0- to 3-point scale. Then, 28 out of 30 questions were summed, with higher scores indicating more severe and frequent depressive symptoms during the past week. High convergent validity was found between the IDS-C and Beck Depression Inventory and Hamilton Rating Scale for Depression (all r’s>0.85) (Rush, Gullion, Basco, Jarrett, & Trivedi, 1996). MDD participants were required to score greater than or equal to 24 (moderate depression), while HCs scored less than 11 (no depression) on the IDS-C.
Ruminative Response Scale (RRS)
The Ruminative Response Scale is a 22-item self-report questionnaire that assesses frequency of ruminative thoughts and behaviors in response to sad or depressed mood (Treynor et al., 2003). Individuals record their responses on a 1- to 4-point scale, with higher scores indicating generally more frequent ruminative thoughts and behaviors. Items are summed to create subscales for divergent types of rumination: depression-related (12 items), brooding (five items), and reflective (five items). The RRS has been found to be a valid and reliable measure of rumination in healthy and depressed individuals (Luminet, 2004). For the current study, only the brooding and reflective ruminative subscales were utilized. As the items in the depression-related subscale are highly correlated with items in a depression severity measure (Beck Depression Inventory) (Treynor et al., 2003) and because we hoped to understand FC associated with the unique construct of rumination independent of clinical status, this subscale was not examined.
Statistical analyses
Demographic and clinical differences between the total sample, and subset, of HC and MDD participants were examined using chi-squared and independent sample t-tests (M, χ2, t, p (at 0.05 level)).
Imaging protocol and preprocessing
Anatomical and functional neuroimaging data were acquired using a 3-Tesla Tim Trio scanner (Siemens) with a 32-channel head coil. Foam inserts and earplugs were implemented to reduce head movement and to decrease scanner noise. Whole-brain data were obtained along the anterior-posterior commissure. High-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) anatomical images were acquired during a 9 min 50 s scan sequence (repetition time (TR)=2,200 ms, echo time (TE)=2.91 ms, flip angle=9°, field of view (FOV)=256 × 256 mms, voxel dimension=1 mm isotropic). Resting-state functional images were acquired during a 10-min echo planar imaging (EPI) sequence (TR=2,000 ms, TE=2.00 ms, flip angle=9°, FOV=220 mm, resolution=1.7 × 1.7 × 3 mm, slice thickness=3 mm, 35 slices, 300 volumes). Participants were asked to lie still with their eyes open and to fixate on a computer screen during the resting-state scan.
Preprocessing of anatomical and functional data was conducted using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK). Functional images were reoriented to the Anterior and Posterior Commissures, slice timing corrected, realigned, and normalized. Each participant’s functional data was co-registered to their anatomical image and then normalized to the MNI152 template using the VBM toolbox (Gaser, 2010). Then functional data was resampled to 2-mm isotropic voxels and spatially smoothed with a 6-mm full-width half-maximum (FWHM) Gaussian kernel.
Functional connectivity analyses
Seed creation
Six seeds were chosen from previous resting-state fMRI literature and/or from the Harvard-Oxford cortical and subcortical atlases (Desikan et al., 2006; Fox et al., 2005; Hamilton, Chen, Thomason, Schwartz, & Gotlib, 2011; Makris et al., 2006) (see Fig. 2). The left dlPFC and mPFC seeds were chosen from a resting-state study of region-specific differences between healthy and depressed participants (dlPFC, MNI: –31, 46, 23; mPFC, MNI: –1, 47, –4) (Fox et al., 2005; Hamilton, Chen, et al., 2011). Peak coordinates from each region were used to draw spherical seeds (6-mm radius) in the FMRIB Software Library (FSL) using the MNI152 template (average152T1.nii.gz) (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). Left and right amygdala and anterior and posterior cingulate cortex seeds were obtained from the Harvard-Oxford cortical and subcortical atlases.
Denoising
Denoising and FC analyses were implemented using the functional connectivity toolbox (CONN) (v. 15.h) (Whitfield-Gabrieli & Nieto-Castanon, 2012). Functional denoising, or nuisance regression, in CONN involves a component-based noise correction method (aCompCorr) to estimate sources of noise in the BOLD signal (Behzadi, Restom, Liau, & Liu, 2007). Eroded tissue masks were created for each participant’s cerebrospinal fluid (CSF) and white matter (WM) using the anatomical data. The first five principal components of CSF and WM signals were extracted from these masks. Then, effects of nuisance variables (the effect of rest, a linear detrending term, CSF and WM principal components, and the six affine motion parameters with first temporal derivatives (Power et al., 2014) were regressed out of the functional data. Finally, bandpass filtering (0.008–0.1 Hz) was applied to the data after regression (Hallquist, Hwang, & Luna, 2013). No global signal regression was used, as this procedure may introduce spurious anti-correlations in FC analyses (Chai, Castanon, Ongur, & Whitfield-Gabrieli, 2012).
Statistical analyses
We chose to use seed-to-voxel (S2V) analyses to highlight regions associated with rumination but also to allow for a more data-driven approach and inclusion of other regions that may be distinctly related to subtypes of rumination. This approach is a commonly employed technique and is a standard analysis technique in CONN (Rogers, Morgan, Newton, & Gore, 2007; Whitfield-Gabrieli & Nieto-Castanon, 2012). We did not choose to employ ROI-ROI analyses, as this technique would increase the number of multiple comparisons that would need to be controlled for in the analyses.
Functional connectivity was estimated by calculating Fisher’s z-transformed Pearson’s correlation coefficients (z) between the time series of seed and individual voxels. These scores were then entered as criterion variables into three general linear model analyses with different predictors: (1) examining the main effect of each type of rumination (reflective and brooding), (2) including the contrast between the two rumination types (reflective > brooding), and (3) utilizing the contrast between diagnostic groups (MDD > HC). Clusters of voxels wherein S2V was significantly predicted by a given predictor were further examined (height threshold p < .001, cluster threshold p-FWE < .05). T-statistics and family-wise error corrected p-values are reported (p-FWE) (Bennett, Wolford, & Miller, 2009). Finally, post-hoc correlation and regression analyses were employed to more fully examine the relationship between FC by rumination type and diagnosis group (r, t, and p-values reported). As these post hoc analyses were single statistical tests, no correction was made to the p-values.
Results
Participant characteristics
Demographic and clinical variables for the entire sample (N = 83) were compared across diagnoses. MDD and HC groups were compared and found to be similar in age, race, education, and marital status (all p’s > .05; see Tables 2 and 3). The MDD group had an increased proportion of Hispanic or Latino participants compared to the HC group (χ2(1)=7.27, p = .007). There were nine individuals who were diagnosed with MDD and a co-morbid anxiety disorder (MDD only n=24; MDD + anxiety n=9). The anxiety disorders included social phobia, agoraphobia, specific phobia, generalized anxiety disorder, and anxiety not otherwise specified. About half of the participants with MDD were experiencing their first-episodes, while the other half had recurrent MDD with a range of two to “too many to count” previous episodes (first-episode MDD n=17; recurrent MDD n=16). Depression severity was found to be significantly greater in the MDD compared to the HC group (t(42.46) =–25.76, p <.001) (see Table 3).
The subset of depressed and HC participants who completed the behavioral rumination measure (N = 63) were comparable in age, race, education, and marital status (all p’s > .05) (see Tables 2 and 3). The MDD group had an increased proportion of Hispanic or Latino participants compared to the HC group (χ2(1)=7.36, p = .007). Post hoc independent samples t-tests comparing Hispanic and Latino with non-Hispanic participants revealed no differences in level of brooding or reflective rumination between the groups (all p’s > .05). Depression severity, brooding rumination, and reflective rumination were all found to be significantly greater in the MDD compared to the HC group (IDS-C t(32.10)=–23.63, p < .001; RRS Brooding t(62)=–6.81, p < .001; RRS Reflective t(62)=–6.25, p < .001) (see Table 3).
Seed-to-voxel (S2V) results
Initially, participants, collapsed across diagnostic group, who completed the behavioral measure of rumination (N=63; HC: n=37, MDD: n=26) were examined for S2V differences related to dimensions of rumination.
Main effect of brooding and reflective rumination
Brooding rumination was associated with decreased FC between the left amygdala and the right temporal pole (MNI: [60, 14, –04], t(61)=–4.53, p-FWE = .002) (see Table 4, Fig. 3). Reflective rumination was associated with increased FC between the mPFC and the posterior cingulate (MNI: [–08, –48, 24], t(61)=4.24, p-FWE = .012). Moreover, reflective rumination was associated with decreased FC between the PCC and the anterior cingulate (MIN: [08, 20, 28], t(61) =–4.72, p-FWE = .049) as well as mPFC and left (MIN: [–28, –92, 32], t(61)= –4.47, p-FWE = .003) and right (MNI: [24, –102, 08], t(61)=–4.09, p-FWE = .033) occipital poles (see Table 4, Fig. 4). No other significant results were found for the other seeds for either brooding or reflective rumination (all p-FWE’s > .05).
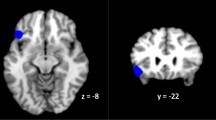
(a) FC between left amygdala and right temporal pole (rTP) associated with brooding rumination (blue connection indicates a negative correlation between rumination and FC). (b) Scatterplot of correlation of brooding rumination (RRS Brooding) and FC between left amygdala and rTP in healthy and depressed participants. Note. This graphic was created using the NeuroViz3D Toolbox (Layden & Berman)
(a) FC between posterior cingulate cortex (PCC) and anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) and PCC and left (lOP) and right occipital pole (rOP) associated with reflective rumination (blue connection indicates a negative correlation between rumination and FC, red connection indicates a positive correlation between rumination and FC). (b) Scatterplot of correlation of reflective rumination (RRS Reflective) and FC between PCC and ACC in healthy and depressed participants. (c) Scatterplot of correlation of reflective rumination (RRS Reflective) and FC between mPFC and PCC in healthy and depressed participants. (d) Scatterplot of correlation of reflective rumination (RRS Reflective) and FC between mPFC and lOP in healthy and depressed participants. (e) Scatterplot of correlation of reflective rumination (RRS Reflective) and FC between mPFC and rOP in healthy and depressed participants. Note. This graphic was created using the NeuroViz3D Toolbox (Layden & Berman)
Reflective > brooding rumination
In order to investigate whether any S2V features were specific to either brooding or reflective rumination, we examined clusters of S2V that were more related to reflective rumination than to brooding rumination. As these two subscales are highly correlated, this analysis was able to tease out any unique contributions of each subtype of rumination. There was a significant main effect for reflective rumination compared to brooding rumination for FC between the ACC seed and a region in the right supramarginal gyrus (rSMG) (MNI: [62, –34, 48], t(60)=5.48, p-FWE = .029). This indicates that there was a stronger association between ACC—rSMG FC and reflective versus brooding rumination (see Table 4, Fig. 5).
(a) FC between anterior cingulate cortex (ACC) and right supramarginal gyrus (rSMG) more strongly associated with reflective, as compared with brooding, rumination (red connection indicates a stronger correlation of reflective rumination and FC). (b) Scatterplot of correlation of reflective rumination (RRS Reflective) and FC between ACC and rSMG in healthy and depressed participants. (c) Scatterplot of correlation of brooding rumination (RRS Brooding) and FC between ACC and rSMG in healthy and depressed participants. Note. This graphic was created using the NeuroViz3D Toolbox (Layden & Berman)
Post hoc analyses further explicated how ACC to rSMG FC was related to both rumination type and diagnosis group (HC versus MDD). First, we performed a correlation analysis of each rumination type and ACC–rSMG FC. We found a significant positive relationship between reflective rumination and FC but not between brooding rumination and FC (Reflective: r=.429, p < .001; Brooding: r = .032, p = .802). We then performed regression analyses to examine if the relationship between ACC–rSMG FC was still significant when diagnosis group was included in the model. For both reflective and brooding rumination, there were no significant differences in the regression model when diagnosis was added as a categorical covariate (p’s > .05).
Next, the entire sample of participants was analyzed (N=83) to compare overall S2V differences between the two groups (MDD: n=33, HC: n=50).
MDD > HC
A significant connection was found between the PCC seed and a region in the right middle frontal gyrus (rMFG) such that depressed participants had decreased FC between these two regions (MNI: [32, 32, 30], t(81)=–4.49, p-FEW = .030). This indicates that depressed participants had decreased functional coupling between these two regions compared to HCs (see Table 4, Fig. 6). This result remained significant when the subset of participants who completed the rumination measure was used (N=63).
Discussion
This study characterized S2V functional connectivity related to reflective and brooding rumination in a sample of healthy and depressed women. Connectivity between six seed regions in limbic, cingulate, and medial and dorsolateral prefrontal regions was examined: left amygdala, right amygdala, ACC, PCC, medial PFC, and left dlPFC. Decreased connectivity between the left amygdala and the right temporal pole was associated with increased brooding rumination. Also, increased connectivity between the mPFC and the PCC was associated with increased reflective rumination. Moreover, decreased connectivity between the PCC and the ACC as well as between the mPFC and the occipital pole was associated with increased reflective rumination. When directly comparing rumination type, increased connectivity between the ACC and the right supramarginal gyrus was found to be uniquely associated with increased reflective rumination. This relationship did not when clinical status was included as a covariate. No other significant FC differences were found when comparing reflective and brooding rumination in this sample of healthy and depressed women. Between diagnoses, depressed participants were found to have decreased FC between the PCC and a region in the right middle frontal gyrus. As this study did not implement global signal regression that may help improve the specificity of positive correlations, there may some additional results for the other seeds that were not detected using our analysis method (Murphy & Fox, 2017). However, we chose not to implement this method due to the potential of detection of spurious anti-correlations (Chai et al., 2012). This study provides unique analyses of FC associated with subtypes of rumination across healthy and depressed participants, independent of clinical status, which is in line with the National Institutes of Health’s recommendation of the examination of rumination on a continuum within the negative valence system (National Institues of Health, 2011).
The result of decreased FC between the left amygdala and the right temporal pole associated with increased brooding rumination contributes to research on brain regions that may be associated with self-critical and threatening interpretations of emotional stimuli. The temporal pole has previously been linked with mentalizing and memory processes (Li et al., 2016). Previous work has found increased FC between these two regions in participants with generalized anxiety disorder and has proposed that this connection underlies integration of emotion regulation with metalizing (Li et al., 2016). Additionally, both the amygdala and temporal pole have previously been associated with rumination in depressed participants (A. B. Nejad et al., 2013). Individuals with high levels of neuroticism have been found to have decreased connectivity between the amygdala and temporal pole (Aghajani et al., 2014; Madsen et al., 2016). Neuroticism has previously been found to be associated with depressive symptoms and mediated by brooding rumination in MDD (Merino, Senra, & Ferreiro, 2016). Consequently, the decreased connectivity found in the current study may be associated with the negative and self-critical agitation that is associated with brooding rumination. However, future research should continue to clarify the role of functional connections between the amygdala and temporal pole in rumination.
The result of decreased FC between the PCC and a region in the dorsal ACC (dACC) associated with increased reflective rumination contributes to research on brain regions associated with cognitive-affective processes. The dorsal portion of the posterior ACC (dACC) is associated with incorporating affective into cognitive control (de la Vega et al., 2016; F. Wang et al., 2016). The PCC has many functions, but it is most associated with retrieving autobiographical memories as well as switching attention between internal and external stimuli (Leech & Sharp, 2014). Increased FC between the PCC and sgACC has been linked with MDD diagnosis and brooding rumination, though not reflective (Berman et al., 2011). Thus, our result of decreased connectivity between the dACC and the PCC and increased reflective rumination provide novel information about FC between these two regions and rumination. We hypothesize our findings could point to the process of internal attention comparing an individual’s current distress with memories with the goal of alleviating current distress that is found in reflective rumination.
The correlation between reflective rumination and FC of the medial prefrontal region (mPFC) adds to research examining self-related thinking. The result of a positive association between reflective rumination and the FC between the mPFC and PCC likely relates to the function of the DMN in internal self-related cognition. These two regions are the main nodes of the DMN, so our result adds to the extensive literature on the role of the DMN in self-focused thinking, social cognition, and episodic memory (Davey, Pujol, & Harrison, 2016; Moran, Kelley, & Heatherton, 2013; A. B. Nejad et al., 2013; Northoff et al., 2006). The result of the association between reflective rumination and decreased connectivity between the mPFC and left and right occipital cortices is a novel finding. The regions we found represent the left and right BA 19 visual association areas. Some studies have found aberant FC of the left and right BA19 regions in depressed compared with healthy participants (Liu et al., 2014; Sheline, Price, Yan, & Mintun, 2010), but there is very little evidence to link FC in the occipital pole regions with rumination. Of note, one study did find increased activity in the middle occipital region when participants performed a rumination task (Cooney et al., 2010). Therefore, more research is needed to understand the link between FC between mPFC and occipital areas associated with reflective rumination.
The positive correlation between reflective rumination and FC between the ACC and right supramarginal gyrus (rSMG) adds to the literature examining the neural basis of self/other distinctions. The rSMG comprises part of the anterior aspect of the right temporal parietal junction, an area that has now been found to be composed of two distinct sub-regions (Bzdok et al., 2013; Mars et al., 2012). This anterior portion has been found to be functionally connected to areas related to affective regulation, such as the medial and anterior cingulate (M/ACC) and insula in healthy participants (Lamm, Decety, & Singer, 2011; Vogt, 2005). The ACC has been found to play a role in self/other distinctions in empathy and vicarious experience of pain and reward in healthy individuals (de la Vega et al., 2016; Jackson, Brunet, Meltzoff, & Decety, 2006; Lockwood, 2016).
Furthermore, activity in the rSMG has previously been related to awareness of an individual’s internal states and an ability to overcome an emotional egocentricity bias (EEB) when making empathic judgments in healthy individuals (Nejad et al., 2015; Silani, Lamm, Ruff, & Singer, 2013; Steinbeis, Bernhardt, & Singer, 2015). EEB is defined as the tendency to project our own emotional perspective when interpreting and making decisions about another individual’s emotional state. Transcranial magnetic stimulation (TMS) of the rSMG, to disrupt the functioning of the region, has been shown to increase an individual’s egocentricity bias (Silani et al., 2013). Interestingly, the rSMG cluster identified in our study was very close to the location found in these studies, which examined EEB (MNI: [68, –38, 36]). Thus, our finding that reflective rumination, as compared to brooding rumination, is associated with increased FC between the ACC and rMFG may be related to a process of examining negative thoughts and emotions about the self from a more neutral or “other” viewpoint. This pattern of increased FC associated with increased reflective rumination did not significantly differ when diagnostic status was considered, so it is not simply a maladaptive process specific to depressive rumination. Notably, the process of reflective rumination is conceptualized as a more affectively neutral process as compared to the negative affective content of brooding rumination. Brooding rumination may cause an individual to get “caught up” in his or her own negative mood, which only serves to perpetuate the negative mood state and leads to passive coping strategies (Lyubomirsky & Nolen-Hoeksema, 1993, 1995). This study is the first to find a clear connection between FC and levels of reflective rumination independent of clinical status. As this study was conducted using resting-state FC, future studies may consider using a rumination induction task to assess fMRI activity associated with these regions.
The result of decreased FC between the PCC and the right middle frontal gyrus (rMFG) in depressed participants contributes to the work examining frontal executive control regions’ interactions with the DMN. The rMFG (BA9) is involved in executive functioning tasks, such as inhibition and working memory, and is a part of the frontoparietal control network (FPN) (Niendam et al., 2012). Previous meta-analytic research has found aberrant associations between regions of the DMN and the FPN in depressive disorders (Chen, Wang, Xueling, Tan, & Zhong, 2015; Kaiser, Andrews-Hanna, Wager, & Pizzagalli, 2015). The hypoconnectivity found in our study thus may represent depressed participants’ difficulty in inhibiting self-focused cognitions and engage in goal-directed behaviors. Interestingly, one study found increased connectivity between the PCC and the rMFG in remitted depressed adolescents that was inversely correlated with rumination (Jacobs et al., 2014). Thus, the hyperactivity in remitted depressed participants may be an adaptive mechanism that counteracts the decreased connectivity found in currently depressed individuals. Future studies should continue to examine how connectivity between the FPN and the DMN is related to rumination in individuals with varying levels of depressive symptoms.
There are a few limitations of the current study, including the female-only sample without co-morbid Axis II disorders, the trait measure of rumination, and the non-participant specific seed masks utilized in the FC analyses. Male and female brains have been found to be anatomically and functionally different (Zagni, Simoni, & Colombo, 2016). However, there is no consensus in the literature about FC differences between women and men (Hjelmervik, Hausmann, Osnes, Westerhausen, & Specht, 2014; Kilpatrick, Zald, Pardo, & Cahill, 2006; Weissman-Fogel, Moayedi, Taylor, Pope, & Davis, 2010). Also, previous studies have found FC differences in regions, such as the DMN and amygdala, in individuals with Axis II disorders (Cullen et al., 2011; Tang et al., 2016; Wolf et al., 2011). Thus, as we hoped to recruit the cleanest possible sample of healthy and depressed women, it is unclear to what extent our findings might generalize to males and individuals with co-morbid Axis II disorders. Future investigations may be warranted to investigate these questions. Notably, the use of only women in this study of rumination was justified by the increased rates of rumination in women—particularly brooding rumination—as well as the gender differences in vulnerability for depressive disorders.
The Ruminative Response Scale is a trait measure of ruminative thinking and behavior. During the fMRI scan, participants were asked to focus their gazes on a fixation point and to not think about anything in particular. Thus, it cannot be determined if the participants in the current study were ruminating during the resting-state scan. Recent research indicates that only some depressed individuals report ruminating during resting-state scans. However, both state and trait measures of rumination have been found to be associated with functional connectivity (Kaiser et al., 2016; Rosenbaum et al., 2017). Accordingly, future studies should consider implementing a state measure of rumination assessing cognition during the resting-state scan to provide a link between the ruminative state and neural underpinnings during functional connectivity scans. The results of this study add to the literature about the connection between intrinsic brain functioning and trait brooding and reflective rumination.
The current study’s use of non-participant-specific seed masks in the FC analyses may also be a limitation. The use of specific seeds for FC analyses in this study was implemented to reduce incorrect rejection of the null hypothesis (type I error) associated with whole brain analyses as well as to investigate connectivity between regions previously found to relate to rumination (Poldrack, 2007). However, the use of atlas-based seeds delineations has been shown to decrease sensitivity and specificity of FC analyses, and the use of participant-specific seeds is sometimes advised to address these issues (Huang et al., 2016; Nieto-Castanon & Fedorenko, 2012). However, the creation of participant-specific seeds requires either a task-based functional localizer (fMRI) or clear anatomical boundaries delineated by an expert. Such approaches may also require volume correction among participants and may reduce the generalizability of findings. Thus, future studies examining FC related to reflective and brooding rumination may consider creating participant-specific masks for regions of interest, but this strategy was not adopted here for the reasons outlined.
This study contributes to our growing understanding of the neural mechanisms underlying reflective and brooding rumination in healthy and depressed women. The strengths include the use of an unmedicated depressed and healthy sample that was recruited from the community. Brooding rumination was found to be associated with left amygdala and temporal pole functional connectivity. Reflective rumination was found to be associated with PCC and dACC FC as well as the mPFC and PCC and occipital pole FC. Additionally, we identified a pattern of stronger FC between the ACC and rSMG, which was uniquely associated with increased reflective rumination. Notably, this association did not significantly differ when diagnostic status was included as a covariate. Also, MDD participants had decreased FC between the PCC and the rMFG compared to HC participants. This study contributes to the current literature examining limbic, cingulate, and medial and dorsolateral prefrontal regions as they relate to subtypes of rumination in healthy and depressed women. Future studies may consider recruiting a sample of healthy and depressed male and female participants, using a state measure of reflective and brooding rumination, or implementing participant-specific seeds to further examine FC differences that may contribute to these processes. Our hope is that the current study informs and provides some groundwork for such studies.
References
Abela, J. R., & Hankin, B. L. (2011). Rumination as a vulnerability factor to depression during the transition from early to middle adolescence: A multiwave longitudinal study. Journal of Abnormal Psychology, 120(2), 259–271. https://doi.org/10.1037/a0022796
Aghajani, M., Veer, I. M., van Tol, M. J., Aleman, A., van Buchem, M. A., Veltman, D. J., … van der Wee, N. J. (2014). Neuroticism and extraversion are associated with amygdala resting-state functional connectivity. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 836–848. https://doi.org/10.3758/s13415-013-0224-0
Alleva, J., Roelofs, J., Voncken, M., Meevissen, Y., & Alberts, H. (2014). On the relation between mindfulness and depressive symptoms: Rumination as a possible mediator. Mindfulness, 5, 72–79.
American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (DSM-IV) (4 ed.). Washington DC, American Psychiatric Association.
Amodio, D. M., & Frith, C. D. (2006). Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews. Neuroscience, 7(4), 268–277. https://doi.org/10.1038/nrn1884
Banks, S. J., Eddy, K. T., Angstadt, M., Nathan, P. J., & Phan, K. L. (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–312. https://doi.org/10.1093/scan/nsm029
Behzadi, Y., Restom, K., Liau, J., & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. https://doi.org/10.1016/j.neuroimage.2007.04.042
Bennett, C. M., Wolford, G. L., & Miller, M. B. (2009). The principled control of false positives in neuroimaging. Social Cognitive and Affective Neuroscience, 4(4), 417–422. https://doi.org/10.1093/scan/nsp053
Berman, M. G., Peltier, S., Nee, D. E., Kross, E., Deldin, P. J., & Jonides, J. (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6(5), 548–555. https://doi.org/10.1093/scan/nsq080
Brennan, K., Barnhofer, T., Crane, C., Duggan, D., & Williams, J. M. (2015). Memory specificity and mindfulness jointly moderate the effect of reflective pondering on depressive symptoms in individuals with a history of recurrent depression. Journal of Abnormal Psychology, 124(2), 246–255. https://doi.org/10.1037/abn0000027
Burwell, R. A., & Shirk, S. R. (2007). Subtypes of rumination in adolescence: Associations between brooding, reflection, depressive symptoms, and coping. Journal of Clinical Child and Adolescent Psychology, 36(1), 56–65. https://doi.org/10.1080/15374410709336568
Bzdok, D., Langner, R., Schilbach, L., Jakobs, O., Roski, C., Caspers, S., … Eickhoff, S. B. (2013). Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. NeuroImage, 81, 381–392. https://doi.org/10.1016/j.neuroimage.2013.05.046
Cavanna, A. E., & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564–583. https://doi.org/10.1093/brain/awl004
Chai, X. J., Castanon, A. N., Ongur, D., & Whitfield-Gabrieli, S. (2012). Anticorrelations in resting state networks without global signal regression. NeuroImage, 59(2), 1420–1428. https://doi.org/10.1016/j.neuroimage.2011.08.048
Chechko, N., Kellermann, T., Augustin, M., Zvyagintsev, M., Schneider, F., & Habel, U. (2016). Disorder-specific characteristics of borderline personality disorder with co-occurring depression and its comparison with major depression: An fMRI study with emotional interference task. NeuroImage: Clinical, 12, 517–525. https://doi.org/10.1016/j.nicl.2016.08.015
Chen, Y., Wang, C., Xueling, Z., Tan, Y., & Zhong, Y. (2015). Aberrant connectivity within the default mode network in first-episode, treatment-naive major depressive disorder. Journal of Affective Disorders, 183, 49–56.
Cooney, R. E., Joormann, J., Eugene, F., Dennis, E. L., & Gotlib, I. H. (2010). Neural correlates of rumination in depression. Cognitive, Affective, & Behavioral Neuroscience, 10(4), 470–478. https://doi.org/10.3758/CABN.10.4.470
Cox, S., Funasaki, K., Smith, L., & Mezulis, A. H. (2011). A prospective study of brooding and reflection as moderators of the relationship between stress and depressive symptoms in adolescence. Cognitive Therapy and Research, 36(4), 290–299.
Cox, S., Funasaki, K., Smith, L., & Mezulis, A. H. (2012). A prospective study of brooding and reflection as moderators of the relationship between stress and depressive symptoms in adolescence. Cognitive Therapy and Research, 36(4), 290–299.
Cullen, K. R., Vizueta, N., Thomas, K. M., Han, G. J., Lim, K. O., Camchong, J., … Schulz, S. C. (2011). Amygdala functional connectivity in young women with borderline personality disorder. Brain Connectivity, 1(1), 61–71. https://doi.org/10.1089/brain.2010.0001
Davey, C. G., Harrison, B. J., Yucel, M., & Allen, N. B. (2012). Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychological Medicine, 42(10), 2071–2081.
Davey, C. G., Pujol, J., & Harrison, B. J. (2016). Mapping the self in the brain's default mode network. NeuroImage, 132, 390–397. https://doi.org/10.1016/j.neuroimage.2016.02.022
Davis, M., & Whalen, P. J. (2000). The amygdala: Vigilence and emotion. Molecular Psychiatry, 6(13–34).
de la Vega, A., Chang, L. J., Banich, M. T., Wager, T. D., & Yarkoni, T. (2016). Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. The Journal of Neuroscience, 36(24), 6553–6562. https://doi.org/10.1523/JNEUROSCI.4402-15.2016
Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., …, Kiliany, R. J. (2006). An automated labeling system for subdividing the human cerebral coretex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980.
Drevets, W. C., Savitz, J., & Trimble, M. (2008). The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums, 13(8), 663–681.
Duque, A., Sanchez, A., & Vazquez, C. (2014). Gaze-fixation and pupil dilation in the processing of emotional faces: The role of rumination. Cognition and Emotion, 28(8), 1347–1366. https://doi.org/10.1080/02699931.2014.881327
Erk, S., Mikschl, A., Stier, S., Ciaramidaro, A., Gapp, V., Weber, B., & Walter, H. (2010). Acute and sustained effects of cognitive emotion regulation in major depression. The Journal of Neuroscience, 30(47), 15726–15734. https://doi.org/10.1523/JNEUROSCI.1856-10.2010
Etkin, A., Egner, T., Peraza, D. M., Kandel, E. R., & Hirsch, J. (2006). Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51(6), 871–882. https://doi.org/10.1016/j.neuron.2006.07.029
First, M., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (2002). Structured Clinical Interview for Dsm-IV-TR Axis I Disorders, Research Version (SCID-I/P). Biometrics Research, New York State Psychiatric Institute: New York.
First, M., Spitzer, R. L., Gibbon, M., Williams, J. B. W., Davies, J., Borus, J., … Rounsaville, B. (1995). The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II). Part II: Multi-site test-retest reliability study. Journal of Personality Disorders, 9(2), 92–104.
Fleiss, J. L. (1971). Measuring nominal scale agreement among many raters. Psychological Bulletin, 76(5), 378–382.
Fleiss, J. L., & Cohen, J. (1973). The equivalence of wieghted kappa and the intraclass correlation coefficient as measures of reliability. Educational and Psycholgical Measurement, 33, 613–619.
Foland-Ross, L. C., Hamilton, P., Sacchet, M. D., Furman, D. J., Sherdell, L., & Gotlib, I. H. (2014). Activation of the medial prefrontal and posterior cingulate cortex during encoding of negative material predicts symptom worsening in major depression. NeuroReport, 25(5), 324–329. https://doi.org/10.1097/WNR.0000000000000095
Fox, M. D., Snyder, A. Z., Vincent, J. L., Corbetta, M., Van Essen, D. C., & Raichle, M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. https://doi.org/10.1073/pnas.0504136102
Gaser, C. (2010). VBM8 Toolbox.
Gibb, B. E., Grassia, M., Stone, L. B., Uhrlass, D. J., & McGeary, J. E. (2012). Brooding rumination and risk for depressive disorders in children of depressed mothers. Journal of Abnormal Child Psychology, 40(2), 317–326. https://doi.org/10.1007/s10802-011-9554-y
Greicius, M. D., Flores, B. H., Menon, V., Glover, G. H., Solvason, H. B., Kenna, H., … Schatzberg, A. F. (2007). Resting-state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry, 62(5), 429–437. https://doi.org/10.1016/j.biopsych.2006.09.020
Grossmann, I., & Kross, E. (2010). The impact of culture on adaptive versus maladaptive self-reflection. Psychological Science, 21(8), 1150–1157. https://doi.org/10.1177/0956797610376655
Hallquist, M. N., Hwang, K., & Luna, B. (2013). The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage, 82, 208–225. https://doi.org/10.1016/j.neuroimage.2013.05.116
Hamilton, J. P., Chen, G., Thomason, M. E., Schwartz, M. E., & Gotlib, I. H. (2011). Investigating neural primacy in major depressive disorder: Multivariate Granger causality analysis of resting-state fMRI time-series data. Molecular Psychiatry, 16(7), 763–772. https://doi.org/10.1038/mp.2010.46
Hamilton, J. P., Farmer, M., Fogelman, P., & Gotlib, I. H. (2015). Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biological Psychiatry, 78(4), 224–230. https://doi.org/10.1016/j.biopsych.2015.02.020
Hamilton, J. P., Furman, D. J., Chang, C., Thomason, M. E., Dennis, E., & Gotlib, I. H. (2011). Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry, 70(4), 327–333. https://doi.org/10.1016/j.biopsych.2011.02.003
Hamilton, J. P., & Gotlib, I. H. (2008). Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biological Psychiatry, 63(12), 1155–1162. https://doi.org/10.1016/j.biopsych.2007.12.015
Hasegawa, A., Koda, M., Hattori, Y., Kondo, T., & Kawaguchi, J. (2013). Longitudinal predictions of the Brooding and Reflection subscales of the Japanese Ruminative Responses Scale for depression. Psychological Reports, 113(2), 566–585. https://doi.org/10.2466/02.15.PR0.113x24z5
Hjelmervik, H., Hausmann, M., Osnes, B., Westerhausen, R., & Specht, K. (2014). Resting states are resting traits—An FMRI study of sex differences and menstrual cycle effects in resting state cognitive control networks. PLoS ONE, 9(7), e103492. https://doi.org/10.1371/journal.pone.0103492
Huang, L., Zhou, G., Liu, Z., Dang, X., Yang, Z., Kong, X. Z., … Liu, J. (2016). A multi-atlas labeling approach for identifying subject-specific functional regions of interest. PLoS ONE, 11(1), e0146868. https://doi.org/10.1371/journal.pone.0146868
Jackson, P. L., Brunet, E., Meltzoff, A. N., & Decety, J. (2006). Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia, 44(5), 752–761. https://doi.org/10.1016/j.neuropsychologia.2005.07.015
Jacobs, R. H., Jenkins, L. M., Gabriel, L. B., Barba, A., Ryan, K. A., Weisenbach, S. L., … Welsh, R. C. (2014). Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS ONE, 9(8), e104366. https://doi.org/10.1371/journal.pone.0104366
Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). Fsl. NeuroImage, 62(2), 782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Johnson, D. P., & Whisman, M. A. (2013). Gender differences in rumination: A meta-analysis. Personality and Individual Differences, 55(4), 367–374. https://doi.org/10.1016/j.paid.2013.03.019
Jones, N. P., Siegle, G. J., & Thase, M. E. (2008). Effects of rumination and initial severity on remission to cognitive therapy for depression. Cognitive Therapy and Research, 32(4). https://doi.org/10.1007/s10608-008-9191-0
Joormann, J., Dkane, M., & Gotlib, I. H. (2006). Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavior Therapy, 37(3), 269–280. https://doi.org/10.1016/j.beth.2006.01.002
Joormann, J., Nee, D. E., Berman, M. G., Jonides, J., & Gotlib, I. H. (2010). Interference resolution in major depression. Cognitive, Affective, & Behavioral Neuroscience, 10(1), 21–33. https://doi.org/10.3758/CABN.10.1.21
Junkins, M. B., & Haeffel, G. J. (2017). Rumination: Reflection can amplify the depressogenic effects of brooding. International Journal of Cognitive Therapy, 10(1), 34–46.
Kaiser, R. H., Andrews-Hanna, J. R., Wager, T. D., & Pizzagalli, D. A. (2015). Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry, 72(6), 603–611. https://doi.org/10.1001/jamapsychiatry.2015.0071
Kaiser, R. H., Whitfield-Gabrieli, S., Dillon, D. G., Goer, F., Beltzer, M., Minkel, J., … Pizzagalli, D. A. (2016). Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology, 41(7), 1822–1830. https://doi.org/10.1038/npp.2015.352
Kilpatrick, L. A., Zald, D. H., Pardo, J. V., & Cahill, L. F. (2006). Sex-related differences in amygdala functional connectivity during resting conditions. NeuroImage, 30(2), 452–461. https://doi.org/10.1016/j.neuroimage.2005.09.065
Kim, M. J., Loucks, R. A., Palmer, A. L., Brown, A. C., Solomon, K. M., Marchante, A. N., & Whalen, P. J. (2011). The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research, 223(2), 403–410. https://doi.org/10.1016/j.bbr.2011.04.025
Koenigs, M., & Grafman, J. (2009). The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research, 201(2), 239–243. https://doi.org/10.1016/j.bbr.2009.03.004
Kross, E., Ayduk, O., & Mischel, W. (2005). When asking “why” does not hurt. Distinguishing rumination from reflective processing of negative emotions. Psychological Science, 16(9), 709–715.
Kross, E., Davidson, M., Weber, J., & Ochsner, K. (2009). Coping with emotions past: The neural bases of regulating affect associated with negative autobiographical memories. Biological Psychiatry, 65(5), 361–366. https://doi.org/10.1016/j.biopsych.2008.10.019
Kross, E., Gard, D., Deldin, P., Clifton, J., & Ayduk, O. (2012). “Asking why” from a distance: Its cognitive and emotional consequences for people with major depressive disorder. Journal of Abnormal Psychology, 121(3), 559–569. https://doi.org/10.1037/a0028808
Kuhn, S., Vanderhasselt, M. A., De Raedt, R., & Gallinat, J. (2012). Why ruminators won't stop: The structural and resting state correlates of rumination and its relation to depression. Journal of Affective Disorders, 141(2-3), 352–360. https://doi.org/10.1016/j.jad.2012.03.024
Lamm, C., Decety, J., & Singer, T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–2502. https://doi.org/10.1016/j.neuroimage.2010.10.014
Leech, R., & Sharp, D. J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(Pt 1), 12–32. https://doi.org/10.1093/brain/awt162
Lemogne, C., le Bastard, G., Mayberg, H., Volle, E., Bergouignan, L., Lehericy, S., … Fossati, P. (2009). In search of the depressive self: Extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience, 4(3), 305–312. https://doi.org/10.1093/scan/nsp008
Li, W., Cui, H., Zhu, Z., Kong, L., Guo, Q., Zhu, Y., … Li, C. (2016). Aberrant functional connectivity between the amygdala and the temporal pole in drug-free generalized anxiety disorder. Frontiers in Human Neuroscience, 10, 549. https://doi.org/10.3389/fnhum.2016.00549
Liu, J., Ren, L., Womer, F. Y., Wang, J., Fan, G., Jiang, W., … Wang, F. (2014). Alterations in amplitude of low frequency fluctuation in treatment-naive major depressive disorder measured with resting-state fMRI. Human Brain Mapping, 35(10), 4979–4988. https://doi.org/10.1002/hbm.22526
Lo, C. S., Ho, S. M., & Hollon, S. D. (2008). The effects of rumination and negative cognitive styles on depression: A mediation analysis. Behaviour Research and Therapy, 46(4), 487–495. https://doi.org/10.1016/j.brat.2008.01.013
Lockwood, P. L. (2016). The anatomy of empathy: Vicarious experience and disorders of social cognition. Behavioural Brain Research, 311, 255–266. https://doi.org/10.1016/j.bbr.2016.05.048
Lopez, C. M., Driscoll, K. A., & Kistner, J. A. (2009). Sex differences and response styles: Subtypes of rumination and associations with depressive symptoms. Journal of Clinical Child and Adolescent Psychology, 38(1), 27–35. https://doi.org/10.1080/15374410802575412
Luminet, O. (2004). Measurement of depressive rumination and associated constructs. In C. Papageorgiou, Wells, A. (Ed.), Depressive rumination. Nature, theory and treatment (pp. 187–215). Chichester: Wiley.
Lyubomirsky, S., Caldwell, N. D., & Nolen-Hoeksema, S. (1998). Effects of ruminative and distracting responses to depressed mood on retrieval of autobiographical memories. Journal of Personality and Social Psychology, 75(1), 166–177.
Lyubomirsky, S., & Nolen-Hoeksema, S. (1993). Self-perpetuating properties of dysphoric rumination. Journal of Personality and Social Psychology, 65(2), 339–349.
Lyubomirsky, S., & Nolen-Hoeksema, S. (1995). Effects of self-focused rumination on negative thinking and interpersonal problem solving. Journal of Personality and Social Psychology, 69(1), 176–190.
Madsen, M. K., Mc Mahon, B., Andersen, S. B., Siebner, H. R., Knudsen, G. M., & Fisher, P. M. (2016). Threat-related amygdala functional connectivity is associated with 5-HTTLPR genotype and neuroticism. Social Cognitive and Affective Neuroscience, 11(1), 140–149. https://doi.org/10.1093/scan/nsv098
Makris, N., Goldstein, J. M., Kennedy, D., Hodge, S. M., Caviness, V. S., Faraone, S. V., …, Sidman, L. J. (2006). Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research, 83(2-3), 155–171.
Mandell, D., Siegle, G. J., Shutt, L., Feldmiller, J., & Thase, M. E. (2014). Neural substrates of trait ruminations in depression. Journal of Abnormal Psychology, 123(1), 35–48. https://doi.org/10.1037/a0035834
Marchetti, I., Koster, E. H., Sonuga-Barke, E. J., & De Raedt, R. (2012). The default mode network and recurrent depression: A neurobiological model of cognitive risk factors. Neuropsychology Review, 22(3), 229–251. https://doi.org/10.1007/s11065-012-9199-9
Marroquin, B. M., Fontes, M., Scilletta, A., & Miranda, R. (2010). Ruminative subtypes and coping responces: Active and passive pathways to depressive symptoms. Cognition and Emotion, 24(8), 1446–1455.
Mars, R. B., Sallet, J., Schuffelgen, U., Jbabdi, S., Toni, I., & Rushworth, M. F. (2012). Connectivity-based subdivisions of the human right “temporoparietal junction area”: Evidence for different areas participating in different cortical networks. Cerebral Cortex, 22(8), 1894–1903. https://doi.org/10.1093/cercor/bhr268
Merino, H., Senra, C., & Ferreiro, F. (2016). Are worry and rumination specific pathways linking neuroticism and symptoms of anxiety and depression in patients with generalized anxiety disorder, major depressive disorder and mixed anxiety-depressive disorder? PLoS ONE, 11(5), e0156169. https://doi.org/10.1371/journal.pone.0156169
Michalak, J., Holz, A., & Teismann, T. (2011). Rumination as a predictor of relapse in mindfulness-based cognitive therapy for depression. Psychology and Psychotherapy, 84(2), 230–236. https://doi.org/10.1348/147608310X520166
Moran, J. M., Kelley, W. M., & Heatherton, T. F. (2013). What can the organization of the brain's default mode network tell us about self-knowledge? Frontiers in Human Neuroscience, 7, 391. https://doi.org/10.3389/fnhum.2013.00391
Mori, M., & Tanno, Y. (2015). Mediating role of decentering in the associations between self-reflection, self-rumination, and depressive symptoms. Psychology, 6(5).
Murphy, K., & Fox, M. D. (2017). Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage, 154, 169–173. https://doi.org/10.1016/j.neuroimage.2016.11.052
National Institues of Health. (2011). Negative Valence Systems. Retrieved from https://www.nimh.nih.gov/research-priorities/rdoc/constructs/negative-valence-systems.shtml
Nejad, A. B., Fossati, P., & Lemogne, C. (2013). Self-referential processing, rumination, and cortical midline structures in major depression. Frontiers in Human Neuroscience, 7, 666. https://doi.org/10.3389/fnhum.2013.00666
Nejad, K., Sugiura, M., Nozawa, T., Kotozaki, Y., Furusawa, Y., Nishino, K., … Kawashima, R. (2015). Supramarginal activity in interoceptive attention tasks. Neuroscience Letters, 589, 42–46. https://doi.org/10.1016/j.neulet.2015.01.031
Niendam, T. A., Laird, A. R., Ray, K. L., Dean, Y. M., Glahn, D. C., & Carter, C. S. (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience, 12(2), 241–268. https://doi.org/10.3758/s13415-011-0083-5
Nieto-Castanon, A., & Fedorenko, E. (2012). Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. NeuroImage, 63(3), 1646–1669. https://doi.org/10.1016/j.neuroimage.2012.06.065
Nolen-Hoeksema, S. (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology, 109(3), 504–511.
Northoff, G., Heinzel, A., de Greck, M., Bermpohl, F., Dobrowolny, H., & Panksepp, J. (2006). Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage, 31(1), 440–457. https://doi.org/10.1016/j.neuroimage.2005.12.002
Ochsner, K. N., Silvers, J. A., & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–24. https://doi.org/10.1111/j.1749-6632.2012.06751.x
Owens, M., & Gibb, B. E. (2017). Brooding rumination and attentional biases in currently non-depressed individuals: An eye-tracking study. Cognition and Emotion, 31(5), 1062–1069. https://doi.org/10.1080/02699931.2016.1187116
Padilla Paredes, P., & Calvete Zumalde, E. (2015). A test of the vulnerability-stress model with brooding and reflection to explain depressive symptoms in adolescence. Journal of Youth and Adolescence, 44(4), 860–869.
Poldrack, R. A. (2007). Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience, 2(1), 67–70. https://doi.org/10.1093/scan/nsm006
Power, J. D., Mitra, A., Laumann, T. O., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341.
Raes, F., & Hermans, D. (2008). On the mediating role of subtypes of rumination in the relationship between childhood emotional abuse and depressed mood: Brooding versus reflection. Depression and Anxiety, 25(12), 1067–1070. https://doi.org/10.1002/da.20447
Randolph, J. (2016). Online Kappa Calculator. Retrieved from http://justus.randolph.name/kappa
Ray, R. D., Ochsner, K. N., Cooper, J. C., Robertson, E. R., Gabrieli, J. D., & Gross, J. J. (2005). Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience, 5(2), 156–168.
Rogers, B. P., Morgan, V. L., Newton, A. T., & Gore, J. C. (2007). Assessing functional connectivity in the human brain by fMRI. Magnetic Resonance Imaging, 25(10), 1347–1357. https://doi.org/10.1016/j.mri.2007.03.007
Rosenbaum, D., Hagen, K., Deppermann, S., Kroczek, A. M., Haeussinger, F. B., Heinzel, S., … TREND Study Consortium (2016). State-dependent altered connectivity in late-life depression: A functional near-infrared spectroscopy study. Neurobiology of Aging, 39, 57–68. https://doi.org/10.1016/j.neurobiolaging.2015.11.022
Rosenbaum, D., Haipt, A., Fuhr, K., Haeussinger, F. B., Metzger, F. G., Nuerk, H. C., … Ehlis, A. C. (2017). Aberrant functional connectivity in depression as an index of state and trait rumination. Scientific Reports, 7(1), 2174. https://doi.org/10.1038/s41598-017-02277-z
Rude, S. S., Maestas, K. L., & Neff, K. (2007). Paying attention to distress: What's wrong with rumination? Cognition and Emotion, 21(4), 843–864.
Rush, A. J., Carmody, T., & Reimitz, P. E. (2000). The Inventory of Depressive Sypmtomatology (IDS): Clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. International Journal of Methods in Psychiatric Research, 9, 45–59.
Rush, A. J., Gullion, C. M., Basco, M. R., Jarrett, R. B., & Trivedi, M. H. (1996). The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine, 26(3), 477–486.
Sheline, Y. I., Price, J. L., Yan, Z., & Mintun, M. A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America, 107(24), 11020–11025. https://doi.org/10.1073/pnas.1000446107
Siegle, G. J., Moore, P., & Thase, M. (2004). Rumination: One construct, many feaures in healthy individuals, depressed individuals, and individuals with lupus. Cognitive Therapy and Research, 28(5), 645–668.
Siegle, G. J., Steinhauer, S. R., Thase, M. E., Stenger, V. A., & Carter, C. S. (2002). Can't shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry, 51(9), 693–707.
Siegle, G. J., Thompson, W., Carter, C. S., Steinhauer, S. R., & Thase, M. E. (2007). Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biological Psychiatry, 61(2), 198–209. https://doi.org/10.1016/j.biopsych.2006.05.048
Silani, G., Lamm, C., Ruff, C. C., & Singer, T. (2013). Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. The Journal of Neuroscience, 33(39), 15466–15476. https://doi.org/10.1523/JNEUROSCI.1488-13.2013
Steinbeis, N., Bernhardt, B. C., & Singer, T. (2015). Age-related differences in function and structure of rSMG and reduced functional connectivity with DLPFC explains heightened emotional egocentricity bias in childhood. Social Cognitive and Affective Neuroscience, 10(2), 302–310. https://doi.org/10.1093/scan/nsu057
Takano, K., & Tanno, Y. (2009). Self-rumination, self-reflection, and depression: Self-rumination counteracts the adaptive effect of self-reflection. Behaviour Research and Therapy, 47(3), 260–264. https://doi.org/10.1016/j.brat.2008.12.008
Tang, Y., Long, J., Wang, W., Liao, J., Xie, H., Zhao, G., & Zhang, H. (2016). Aberrant functional brain connectome in people with antisocial personality disorder. Scientific Reports, 6, 26209. https://doi.org/10.1038/srep26209
Treynor, W., Gonzalez, R., Nolen-Hoeksema, S. (2003). Rumination reconsidered: A psychometric analaysis. Cognitive Therapy and Research, 27(3), 247–259.
Uddin, L. Q., Kelly, A. M., Biswal, B. B., Castellanos, F. X., & Milham, M. P. (2009). Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping, 30(2), 625–637. https://doi.org/10.1002/hbm.20531
Vanderhasselt, M. A., Baeken, C., Van Schuerbeek, P., Luypaert, R., De Mey, J., & De Raedt, R. (2013). How brooding minds inhibit negative material: An event-related fMRI study. Brain and Cognition, 81(3), 352–359. https://doi.org/10.1016/j.bandc.2013.01.007
Vanderhasselt, M. A., Kuhn, S., & De Raedt, R. (2011). Healthy brooders employ more attentional resources when disengaging from the negative: An event-related fMRI study. Cognitive, Affective, & Behavioral Neuroscience, 11(2), 207–216. https://doi.org/10.3758/s13415-011-0022-5
Vogt, B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews. Neuroscience, 6(7), 533–544. https://doi.org/10.1038/nrn1704
Wang, F., Peng, K., Bai, Y., Li, R., Zhu, Y., Sun, P., … Sui, J. (2016). The dorsal anterior cingulate cortex modulates dialectical self-thinking. Frontiers in Psychology, 7, 152. https://doi.org/10.3389/fpsyg.2016.00152
Wang, K., Wei, D., Yang, J., Xie, P., Hao, X., & Qiu, J. (2015). Individual differences in rumination in healthy and depressive samples: Association with brain structure, functional connectivity and depression. Psychological Medicine, 45(14), 2999–3008. https://doi.org/10.1017/S0033291715000938
Watkins, E., & Teasdale, J. D. (2004). Adaptive and maladaptive self-focus in depression. Journal of Affective Disorders, 82(1), 1–8. https://doi.org/10.1016/j.jad.2003.10.006
Weissman-Fogel, I., Moayedi, M., Taylor, K. S., Pope, G., & Davis, K. D. (2010). Cognitive and default-mode resting state networks: Do male and female brains “rest” differently? Human Brain Mapping, 31(11), 1713–1726. https://doi.org/10.1002/hbm.20968
Whiteman, R. C., & Mangels, J. A. (2016). Rumination and rebound from failure as a function of gender and time on task. Brain Sciences, 6(1). https://doi.org/10.3390/brainsci6010007
Whitfield-Gabrieli, S., & Nieto-Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. https://doi.org/10.1089/brain.2012.0073
Wolf, R. C., Sambataro, F., Vasic, N., Schmid, M., Thomann, P. A., Bienentreu, S. D., & Wolf, N. D. (2011). Aberrant connectivity of resting-state networks in borderline personality disorder. Journal of Psychiatry & Neuroscience, 36(6), 402–411. https://doi.org/10.1503/jpn.100150
Woody, M. L., Kudinova, A. Y., McGeary, J. E., Knopik, V. S., Palmer, R. H., & Gibb, B. E. (2016). Influence of maternal depression on children's brooding rumination: Moderation by CRHR1 TAT haplotype. Cognition and Emotion, 30(2), 302–314. https://doi.org/10.1080/02699931.2014.998631
Yoshimura, S., Okamoto, Y., Onoda, K., Matsunaga, M., Ueda, K., Suzuki, S., & Shigetoyamawaki. (2010). Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. Journal of Affective Disorders, 122(1-2), 76–85. https://doi.org/10.1016/j.jad.2009.06.017
Zagni, E., Simoni, L., & Colombo, D. (2016). Sex and gender differences in central nervous system-related disorders. Neuroscience Journal, 2827090. https://doi.org/10.1155/2016/2827090
Zhu, X., Wang, X., Xiao, J., Liao, J., Zhong, M., Wang, W., & Yao, S. (2012). Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry, 71(7), 611–617. https://doi.org/10.1016/j.biopsych.2011.10.035
Acknowledgements
This research was supported by grants from Northwestern Memorial Hospital Women’s Board and The Davee Family Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Satyshur, M.D., Layden, E.A., Gowins, J.R. et al. Functional connectivity of reflective and brooding rumination in depressed and healthy women. Cogn Affect Behav Neurosci 18, 884–901 (2018). https://doi.org/10.3758/s13415-018-0611-7
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-018-0611-7