Abstract
The aim of the present study was to use fMRI to examine the neural correlates of engaging in rumination among a sample of remitted depressed adolescents, a population at high risk for future depressive relapse. A rumination induction task was used to assess differences in the patterns of neural activation during rumination versus a distraction condition among 26 adolescents in remission from major depressive disorder (rMDD) and in 15 healthy control adolescents. Self-report depression and rumination, as well as clinician-rated depression, were also assessed among all participants. All of the participants recruited regions in the default mode network (DMN), including the posterior cingulate cortex, medial prefrontal cortex, inferior parietal lobe, and medial temporal gyrus, during rumination. Increased activation in these regions during rumination was correlated with increased self-report rumination and symptoms of depression across all participants. Adolescents with rMDD also exhibited greater activation in regions involved in visual, somatosensory, and emotion processing than did healthy peers. The present findings suggest that during ruminative thought, adolescents with rMDD are characterized by increased recruitment of regions within the DMN and in areas involved in visual, somatosensory, and emotion processing.
Similar content being viewed by others
Major depressive disorder (MDD) during adolescence is associated with several debilitating outcomes, including a five-fold increased risk for suicide attempts, increased occurrence of physical and medical hospitalizations, and impaired functioning in the school, social, and family domains (e.g., Dunn & Goodyer, 2006). Adolescents with a history of MDD are also at particularly high risk for depressive relapse, with a cumulative probability of recurrence of 40% after 2 years, and 70% after 5 years (Avenevoli, Stolar, Li, Dierker, & Merikangas, 2001). Identifying risk factors that are present among adolescents during periods of remission from MDD may help shed light on the mechanisms implicated in depressive relapse.
One mechanism associated with risk for MDD relapse is rumination, a perseverative negative thought pattern characterized by repetitive, prolonged, self-reflective, and uncontrollable focus on sad mood and its causes and consequences (Nolen-Hoeksema, Larson, & Grayson, 1999). Research suggests that rumination is associated with greater severity and duration of depressive episodes in adults (for a review, see Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008), and prospectively increases the risk of depressive relapse (Marchetti et al. 2012; Roberts, Gilboa, & Gotlib, 1998). Notably, adolescents with a past history of MDD also exhibit higher levels of self-report rumination relative to healthy controls (e.g., Beevers, Rohde, Stice, & Nolen-Hoeksema, 2007; Hong et al., 2010). Given the robust relation between extent of rumination and recurrent depressive course, identifying the neural mechanisms supporting rumination is essential to provide greater insight into how this mechanism may be modified to stave off treatment refractoriness.
To date, the most consistent neural correlates of rumination have been regions within the default mode network (DMN) and subgenual prefrontal cortex (sgPFC; for reviews, see Hamilton, Farmer, Fogelman, & Gotlib, 2015; Nejad, Fossati, Lemogne, 2013). The DMN includes the posterior cingulate cortex (PCC), ventro-medial prefrontal cortex (mPFC), medial temporal gyrus (MTG), and inferior parietal cortex (IPC), and is activated during rest, mind-wandering, or self-reflective thought (for a review, see Whitfield-Gabrieli & Ford, 2012). Studies have suggested that adults with current MDD exhibit greater activation in the subgenual anterior cingulate cortex (ACC), PCC, mPFC, and MTG, encompassing the parahippocampus, when rumination is induced during functional magnetic resonance imaging (fMRI; Cooney, Joormann, Eugène, Dennis, & Gotlib, 2010). Moreover, greater dominance of the DMN relative to a task positive network during the resting state was associated with higher levels of depressive rumination in adults (Hamilton et al., 2011). Finally, meta-analytical findings have demonstrated that increased functional connectivity between the DMN and sgPFC predicts levels of depressive rumination in adults (for reviews, see Hamilton et al., 2015; Nejad, Fossati, Lemogne, 2013).
Laboratory studies have also shown that ruminative thought exacerbates negative mood among dysphoric and depressed individuals (e.g., Nolen-Hoeksema & Morrow, 1993). Given this, it is not surprising that rumination is also correlated with emotional processing networks (for a review, see Nejad, Fossati, & Lemogne, 2013). For example, self-report rumination is associated with amygdala activation when healthy controls are instructed to increase their levels of negative affect (Ray et al., 2005). Several studies have also suggested that rumination is correlated with greater sustained activation of the amygdala to emotional stimuli among depressed adults (Mandell, Siegle, Shutt, Feldmiller, & Thase, 2014; Siegle, Carter, & Thase, 2006; Siegle, Steinhauer, Thase, Stenger, & Carter, 2002). Moreover, this link has been replicated experimentally, with depressed adults exhibiting greater activation in the amygdala while engaging in self-referential processing than during distraction (Cooney et al., 2010). Thus, regions supporting the encoding, processing, interpretation, and regulation of emotional information, such as the amygdala, are also implicated in supporting ruminative processes, but have not yet been studied among adolescents.
Given that rumination is a known mechanism increasing the risk of depressive relapse (Roberts et al., 1998), it is striking that no study to date has explored whether abnormalities in DMN and emotion processing regions during ruminative thought are present in adolescents with remitted MDD (rMDD). However, findings from neuroimaging studies exploring the neural correlates of emotion processing in adolescent depression may provide insight into the regions that may support rumination and adolescent MDD. For instance, several studies have highlighted overactivation of the amygdala in response to processing negative stimuli in adolescents with current MDD (Beesdo et al., 2009; Yang et al., 2010), and among youth at high risk for MDD (Monk et al., 2008). Moreover, adolescents of depressed parents, a population at high risk for developing MDD, exhibited greater amygdala and prefrontal activation in response to a negative mood induction (Joormann, Cooney, Henry, & Gotlib, 2012). Joormann and colleagues (2012) found that, in addition to recruiting regions involved in emotional reactivity and regulation, adolescents at high risk for MDD also recruited regions that are more characteristic of self-referential processing and rumination, such as the MTG, during attempts to repair their negative mood. A critical, unanswered question is whether these regions may also be implicated in ruminative thought among adolescents with rMDD, a population at particularly high risk for relapse (Avenevoli, Stolar, Li, Dierker, & Merikangas, 2001).
In the present study, therefore, we sought to examine the neural correlates of engaging in ruminative thought versus distraction among youth in the remitted phase of depressive illness, as compared to their peers. The present task used to induce ruminative thought was adapted from previous experimental psychology studies (i.e., Cooney et al., 2010; Johnson et al., 2006; Lyubomirsky & Nolen-Hoeksema, 1993), and modified for an adolescent population. We hypothesized that adolescents with rMDD would demonstrate increased activation in the subgenual ACC and DMN regions, including activation in regions such as the PCC, IPC, and the mPFC, relative to healthy control adolescents, during ruminative thought relative to distraction. Consistent with previous studies that have examined the neural correlates of emotion processing in adolescent MDD (Beesdo et al., 2009; Yang et al., 2010), we also predicted that adolescents with rMDD would exhibit greater activation in the amygdala during ruminative thought, relative to distraction. Finally, to assess the study’s clinical relevance, exploratory correlations were run to examine the extent to which activations in regions associated with rumination versus distraction were associated with clinical ratings of depression and rumination.
Method
Procedure
Participants were recruited using flyers, multiple forms of posting on the Internet, and in-person interviews conducted at a pediatric psychiatry clinic. The patients with rMDD were recruited, as part of a pilot clinical trial that involved randomization, for eight weeks of adjunctive treatment with rumination-focused cognitive behavior therapy (Watkins, 2015; Watkins et al., 2011) or for assessment only. The healthy control (HC) participants were recruited for a baseline eligibility evaluation and one fMRI scan. The study was approved by the University of Illinois at Chicago’s Institutional Review Board, and all participants and one guardian signed assent and consent, respectively. The participants and their parents were then interviewed using the Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version (K-SADS-PL; Kaufman, Birmaher, Brent, & Rao, 1997) and the Children’s Depression Rating Scale–Revised (CDRS-R; Poznanski & Mokros, 1996) by two trained research assistants supervised by a licensed psychologist. The adolescents also completed self-report measures including the Reynold’s Adolescent Depression Scale (RADS; Reynolds, 1988) and the Ruminative Response Scale (RRS; Treynor, Gonzalez, & Nolen-Hoeksema, 2003). Within two weeks of this initial assessment, all participants underwent an fMRI scan.
Participants
The participants included adolescents in full or partial remission from MDD (n = 26) and adolescents with no lifetime history of any DSM-IV disorder (n = 15). Participants were considered in remission from MDD if they had previously met criteria for at least one major depressive episode (MDE) but had not met full criteria for an MDE in the past two weeks. This was operationally defined as three or fewer full-threshold symptoms rated as clinically significant on the K-SADS-PL (Kaufman et al., 1997). Participants with more than three symptom criteria of depression at subthreshold level (item rating of 2) on the K-SADS-PL were required to be enrolled in current mental health services. Of the 26 adolescents in remission from MDD, two endorsed one current full-threshold depression symptom, and one endorsed three current full-threshold depression symptoms on the K-SADS-PL (the remaining 23 participants endorsed zero full-threshold depression symptoms at the time of the study). As is shown in Table 1, the average number of days since last MDE among adolescents in remission from MDD was 361.21. Participants were not eligible if they endorsed any signs of active suicidality or substance abuse or dependence within the previous six months. rMDD participants were permitted to be on a maintenance antidepressant or stimulant medication, but they were only eligible if they had been on these medications for a minimum of 12 weeks with no dose changes in the last two weeks. HC participants could not meet the current or past criteria for MDD or any other DSM-IV psychiatric disorder. The average age of our sample was 15.55 (SD = 1.78, range = 12–18), 56% were Caucasian, and 56% were female. The participant demographics and clinical characteristics by group are presented in Table 1.
Clinical measures
Children’s Depression Rating Scale–Revised (Poznanski & Mokros, 1996)
The CDRS-R, a well validated 17-item clinician-rated depression severity measure based on interview of both the parent and child, was completed by an independent evaluator at baseline. The reliability and validity of the CDRS-R are well documented (Brooks & Kutcher, 2001). In this study, the interrater reliability (intraclass correlations [ICC] = 98%) on the CDRS-R was excellent.
Reynolds Adolescent Depression Scale (Reynolds, 1988)
Adolescents at baseline completed the RADS, a 30-item self-report measure of current depressive symptoms. This measure is rated on a 4-point Likert scale and has excellent internal consistency and good retest reliability (Reynolds, 1988). Higher total scores reflected higher levels of depression. In the present study, α = .87.
Ruminative Response Scale (Treynor, Gonzalez, & Nolen-Hoeksema, 2003)
Self-report rumination was assessed using the RRS. The RRS was used as a measure of depressive rumination in view of the sample’s history of rumination and in order to compare the current findings to the extant literature among adults. The RRS consists of 22 possible responses to sad mood that are focused on the self, one’s symptoms, and the possible causes and consequences of the mood state. The RRS had been shown to be a reliable and valid measure of rumination among young adolescent populations (Burwell & Shirk, 2007). In the present study, α = .94.
fMRI stimulus presentation
Rumination paradigm
The fMRI rumination task was based on the principles of previous studies (i.e., Cooney et al., 2010; Johnson et al., 2006; Lyubomirsky & Nolen-Hoeksema, 1993) and was modified to include a negative mood induction to facilitate the emergence of ruminative thought, and therefore decrease the amount of time the adolescent spent in the scanner. The task consisted of four blocks of mood induction, rumination induction, and distraction induction. At the baseline visit, participants were asked to generate four negative life events (failure event, sad family event, hurtful event, and frustrating event). Approximately two weeks later, they completed the fMRI scan. Following completion of a 6-min resting-state scan (reported elsewhere), the rumination task began. The task proceeded with a mood induction instruction, rumination prompt, self-rating question, distraction prompt, and self-rating question. This order was repeated three additional times. The task was run in the same order with the same prompts for all participants. First, participants were given mood induction instructions for 25 s: “1) Remember the time when someone badly hurt your feelings; 2) Remember the saddest event in your family; 3) Remember the time you were so sad/frustrated that you felt there was no hope for you; or 4) Remember when you failed badly at something.” For each of these memories, adolescents were asked to “Use your imagination to bring this fully into your mind and picture the event.” Immediately following each mood induction instruction, a rumination prompt was displayed for 30 s using Nolen-Hoeksema’s standard rumination protocols and those adapted for a younger population (Hilt & Pollak, 2012), such as “think about what your feelings mean.” The rumination prompt was followed immediately by two self-report questions (“How sad do you feel right now?”) and (“How much are you focused on your feelings right now?”) on a 1 (not at all) to 4 (a lot) Likert scale; each question was displayed for 6 s, for a total of 12 s. Next, a 10- to 15-s jittered crosshair interval was displayed, followed by a 30-s distraction prompt. An example distraction prompt was to think about “a truck full of watermelons.” The task length was 8.5 min. After the scan was completed, a positive mood induction was used to remedy low mood. During the positive mood induction, participants were given detailed instructions to think of a positive memory and describe the memory in detail. They were then asked to rate their current mood; if low positive mood was endorsed, the clinical psychologist on call was notified to check in with the participant.
fMRI acquisition and preprocessing
The rumination/distraction task was completed in a 3.0-T GE Discovery scanner (Milwaukee, WI) using parallel imaging with ASSET and T2* gradient-echo axial echoplanar imaging, with the following parameters: 90-deg flip, field of view 22, matrix size = 64×64, slice thickness = 3 mm, 22.2-ms echo time, 44 slices, repetition time (TR) of 2,000 ms, and a total of 265 volumes. Prior to the rumination/distraction task, an eyes-open resting scan was acquired over 6 min with the same parameters described above and a total of 240 volumes. High-resolution anatomic T1 SPGR scans were obtained for spatial normalization. Motion was minimized with foam pads and a cross on the display, and by conveying the importance of holding still to participants. Several steps were taken to reduce potential sources of noise and artifact. Specifically, slice-timing correction was completed with SPM8 (www.fil.ion.ucl.ac.uk/spm/doc/), and motion detection algorithms (MCFLIRT; Jenkinson, Bannister, Brady, & Smith, 2002) were applied using FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Coregistration of the structural images to functional images was followed by spatial normalization of the coregistered T1-SPGR to the Montreal Neurological Institute (MNI) template. The resulting normalization matrix was then applied to the slice-time-corrected, physiologically corrected, time-series data. These normalized T2* time-series data were spatially smoothed with a 5-mm Gaussian kernel, resulting in T2* images with isotropic voxels, 2 mm on a side.
Movement was addressed with the following strategies: First, blocks were identified in which individual participants had any TR to TR movement exceeding 1.5 mm, or three consecutive TRs exceeding the same in any direction—pitch, roll, or yaw. In addition, normality plots of the average standard deviations of the movement values were examined for outliers. Rumination and distraction blocks in which participants had movement values greater than two standard deviations beyond the mean were not modeled. This resulted in three participants having one rumination block omitted, and three participants having one distraction block omitted from the respective models. These five participants (three rMDD and two HC) did not differ from the participants without blocks omitted in terms of age, depressive severity, self-report rumination, sex, or depression diagnostic history (lowest p = .21). Furthermore, the results described below remained unchanged when these participants were removed from the analysis.
Consistent with previous research using a similar version of the rumination task in adults (Cooney et al., 2010; Johnson et al., 2006), contrast images were derived based on the rumination-versus-distraction contrast. These were computed by using the standard hemodynamic response function in SPM to model the blood oxygenation level dependent (BOLD) signal for all rumination blocks, and subtracting similar BOLD signal changes for distraction blocks for each individual in a first-level analysis. One-sample t tests were conducted using whole-brain analyses from the individual group contrasts between the rMDD and control groups in SPM8. Whole-brain correction was achieved at p < .05 by using AlphaSim with 1,000 Monte Carlo simulations to determine a joint threshold of height and extent (p < .005, cluster extent of 440 mm3). Post-hoc analyses included using a DMN mask that was derived from the seed-based connectivity of the left PCC, a DMN hub, among the same sample of adolescents (Jacobs et al., 2016). The DMN mask was entirely subsumed within an adult mask reported previously by Yeo et al. (2011). We created our own mask to be certain about its developmental age relevance.
Behavioral analyses
Participants completed ratings of state sadness and self-focus following each rumination and distraction block. These ratings were averaged across each condition (rumination, distraction), and a two-way (group: rMDD, HC) repeated condition (rumination, distraction) analysis of variance (ANOVA) was conducted to examine all differences in sadness and self-focus.
Exploratory factor analysis
Exploratory factor analysis was conducted on the extracted z values from each cluster of significant differences between groups (rMDD, HC) for the rumination-versus-distraction contrast. The number of factors retained was determined by using maximum likelihood as an extraction method and a threshold of eigenvalue > 1, followed by oblique rotation. We also verified that the extracted factors surpassed 50% of the total variance. Bivariate correlations were then conducted to examine the relation between the extracted factors and self-report measures of depression and rumination. To rule out potential additional relationships with clinical and demographic features, we also examined whether participants’ gender, medication status, and current anxiety comorbidity were related to the extracted factors during the rumination versus distraction contrast.
Results
Behavioral results
When we examined the differences between the HC and rMDD groups in self-report sadness following the rumination and distraction blocks, our results revealed significant main effects of group [F(1, 35) = 5.82, p = .02], reflecting that adolescents with rMDD reported higher levels of sadness across all four rumination and distraction prompts, and condition [F(1, 35) = 45.38, p < .001], reflecting greater sadness ratings across all participants during the rumination versus the distraction condition, replicating previous adult studies using these inductions (Lyubomirsky & Nolen-Hoeksema, 1993), and confirming that the manipulations were effective inside the fMRI scanner. The Group × Condition interaction was not significant [F(1, 35) = 0.29, p = .59]. An identical ANOVA was conducted with the self-focus ratings, and the results revealed a significant main effect of condition [F(1, 35) = 39.13, p < .001], reflecting that adolescents reported greater levels of focus on their feelings during the rumination versus the distraction condition, again confirming the efficacy of the manipulations in the scanner. The main effect of group [F(1, 35) = 0.01, p = .96] and the Group × Condition interaction [F(1, 35) = 1.99, p = .17] were not significant.
fMRI results
Rumination-versus-distraction across all participants
As is shown in Table 2, across all participants, the rumination-versus-distraction contrast resulted in activations in default-mode regions such as the PCC (BA = 23), mPFC (BA = 10), IPL (BA = 47), and MTG (BA = 21). In addition, regions known to support the processing of emotional stimuli, such as the hippocampus, and of visual stimuli, including the occipital gyrus (BA = 18), were activated during rumination as compared to distraction. We found no statistically significant positive activations for the distraction versus the rumination condition. A post-hoc analysis was conducted to determine whether the regions activated during the rumination induction were inside or outside the DMN by using a mask created from the PCC seed of a resting-state connectivity analysis within the same dataset (Jacobs et al., 2016). Regions activated within and outside the DMN during the rumination induction are presented in Fig. 1.
(A) The default mode network (DMN), based on bilateral posterior cingulate cortex, parahippocampal gyrus, and dorsomedial prefrontal cortex, from rs-fMRI seeds (t > 15, k < 55) with our adolescent sample only (fuchsia in online electronic version); the DMN network from Yeo et al. (2011) only (cyan online); and the intersection of the two masks (violet online). (B) Activation for the rumination–distraction contrast, with regions within the adolescent DMN mask highlighted cyan online, and regions in red online showing areas outside the adolescent DMN mask
Group differences in rumination versus distraction
Adolescents in the rMDD group demonstrated greater activation in default mode regions, including the left precuneus (BA = 7) and the right IPL (BA = 40), relative to HCs in the rumination–distraction contrast (Fig. 2 and Table 3). In addition, the left MTG (BA = 30) was activated to a greater extent in rMDD participants than in HCs. Individuals with rMDD also recruited regions involved in visual processing, such as the bilateral inferior occipital gyri (BA = 19), bilateral lingual gyri (BA = 19), and left fusiform (BA = 19), to a greater extent than did HCs. Emotion processing regions including the amygdala, thalamus, and insula were also activated to a greater extent in rMDD individuals than in HCs during rumination versus distraction. Finally, somatosensory areas, including bilateral precentral gyri (BAs = 4 and 44), were also hyperactive among rMDD individuals relative to HCs during rumination.Footnote 1 In contrast, no significant findings emerged wherein HCs activated regions to a greater extent than rMDD youth in the rumination–distraction contrast.
We reutilized the previously described DMN mask to examine whether the regions activated during the rumination–distraction contrast among the rMDD adolescents were within or outside the DMN. Apart from small overlaps, the majority of group differences during the rumination induction were outside of the DMN mask and included the previously described regions, such as bilateral occipital gyri, thalamus, amygdala, insula, and bilateral precentral gyri.
Data reduction with exploratory factor analyses
The results from exploratory factor analysis suggested a two-factor solution for activations in the rumination–distraction contrast for the rMDD adolescents. Factor 1 had an eigenvalue of 10.60 and accounted for 42% of the variance after rotation, whereas Factor 2 has an eigenvalue of 1.30 and accounted for 37% of the variance after rotation. Factors 1 and 2 were positively related (r = .74, p < .001). As is shown in Table 3, Factor 1 had higher loadings on seven of the 14 clusters, whereas Factor 2 included the remaining seven of the 14 clusters.<>
Clinical correlates
As is shown in Table 1, the rMDD adolescents reported higher residual levels of rumination (t = 3.70, p < .01), as well as higher levels of self-report (t = 3.70, p < .001) and interviewer-rated (t = 5.77, p < .001) depressive symptoms, relative to HC adolescents. Across all adolescents, Factor 1 during the rumination induction was associated with higher levels of rumination (r = .42, p < .01; Fig. 3a) and interviewer-rated depressive symptoms (r = .32, p = .04); a nonsignificant trend emerged between Factor 1 and self-report depressive symptoms (r = .28, p = .09). Factor 2 was also associated with higher levels of self-report rumination (r = .38, p = .02) and depressive symptoms (r = .32, p = .048), and greater clinician-rated depressive symptoms (r = .43, p < .01; Fig. 3b).
Relationships between rumination–distraction activation and rumination and depression symptoms. (a) Rumination induction Factor 1 is positively related to self-report rumination among adolescents. (b) Rumination induction Factor 2 is positively related to clinician-rated depressive symptoms among adolescents
Potential confounds
Finally, participants’ gender, medication status, and current anxiety comorbidity were unrelated to the two extracted factors during the rumination versus distraction contrast (ps > .19). The rMDD adolescents continued to differ significantly from the HC adolescents across the two factors during the rumination versus distraction contrast when we adjusted for behavioral ratings of sadness during the fMRI task (ps < .01) and for current anxiety symptoms (ps < .01), and after we excluded rMDD participants currently on medication (ps < .04).
Discussion
The primary aim of the present study was to examine the neural correlates of rumination among youth in the remitted phase of depressive illness, as compared to healthy controls. In the present study, we used an experimental task modified for fMRI that is known to induce rumination and distraction (Cooney et al., 2010), with slightly adapted language for use with adolescents. Across all participants, our results indicated that adolescents reported greater levels of state sadness and self-focus while engaging in rumination relative to distraction, indicating that the experimental manipulation was successful in inducing ruminative mood. Reports of rumination were equivalent for both groups, whereas the depressed adolescents reported greater state sadness during the rumination induction than did nondepressed adolescents. Engaging in rumination relative to distraction resulted in greater neural recruitment of regions in the DMN, including the PCC, mPFC, IPL, and MTG, across all adolescents. Additional areas were also activated during the rumination induction among all adolescents, including areas involved in emotion processing, such as the hippocampus, as well as areas involved in visual processing. Beyond these main effects of condition that were predominantly located within the DMN, rMDD adolescents exhibited greater activation than did HCs in a few DMN regions, including the precuneus, IPL, and MTG. In addition, the majority of group differences (rMDD > HC) during the rumination induction were outside the DMN, and instead located in limbic, visual-processing, and somatosensory areas. Notably, recruitment of these neural regions during rumination was also associated with greater levels of rumination and interviewer-rated depressive symptoms across participants. The present findings suggest that hyperactivation of these neural regions during rumination may be a residual consequence of adolescent-onset MDD or may be a risk factor for relapse. Given that rumination is a known predictor of depressive relapse (Marchetti et al., 2012; Roberts, Gilboa, & Gotlib, 1998), the present findings raise the possibility that these neural patterns may depict this risk.
The present results provide further support for the role of the DMN in rumination, and they extend previous findings by documenting this pattern in an adolescent population. Specifically, our finding that all adolescents exhibited greater DMN activation during ruminative thought relative to distraction is consistent with a previous study showing that adults exhibit greater activation of the PCC and precuneus during self-reflective thought (Johnson et al., 2006). The increased activation within regions of the DMN, such as the MTG, is also consistent with previous findings from currently depressed adults (Cooney et al., 2010), and may suggest that adolescents in remission from MDD may be engaging in more mood-congruent memory retrieval during rumination than do HCs. Adolescents in remission from MDD recruited several areas outside the DMN to a greater extent than did HCs while engaging in rumination. Whereas links between emotional salience regions and rumination have been repeatedly observed in the actively depressed state (Cooney et al., 2010; Mandell et al., 2014; Siegle et al., 2006; Siegle et al., 2002), the present study has been the first to illustrate heightened amygdala, insular, and thalamic activity during rumination that occurs during adolescence and persists during remission from a depressive episode.
It is also noteworthy that relative to HCs, adolescents in remission from MDD exhibited greater activation in visual processing and somatosensory areas during rumination relative to distraction, such as in the bilateral inferior occipital, bilateral lingual, left fusiform, and bilateral precentral gyri. Since we know that remitted depressed adolescents engage in higher levels of rumination relative to HCs (e.g., Beevers, Rohde, Stice, & Nolen-Hoeksema, 2007; Hong et al., 2010), rumination may be more elaborative for these individuals. In other words, adolescents in remission from depression may be more likely to ruminate on negative information more vividly, with visual and somatic brain regions recruited. Importantly, these findings are consistent with and extend those of a previous study highlighting the role of increased recruitment of visual-processing areas, including the fusiform gyrus, in the retrieval of memories among remitted depressed adults, relative to HCs (van Wingen et al., 2010). The neural processes involved in rumination may parallel processes involved in the retrieval of negative emotional memories, and may be one mechanism placing these individuals at high risk for relapse.
Contrary to our hypothesis, the present study revealed no evidence for the role of the subgenual ACC being implicated in ruminative thought among remitted depressed adolescents at the corrected level. This is inconsistent with previous adult depression and rumination studies (Cooney et al., 2010; Grimm et al., 2009; Hamilton et al., 2015; Lemogne, Delaveau, Freton, Guionnet, & Fossati, 2012), although an uncorrected region-of-interest analysis did show the expected effect. One possible explanation for this may be our focus on an adolescent population that was currently in remission from depression. That is, previous adult studies examining the neural correlates of ruminative thought in depression have not focused on individuals in remission from the disorder. Moreover, previous volumetric (Hasler, Drevets, Manji, & Charney, 2004) and functional imaging (Matthews et al., 2009) studies suggested that abnormalities in the size and function of the subgenual ACC in depression are exacerbated by illness severity and chronicity. Thus, one possibility is that these regions are more likely to be implicated in rumination among adults with chronic MDD or multiple depressive episodes in the active state. Additionally, in the present study we did not examine connectivity between regions during rumination versus distraction. Given that previous studies have consistently demonstrated that increased functional connectivity between the DMN and sgPFC is predictive of levels of depressive rumination in adults (for a review, see Hamilton et al., 2015; Nejad, Fossati, & Lemogne, 2013), it is possible that connectivity analyses may reveal regions involved in rumination among adolescents in remission from MDD. Future studies will be needed to test this possibility.
Interestingly, both healthy and rMDD adolescents appeared to engage in the rumination task to a similar degree, as reflected by their self-monitoring responses during the scan. This self-monitoring response assessed the extent to which adolescents engaged in rumination during short blocks in the scanner, which is a different phenomenon than a tendency to ruminate as a response style in real life (as measured by the RRS). Thus, consistent with other previous research (Beevers, Rohde, Stice, & Nolen-Hoeksema, 2007; Hong et al., 2010), these behavioral data suggest that rumination appears to be a trait-like factor implicated in adolescent MDD. The similar state-rumination responses in both groups might also suggest that rumination ratings of the self may have qualitatively different scales in MDD relative to HC. It is also noteworthy that the imaging results appear to be more robust and extensive than those observed in a similarly sized sample of adults with a similar task (Cooney et al., 2010), which may reflect developmental changes in the capacity for and richness of rumination. Indeed, we were unable to address some developmental considerations pertaining to rumination (Jose & Brown, 2008), as well as to rumination during adolescent depression, in this cross-sectional study. Therefore, it will be important for future, longitudinal studies to examine the stability and plasticity of these neural regions during ruminative thought from adolescence to adulthood.
Additional limitations of the present study provide some important directions for future research. First, prospective designs are needed, to determine whether these neural abnormalities during rumination are present prior to the first onset of MDD, thereby representing a putative vulnerability factor. Second, the rMDD adolescents in the present sample were not medication naïve and had a high rate of psychiatric comorbidity. On the other hand, it is also possible that this may have resulted in a more generalizable sample, given the high rates of comorbidity among adolescents with MDD (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Kilpatrick et al., 2003). Third, the present study was preliminary in nature and included a small sample. Therefore, we were unable to examine the effects of age and gender. Given that past studies have shown that rumination helps explain the gender difference in adolescent depression (Jose & Brown, 2008), future studies with larger sample sizes will be needed to examine whether gender moderates the relation between adolescents’ depression histories and neural activation during ruminative thought. Additionally, in the present study we were also unable to examine how neural activation during rumination correlated with the different subscales of the RRS, since these subscales tend to be highly correlated among small samples. Thus, future research should examine the extent to which these neural regions during rumination are related to adaptive (self-reflective) versus maladaptive (brooding) forms of rumination among adolescents. Moreover, future studies should seek to discriminate the neural regions involved in mind-wandering, background processing (e.g., to-do lists), distraction, and rumination, which are various forms of self-directed thought that vary in the degrees to which they focus on concrete, abstract, and perseverative content (Watkins, 2008).
Finally, the task used in the present study to induce rumination had some limitations worth noting. First, the number of events in each condition was lower than in typical fMRI studies, so as to reduce the length of the experiment. In this initial study, we attempted to mirror adult studies that have utilized a rumination induction. In subsequent studies, multiple control condition blocks might be employed to better segregate the unique pattern of activations associated with rumination. Probes could also be employed that provide qualitative information about the experience of rumination (e.g., intensity, vividness). Second, to facilitate comparisons with previous adult studies in which rumination has been induced in the scanner (Cooney et al., 2010), the contrast used in the present study compared rumination with distraction. Thus, we cannot definitively conclude that the regions differing between the rMDD and HC adolescents were specific to rumination, since they may reflect group differences in distraction, or order effects. Future studies that include a baseline condition, such as a rest condition, or a divided-attention task will be needed to parse the regions specific to rumination versus distraction.
Finally, the present version of the rumination task included a negative mood induction to reduce the amount of time required to begin ruminating. A limitation of this design is that the neural regions activated in response to rumination cannot be discriminated from those deriving from the negative mood induction or memory of the events and conditions leading to rumination. Research suggests that girls at high risk for depression exhibit greater amygdala and prefrontal activation in response to a negative mood induction, and increased MTG activation during attempts to repair their negative mood (Joormann et al., 2012). Data from the self-report validity questions administered immediately following each rumination and distraction block indicated that the remitted depressed youth did not differ from healthy peers in their ratings of sadness following rumination. These data provide some assurance that the results obtained do not derive solely from the fact that the mood induction was more potent among the rMDD group. In addition, rumination may, in the regular course of daily life, be triggered by negative affect or stressful life events. Thus, this paradigm may provide a useful window into how that process affects those at risk for depression differently. Future research might include alternative rumination tasks to further explore the similar and distinct brain regions supporting sad mood versus rumination.
Conclusion
In summary, the present results suggest that hyperactivity of neural regions involved in the DMN, somatic and visual processing, and the processing of emotional information is observed during ruminative thought among adolescents with rMDD, relative to HC adolescents. Future studies will be needed to examine the extent to which rMDD adolescents differ from HC youth in their functional connectivity between neural regions involved in rumination, including regions of the DMN as well as the amygdala. Additionally, the use of more nuanced methods for examining networks, including connectomics, will be an important next step toward determining the neural networks involved in switching between rumination and distraction among adolescents, and toward determining whether intervention efforts may be implemented to enhance the ability to disengage from rumination. The present examination of the neural signature of rumination using an fMRI task among a sample of adolescents during a period of relative wellness is innovative and important, and it suggests that enhanced recruitment of the neural regions involved in multisensory and self-referential processes while engaging in rumination may be a residual consequence of adolescent-onset MDD or a risk factor for relapse. Future studies should examine how targeting rumination through prevention or intervention may alter these neural mechanisms and, in turn, reduce the risk of future depressive relapse.
Notes
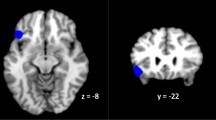
Given the well-documented link between ruminative thought and subgenual ACC activation (for reviews, see Hamilton et al., 2015; Nejad, Fossati, Lemogne, 2013), exploratory analyses were conducted to determine whether this region was activated to a greater extent among rMDD adolescents than among HC adolescents during rumination versus distraction, utilizing an uncorrected p value. Within the BA 25 mask from the Wake Forest Pickatlas, and using uncorrected thresholds of p < .05 and k > 15, rMDD adolescents exhibited greater activation during ruminative thought than did HC adolescents (Z = 3.55, p < .0002, k = 178, {–4, 4, 10}; see Supplementary Fig. 1).
References
Avenevoli, S., Stolar, M., Li, J., Dierker, L., & Merikangas, K. R. (2001). Comorbidity of depression in children and adolescents: Models and evidence from a prospective high-risk family study. Biological Psychiatry, 49, 1071–1081.
Beesdo, K., Lau, J. Y., Guyer, A. E., McClure-Tone, E. B., Monk, C. S., Nelson, E. E.,…Pine, D. S. (2009). Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry, 66, 275–285. doi:10.1001/archgenpsychiatry.2008.545
Beevers, C. G., Rohde, P., Stice, E., & Nolen-Hoeksema, S. (2007). Recovery from major depressive disorder among female adolescents: A prospective test of the scar hypothesis. Journal of Consulting and Clinical Psychology, 75, 888–900. doi:10.1037/0022-006X.75.6.888
Brooks, S. J., & Kutcher, S. (2001). Diagnosis and measurement of adolescent depression: A review of commonly utilized instruments. Journal of Child and Adolescent Psychopharmacology, 11, 341–376.
Burwell, R. A., & Shirk, S. R. (2007). Subtypes of rumination in adolescence: Associations between brooding, reflection, depressive symptoms, and coping. Journal of Clinical Child and Adolescent Psychology, 36, 56–65.
Cooney, R. E., Joormann, J., Eugène, F., Dennis, E. L., & Gotlib, I. H. (2010). Neural correlates of rumination in depression. Cognitive, Affective, & Behavioral Neuroscience, 10, 470–478. doi:10.3758/CABN.10.4.470
Costello, E. J., Mustillo, S., Erkanli, A., Keeler, G., & Angold, A. (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry, 60, 837–844.
Dunn, V., & Goodyer, I. M. (2006). Longitudinal investigation into childhood- and adolescence-onset depression: Psychiatric outcome in early adulthood. British Journal of Psychiatry, 188, 216–222. doi:10.1192/bjp.188.3.216
Grimm, S., Ernst, J., Boesiger, P., Schuepbach, D., Hell, D., Boeker, H., & Northoff, G. (2009). Increased self‐focus in major depressive disorder is related to neural abnormalities in subcortical‐cortical midline structures. Human Brain Mapping, 30, 2617–2627.
Hamilton, J. P., Farmer, M., Fogelman, P., & Gotlib, I. H. (2015). Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biological Psychiatry, 78, 224–230. doi:10.1016/j.biopsych.2015.02.020
Hamilton, J. P., Furman, D. J., Chang, C., Thomason, M. E., Dennis, E., & Gotlib, I. H. (2011). Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biological Psychiatry, 70, 327–333. doi:10.1016/j.biopsych.2011.02.003
Hasler, G., Drevets, W. C., Manji, H. K., & Charney, D. S. (2004). Discovering endophenotypes for major depression. Neuropsychopharmacology, 29, 1765–1781. doi:10.1038/sj.npp.1300506
Hilt, L. M., & Pollak, S. D. (2012). Getting out of rumination: Comparison of three brief interventions in a sample of youth. Journal of Abnormal Child Psychology, 40, 1157–1165.
Hong, W., Abela, J. R., Cohen, J. R., Sheshko, D. M., Shi, X. T., Hamel, A. V., & Starrs, C. (2010). Rumination as a vulnerability factor to depression in adolescents in mainland China: Lifetime history of clinically significant depressive episodes. Journal of Clinical Child & Adolescent Psychology, 39, 849–857. doi:10.1080/15374416.2010.517159
Jacobs, R. H., Watkins, E. R., Peters, A. T., Feldhaus, C. G., Barba, A., Carbray, J., & Langenecker, S. A. (2016). Targeting ruminative thinking in adolescents at risk for depressive relapse: Rumination-focused cognitive behavior therapy in a pilot randomized controlled trial with resting state fMRI. PLOS ONE, 11, e0163952.
Jenkinson, M., Bannister, P., Brady, J. M., & Smith, S. M. (2002). Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841.
Johnson, M. K., Raye, C. L., Mitchell, K. J., Touryan, S. R., Greene, E. J., & Nolen-Hoeksema, S. (2006). Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience, 1, 56–64. doi:10.1093/scan/nsl004
Joormann, J., Cooney, R. E., Henry, M. L., & Gotlib, I. H. (2012). Neural correlates of automatic mood regulation in girls at high risk for depression. Journal of Abnormal Psychology, 121, 61–72. doi:10.1037/a0025294
Jose, P. E., & Brown, I. (2008). When does the gender difference in rumination begin? Gender and age differences in the use of rumination by adolescents. Journal of Youth and Adolescence, 37, 180–192.
Kaufman, J., Birmaher, B., Brent, D., & Rao, U. (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 980–988.
Kilpatrick, D. G., Ruggiero, K. J., Acierno, R., Saunders, B. E., Resnick, H. S., & Best, C. L. (2003). Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: Results from the National Survey of Adolescents. Journal of Consulting and Clinical Psychology, 71, 692–700. doi:10.1037/0022-006X.71.4.692
Lemogne, C., Delaveau, P., Freton, M., Guionnet, S., & Fossati, P. (2012). Medial prefrontal cortex and the self in major depression. Journal of Affective Disorders, 136, 1–11.
Lyubomirsky, S., & Nolen-Hoeksema, S. (1993). Self-perpetuating properties of dysphoric rumination. Journal of Personality and Social Psychology, 65, 339–349. doi:10.1037/0022-3514.65.2.339
Mandell, D., Siegle, G. J., Shutt, L., Feldmiller, J., & Thase, M. E. (2014). Neural substrates of trait ruminations in depression. Journal of Abnormal Psychology, 123, 35–48.
Marchetti, I., Koster, E. H., Sonuga-Barke, E. J., & De Raedt, R. (2012). The default mode network and recurrent depression: A neurobiological model of cognitive risk factors. Neuropsychology Reviews, 22, 229–251. doi:10.1007/s11065-012-9199-9
Matthews, S., Simmons, A., Strigo, I., Gianaros, P., Yang, T., & Paulus, M. (2009). Inhibition-related activity in subgenual cingulate is associated with symptom severity in major depression. Psychiatry Research: Neuroimaging, 172, 1–6.
Monk, C. S., Telzer, E. H., Mogg, K., Bradley, B. P., Mai, X., Louro, H. M.,…Pine, D. S. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65, 568–576. doi:10.1001/archpsyc.65.5.568
Nejad, A. B., Fossati, P., & Lemogne, C. (2013). Self-referential processing, rumination, and cortical midline structures in major depression. Frontiers in Human Neuroscience, 7, 666. doi:10.3389/fnhum.2013.00666
Nolen-Hoeksema, S., Larson, J., & Grayson, C. (1999). Explaining the gender difference in depressive symptoms. Journal of Personality and Social Psychology, 77, 1061–1072. doi:10.1037/0022-3514.77.5.1061
Nolen-Hoeksema, S., & Morrow, J. (1993). Effects of rumination and distraction on naturally occurring depressed mood. Cognition and Emotion, 7, 561–570.
Nolen-Hoeksema, S., Wisco, B. E., & Lyubomirsky, S. (2008). Rethinking rumination. Perspectives on Psychological Science, 3, 400–424. doi:10.1111/j.1745-6924.2008.00088.x
Poznanski, E. O., & Mokros, H. B. (1996). Children’s Depression Rating Scale–Revised manual. Los Angeles, CA: Western Psychological Services.
Ray, R. D., Ochsner, K. N., Cooper, J. C., Robertson, E. R., Gabrieli, J. D. E., & Gross, J. J. (2005). Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience, 5, 156–168. doi:10.3758/CABN.5.2.156
Reynolds, W. M. (1988). Reynolds Adolescent Depression Scale. New York, NY: Wiley.
Roberts, J. E., Gilboa, E., & Gotlib, I. H. (1998). Ruminative response style and vulnerability to episodes of dysphoria: Gender, neuroticism, and episode duration. Cognitive Therapy and Research, 22, 401–423.
Siegle, G. J., Carter, C. S., & Thase, M. E. (2006). Use of fMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry, 163, 735–738. doi:10.1176/appi.ajp.163.4.735
Siegle, G. J., Steinhauer, S. R., Thase, M. E., Stenger, V. A., & Carter, C. S. (2002). Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry, 51, 693–707.
Treynor, W., Gonzalez, R., & Nolen-Hoeksema, S. (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27, 247–259.
van Wingen, G. A., van Eijndhoven, P., Cremers, H. R., Tendolkar, I., Jan Verjkes, R., Buitelaar, J. K., & Fernandez, G. (2010). Neural state and trait bases of mood-incongruent memory formation and retrieval in first-episode major depression. Journal of Psychiatry Research, 44, 527–534.
Watkins, E. R. (2008). Constructive and unconstructive repetitive thought. Psychological Bulletin, 134, 163–206. doi:10.1037/0033-2909.134.2.163
Watkins, E. R. (2015). Psychological treatment of depressive rumination. Current Opinion in Psychology, 4, 32–36.
Watkins, E. R., Mullan, E. G., Wingrove, J., Rimes, K., Steiner, H., Bathurst, N.,…Scott, J. (2011). Rumination-focused cognitive behaviour therapy for residual depression: Phase II randomized controlled trial. British Journal of Psychiatry, 199, 317– 322.
Whitfield-Gabrieli, S., & Ford, J. M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology, 8, 49–76. doi:10.1146/annurev-clinpsy-032511-143049
Yang, T. T., Simmons, A. N., Matthews, S. C., Tapert, S. F., Frank, G. K., Max, J. E.,…Wu, J. (2010). Adolescents with major depression demonstrate increased amygdala activation. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 42–51.
Yeo, B. T., Krienen, F. M., Sepulcre, J., Sabuncu, M. R., Lashkari, D., Hollinshead, M.,…Fischl, B. (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106, 1125–1165.
Author note
The present study was funded by a UL1TR00050 (PI:Azar for UIC CCTS) Professional Development award, the Klingenstein Third Generation Foundation, the UIC Campus Research Board, and a Varela award from the Mind and Life Institute (awarded to R.H.J.). A.T.P. and K.L.B. were supported by National Institute of Mental Health Grant No. T32- MH067631 (Training in the Neuroscience of Mental Health; PI: Mark Rasenick), and S.A.L. was supported by Grant Nos. MH091811 and MH101487. The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 157 kb)
Rights and permissions
About this article
Cite this article
Burkhouse, K.L., Jacobs, R.H., Peters, A.T. et al. Neural correlates of rumination in adolescents with remitted major depressive disorder and healthy controls. Cogn Affect Behav Neurosci 17, 394–405 (2017). https://doi.org/10.3758/s13415-016-0486-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-016-0486-4