Abstract
In this study, we investigated the time course of attentional bias for threat-related (angry) facial expressions under conditions of high versus low cognitive (working memory) load. Event-related potential (ERP) and reaction time (RT) data were recorded while participants viewed pairs of faces (angry paired with neutral face) displayed for 500 ms and followed by a probe. Participants were required to respond to the probe while performing a concurrent task of holding in working memory a sequence of digits that were either in the same order (low memory load) or in a random mixed order (high memory load). The ERP results revealed that higher working memory load resulted in enhanced lateralized neural responses to threatening relative to neutral faces, consistent with greater initial orienting of attention to threatening faces (early N2pc: 180–252 ms) and enhanced maintenance of processing representations of threat (late N2pc, 252–320 ms; SPCN, 320–500 ms). The ERP indices showed significant positive relationships with each other, and also with the behavioral index of attentional bias to threat (reflected by faster RTs to probes replacing angry than neutral faces at 500 ms), although the latter index was not significantly influenced by memory load. Overall, the findings indicate that depletion of cognitive control resources, using a working memory manipulation, increases the capacity of task-irrelevant threat cues to capture and hold attention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Several studies indicate that threat stimuli preferentially attract attention (e.g., Calvo, Nummenmaa, & Hyönä, 2007; Holmes, Bradley, Kragh Nielsen, & Mogg, 2009; Mogg & Bradley, 1999; Nummenmaa, Hyönä, & Calvo, 2009; Öhman, Flykt, & Esteves, 2001). Evolutionary perspectives suggest that a propensity to orient attention rapidly toward a possible cue for threat will prepare an individual to deal with potential sources of danger in the environment (e.g., Davis & Whalen, 2001; Gray, 1982; LeDoux, 1996; McNaughton & Gray, 2000; Öhman, Flykt, & Lundqvist, 2000). Recent theoretical views propose that threat-related attentional capture is mediated by specialized emotion processing systems, supported by neural circuitry centered on the amygdala, which prioritize the attentional selection of stimuli with high motivational significance (e.g., Öhman & Mineka, 2003; Vuilleumier & Huang, 2009). A “biased competition” account argues that the amygdala biases the representation of threat over competing neutral stimuli by means of amygdala feedback to sensory processing areas of the brain (Pessoa, 2009; Pessoa & Adolphs, 2010; Pourtois, Schettino, & Vuilleumier, 2013; Vuilleumier, 2005).
Biased competition models of selective attention discuss not only mechanisms of reflexive biasing, but also mechanisms of top-down frontal control that are engaged to enhance processing of task-relevant stimuli and minimize interference from task-irrelevant distractor stimuli (e.g., Corbetta, Patel, & Shulman, 2008; Desimone & Duncan, 1995). One role of executive control processes is to maintain templates in working memory (WM) that provide top-down biasing signals to support task-relevant processes and suppress task-irrelevant processing (i.e., a goal-directed “attentional set”; de Fockert, Rees, Frith, & Lavie, 2001; Lavie & de Fockert, 2005). The demands on frontal control processes for the inhibition of task-irrelevant distractors are likely to be particularly pronounced when the distractors are of a threatening nature, because of the presumed enhancement of their signal from emotion processing systems.
Following these views, the extent to which attention is allocated to task-irrelevant threat information depends on competition between biasing effects of emotion-mediated processes (supporting threat-related attentional capture) and executive attention control processes (supporting task-relevant processes). If executive control processes are efficient, attention is more likely to remain task-focused and less likely to be grabbed by task-irrelevant information. However, if executive control resources are weak or depleted, task-irrelevant information is less likely to be effectively inhibited—that is, under high concurrent cognitive load, threat-related (relative to neutral) distractors would be more likely to intrude into the focus of attention due to insufficient executive attention resources to suppress their processing. The primary aim of the present study is to investigate this directly, by assessing the effect of concurrent WM load on neural and behavioral measures of the allocation of spatial attention to task-irrelevant threat information.
This research is also guided by Lavie’s (2005, 2010; Lavie, Hirst, de Fockert, & Viding, 2004) load theory of attention, which emphasizes distinct effects of “cognitive control” load (such as WM load) versus perceptual load, on distractor processing. That is, distractor interference is increased by high cognitive control load, but reduced by high perceptual load (see Lavie, 2010, for a review). Several studies have examined effects of perceptual load on emotion processing (e.g., Bishop, Jenkins, & Lawrence, 2007; Fenker et al., 2010; Okon-Singer, Tzelgov, & Henik, 2007; Richards, Hadwin, Benson, Wenger, & Donnelly, 2011), but this work is outside the scope of the present study, which is concerned instead with the effect of executive control (“cognitive”) load on attention to task-irrelevant threat (as discussed earlier). Studies examining the effect of cognitive control load on emotion processing have produced mixed findings. For example, some found no effect of manipulating concurrent WM load on discrimination judgments of emotional versus neutral stimuli (e.g., Phillips, Channon, Tunstall, Hedenstrom, & Lyons, 2008; Van Dillen & Koole, 2009). Others found that high cognitive load (arithmetic task) reduced amygdala response to aversive stimuli that were passively viewed immediately before the cognitive load (Van Dillen, Heslenfeld, & Koole, 2009). However, these studies did not directly examine effects of cognitive load on the allocation of attention to task-irrelevant threat. One exception is Pecchinenda and Heil (2007, Exp. 3) who reported that the interference effect of emotional face distractors on valence judgments of emotional word targets (which was used to index attention) was not significantly affected by concurrent WM load (remembering random sequence of digits). Similarly, a recent study by Berggren, Koster, and Derakshan (2012) has also revealed that the capture of attention by emotional faces in a visual search array was not influenced by a concurrent cognitive load (counting back in multiples of three). Given that previous relevant evidence is very limited, the need to investigate further the effect of manipulating WM load on the allocation of visuospatial attention to task-irrelevant threat cues is clear.
In the present study, we investigated the effect of manipulating executive control resources on selective attention to task-irrelevant threat while participants performed a visual probe task. In order to vary the resources available for top-down attentional control we manipulated WM load by requiring participants to remember, across every two, three, or four visual probe trials, either a fixed order of digits (low load) or a different order of digits (high load). In the visual probe task, on each trial, a threat and neutral stimulus were presented simultaneously (angry and neutral face, side-by-side) and participants were required to respond to a target probe that immediately followed the stimulus pair. Thus, the threat and neutral face in each stimulus pair competed with each other for attention, with both stimuli being task-irrelevant. The visual probe task used here provided both neural and behavioral measures of attentional allocation to threat cues. The behavioral measure of attentional bias to threat is obtained from response times (RTs) to the probes, with faster RTs to probes replacing threat, relative to neutral, stimuli indicating that attention is drawn preferentially to threat cues. The neural measures of attentional bias to threat are obtained from lateralized event-related potentials (ERPs) associated with shifts of attention to the threat versus neutral stimuli in each pair (N2pc, SPCN).
An advantage of the ERP technique is that the time course of attentive processing can be characterized at a fine temporal resolution. The N2pc reflects rapid shifts in spatial attention to cue stimuli appearing in the left or right visual field (e.g., Luck & Hillyard, 1994; Woodman & Luck, 1999). It is typically elicited between 180 and 300 ms poststimulus onset in the hemisphere contralateral to the side of the attended stimulus. Previous research has distinguished between the early and late portions of the N2pc (Eimer & Kiss, 2007; Holmes et al., 2009; Hopf et al., 2000), with evidence of the early N2pc reflecting the initiation of a shift of attention and the late N2pc being involved in the filtering of distractors in order to maintain the focus of attention (Hopf et al., 2000). A subsequent lateralized ERP component is the SPCN, or sustained posterior contralateral negativity (~300 to 650 ms; Dell’Acqua, Sessa, Jolicœur, & Robitaille, 2006; Jolicœur, Sessa, Dell’Acqua, & Robitaille, 2006), which is also known as the CDA, or contralateral delay activity (Vogel & Machizawa, 2004). The SPCN is proposed to reflect selection and maintenance of information in visual short-term memory (Dell’Acqua et al., 2006; Jolicœur et al., 2006). Maintenance of information in visual short-term memory has also been related to holding selected stimuli in the focus of attention—that is, sustained visuospatial attention (Jonides et al., 2008). Indeed, selective attention and working memory are increasingly conceptualized as overlapping constructs, as growing evidence is showing that common neural mechanisms support maintenance of attention on both internal and external stimulus representations (Chun, 2011; Chun, Golomb, & Turk-Browne, 2011; Gazzaley & Nobre, 2012).
Previous research into these neural responses to emotional information has shown rapid initial attentional selection of threat faces, relative to neutral faces, reflected by the early N2pc (~180–250 ms; e.g., Eimer & Kiss, 2007; Holmes et al., 2009), which was maintained across the late N2pc (~250–320 ms) and SPCN (~320–500 ms); consistent with a bias in initial orienting and maintained attention toward threat relative to neutral information over this time-period (Holmes et al., 2009; see also Feldmann-Wüstefeld, Schmidt-Daffy, & Schubö, 2011).
To recap briefly, allocation of attention to task-irrelevant threat cues is assumed to depend on the interplay between emotion-related influences (which automatically direct attention to threat) and top-down influences (which support task-relevant processes and inhibit processing of task-irrelevant information). If executive control resources are depleted by additional cognitive demands (e.g., high WM load), attention to task-irrelevant threat should be less effectively suppressed. Consequently, it is hypothesised that task-irrelevant threat cues will attract greater attention when concurrent WM load is high, relative to low concurrent WM load. It is predicted that this effect will be found for each measure of attentional bias for threat—that is, assessed by early N2pc (Hypothesis 1), late N2pc (Hypothesis 2), SPCN (Hypothesis 3), and manual RTs to probes (Hypothesis 4). Since each measure is assumed to reflect preferential allocation of processing resources to threat relative to neutral cues, it is also hypothesized that these measures will positively correlate with each other (Hypothesis 5).
Method
Participants
The participants were 23 healthy volunteers. One participant was excluded because of excessive eye movement artifacts (≥80 %), so that 22 participants (three male, 19 female; 18–41 years old; average age: 25.6 years) remained in the sample. All participants had normal or corrected-to-normal vision and all were right-handed. The experiment was performed in compliance with relevant institutional guidelines and was approved by the University ethics committee.
Stimuli and apparatus
In the visual probe task, face stimuli consisted of pairs of grayscale photographs of 32 different individuals (16 male, 16 female) taken from the NimStim Face Stimulus Set (Tottenham et al., 2009). Each pair consisted of two pictures of the same individual, with one photograph portraying an angry expression and the other a neutral expression. An additional set of neutral face pairs using photographs of four individuals (two male, two female) from the NimStim Set was used for practice items. Each face was enclosed within a black rectangular frame measuring 8 cm high × 6.2 cm wide, and the centers of the faces were 5 cm from a white central fixation cross. The faces within each pair were equated for mean luminance and root mean square contrast energy using standard routines in MATLAB 7. The probe stimuli were white up- and down-pointing arrows measuring 0.8 cm, and replaced the left or right faces at a position of 3.75 cm from the central fixation cross.
In the memory task, each digit measured 0.3 cm horizontally and 0.5 cm vertically. Each memory set (i.e., string of five digits) subtended 2.8 cm horizontally. All stimuli appeared against a black background. Participants were seated in a dark cabin, and stimuli were presented at a viewing distance of approximately 70 cm on a 17-in ViewSonic G220f computer screen with a refresh rate of 75 Hz, connected to a Dell Precision Pentium IV computer. Stimulus presentation was controlled with E-Prime v2.0 (Psychology Software Tools Inc.; www.pstnet.com/prime). Stimulus parameters were based on those employed by Holmes et al. (2009) and de Fockert et al. (2001).
Procedure
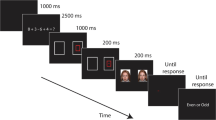
Each WM load trial contained a memory set (digit string) and memory test, interspersed by an unpredictable series of visual probe trials, as is described below (Fig. 1). After a 500-ms fixation cross, the memory set for that trial was presented for 1,500 ms. Under low WM load, the digit string was always “01234.” Under high WM load, the last four digits were in a new random order for each trial—for example, “04312” (“01234” and “04321” were excluded from the high-WM-load condition). Participants were instructed to remember the order of these digits for the memory test at the end of the trial. After each memory set, a fixation display was presented for 850 ms, followed by two, three, or four visual probe trials. The number of visual probe trials within each memory task trial was varied in order to make the onset of the memory probe unpredictable, thus ensuring that the memory set was actively rehearsed throughout each visual probe trial. After the short series of visual probe trials, the memory test was presented, which started with a 500-ms fixation cross, followed by a memory probe for 3,000 ms. Participants were requested to report the digit that followed this probe in the memory set by pressing the appropriate key (labeled “1,” “2,” “3,” or “4”) on the numeric keypad on the computer keyboard using the left hand. In order to ensure that all four responses (including “1” in low-WM-load trials) were used, a “0” was always the first digit in each memory set. On each trial, the probe digit was randomly selected, ensuring that it was not the same as the last digit in the memory set. Immediately following a response, a new WM load trial was presented. The WM load trial sequence was adapted from de Fockert et al. (2001).
Each visual probe trial started with a central fixation cross (see Fig. 1). After 500 ms, an angry–neutral face pair was also displayed for a further 500 ms. Immediately after the offset of the face cues and the fixation cross, a probe was presented until a response was made or until 6 s had elapsed. Participants were instructed to press one of two buttons on a purpose-built response box, using the index finger (upper button) and thumb (lower button) of their right hand, to indicate as quickly and accurately as possible the type of probe (i.e., up-pointing arrow [upper key] or down-pointing arrow [lower key]). Participants were also asked to keep their gaze focused on the central fixation location throughout the task. The intertrial interval (ITI) was variable, ranging from 750 to 1,250 ms. This trial sequence was used because previous behavioral and electrophysiological investigations had shown it to be sensitive to threat-related attentional bias (e.g., Holmes et al., 2009; Mogg & Bradley, 1998). All visual probe trial types (varying as a function of position of angry face, probe position, and probe type) were equiprobable across the experimental trials and were presented in a new mixed random order for each participant within each block.
Participants were given two short practice blocks of trials (one high and one low WM load block); each consisting of five memory task trials and 16 visual probe trials. This was followed by eight experimental blocks (four high WM load alternating with four low WM load). Each experimental block consisted of ten memory task trials and 32 visual probe trials.
EEG data acquisition
EEG was recorded using a Neuroscan 64-channel device (Synamps). Horizontal and vertical electrooculographs (EOGs) were recorded using four facial bipolar electrodes placed on the outer canthi of the eyes and in the inferior and superior areas of the left orbit. Scalp EEG was recorded from 62 Ag–AgCl electrodes mounted in a quickcap (extended 10–20 system). All electrodes were referenced online to one electrode (vertex) and bandpass filtered at 0.01–100 Hz. The impedance for electrodes was generally kept below 5 kΩ, and EEG and EOG were sampled online with a digitization rate of 1000 Hz. Following EEG recording, data were downsampled to 250 Hz to save later computation time, digitally filtered with a low-pass filter at 40 Hz, and all channels were re-referenced using the average of the mastoids (M1 and M2). EEG and horizontal EOGs (HEOGs) were epoched offline relative to a 100-ms prestimulus baseline, and extending for 500 ms after stimulus presentation.
Trials with lateral eye movements (HEOG exceeding ±30 μV), as well as trials with vertical eye movements, eye blinks, or other artifacts (a voltage exceeding ±60 μV at any electrode) measured after target onset were excluded from analysis. This resulted in the rejection of 26 % of trials. No significant differences were revealed by t tests comparing a) the number of horizontal eye movements that were rejected across high-WM (M = 10.9, SD = 8.6) and low-WM load (M = 9.5, SD = 7.3) conditions, t(21) = 1.33, p = .20; or b) the magnitude of HEOG signals across the entire critical time interval of 180–500 ms for both high WM (M = −0.79 μV, SD = 2.4) and low WM load (M = −1.07 μV, SD = 2.8) conditions, t(21) = 0.72, p = .48. These findings indicate that any ERP effects relating to WM load cannot be explained simply in terms of variation in the focusing of gaze on the central fixation cross and thus clarity of perception for the bilateral face stimuli.
Separate averages were computed for all combinations of WM load (high vs. low), angry face location (left vs. right), contralaterality (electrodes ipsilateral vs. contralateral to the location of the emotional face), and component (early N2pc vs. late N2pc vs. SPCN). The ipsilateral waveform was computed as the average of the left-sided electrodes to the left-sided angry face and the right-sided electrodes to the right-sided angry face, and the contralateral waveform was computed as the average of the left-sided electrodes to the right-sided angry face and the right-sided electrodes to the left-sided angry face. Regional activity was analyzed at lateral posterior electrodes P7, PO7 (left hemisphere), P8, PO8 (right hemisphere), within poststimulus time windows of 180–252 ms (early N2pc), 252–320 ms (late N2pc), and 320–500 ms (SPCN). Our selection of time windows was consistent with our previous investigation of attentional selection of emotional faces (Holmes et al., 2009). The selection of electrode sites was also based on this study (P7, P8) and, in addition, we selected P07 and P08 because these had been employed previously by Eimer and Kiss (2007).
Results
Visual probe task: ERP data
Figure 2 shows the ERPs obtained at electrode sites contralateral to the angry face location (solid lines) and ipsilateral to the angry face location (dashed lines) for the high-WM-load (top panel) and low-WM-load (bottom panel) conditions. In the high-load condition, an enhanced negativity appeared contralateral to angry face cues within the early phase of the N2pc (180–252 ms), and remained present throughout the late phase of the N2pc (252–320 ms) and the SPCN (320–500 ms). By contrast, we found no evidence of an enhanced negative contralaterality effect under conditions of low WM load across any of the component time windows. These observations were confirmed using omnibus analysis of variance (ANOVA) and hypothesis-driven contrasts.
Grand averaged event-related potentials (ERPs) for regional analyses of posterior electrode sites (P7, P8, PO7, and PO8), elicited to stimulus pairs containing a neutral and an angry face under high WM load (top panel) and low WM load (lower panel). ERPs are shown at electrodes contralateral (solid lines) and ipsilateral (dashed lines) to the angry face
ERP amplitudes were entered into a 2 × 2 × 3 repeated measures ANOVA, with the factors WM Load (high vs. low), Contralaterality (electrodes ipsilateral vs. contralateral to location of angry face), and Component (early N2pc vs. late N2pc vs. SPCN). We observed significant main effects of component, F(2, 42) = 5.99, p = .005, η p 2 = .22, and contralaterality, F(1, 21) = 13.58, p = .001, η p 2 = .39, as well as a significant interaction between WM load and contralaterality, F(1, 21) = 7.65, p = .01, η p 2 = .27.Footnote 1 No other significant main effects or interactions emerged. Notably, the WM Load × Contralaterality interaction was not significantly influenced by component (F ≤ 1).
To test the specific hypotheses and clarify the significant two-way interaction, contralaterality threat-bias scores (which reflect attentional bias to angry relative to neutral faces) were calculated by taking the mean amplitude contralateral to angry faces minus the mean amplitude ipsilateral to angry faces (since an angry and a neutral face appeared simultaneously in opposite visual fields on each trial). These bias scores were calculated for each WM load condition, ERP component, and participant (see Table 1 for the mean contralaterality threat-bias scores). Hypothesis-driven paired contrasts indicated that attention bias to angry faces was significantly increased for each ERP component in the high-load condition, relative to the low-load condition [early N2pc, t(21) = 3.27, p = .004, d = 0.70; late N2pc, t(21) = 2.76, p = .01, d = 0.59; SPCN, t(21) = 2.14, p = .04, d = 0.46]. These results support Hypotheses 1–3.
Visual probe task: RT data
RTs were excluded from trials with incorrect responses (1.0 % of trials: see Table 1 for the mean errors) and outliers (RTs ≤200 ms or ≥1,000 ms; 1.7 % of trials). RTs were log-transformed before the analyses to reduce skewness. The analyses were conducted on transformed data, whereas descriptive statistics are given for untransformed data for ease of comprehension. The mean RTs in each condition were entered into a 2 × 2 repeated measures ANOVA, with the factors WM Load (high, low) and Probe Location (probe replaces angry face, probe replaces neutral face). We found a significant main effect of probe location, F(1, 21) = 4.32, p = .05, η p 2 = .17, as responses were faster on trials in which the probe and angry face appeared in the same location (M = 557 ms, SD = 72) rather than in opposite locations (M = 561 ms, SD = 71), which is consistent with an attentional bias toward threat relative to neutral faces. No other significant main or interaction effects emerged. Since the effect of probe location on RTs (indicating attentional bias to threat) was not significantly influenced by the WM load manipulation (i.e., Probe Location × WM Load interaction; F ≤ 1), Hypothesis 4 was not supported.
Memory test
Performance on the memory test indicated that the WM load manipulation was effective. Participants gave more correct responses to the memory test questions in the low-load than in the high-load condition, paired t(21) = 5.15, p ≤ .001, d = 1.54, and their responses were also faster in the low-load than in the high-load condition, paired t(21) = 13.93, p ≤ .001, d = 3.04 (see Table 1 for the mean accuracy and RTs).
Correlations between attentional bias measures
Correlations were calculated to test the prediction that the ERP and RT measures of attentional bias would be positively correlated with each other (Hypothesis 5). The ERP measures were the contralaterality threat-bias scores for each of the three ERP components (which reflect enhanced processing of threat relative to neutral faces) described earlier. These scores were highly intercorrelated (i.e., early N2pc bias score correlated .90 with late N2pc bias score, and .82 with SPCN bias score; the late N2pc bias score correlated .82 with SPCN bias score; all ps ≤ .01).
The probe RT measure of threat-related attention bias was calculated as the difference in mean RTs between “probe replaces neutral face” versus “probe replaces angry face” trials (this difference corresponds to the “probe location effect” in the analysis of RT data described earlier). The RT index of attentional bias correlated positively with each ERP measure of threat-related bias (i.e., RT bias correlated .43, .45, and .48 with the early N2pc, late N2pc, and SPCN contralaterality bias scores, respectively; all ps ≤ .05). The RT bias measure significantly correlated .47, p ≤ .05, with the overall ERP bias index (contralaterality bias score for threat relative to neutral faces, averaged across the three ERP components).
Discussion
The aim of the study was to investigate whether threatening faces attract greater attention under high compared with low concurrent cognitive load. The main results can be summarized as follows. The ERP results revealed that threat-related attentional bias was significantly greater under conditions of high than low WM load. This enhanced attentional prioritization of angry faces was found across three time windows following the onset of the face-pair (corresponding to early N2pc, late N2pc and SPCN), supporting the first three experimental hypotheses. The probe RT results showed evidence for an attentional bias toward angry faces; however, this bias was not modified by WM load, providing no support for the fourth hypothesis. Correlational evidence showed that the behavioral index of threat-related attentional bias was significantly associated with each ERP measure of attentional bias, and that the ERP measures were also significantly inter-correlated, as predicted by the fifth hypothesis.
Only a few studies to date have investigated lateralized ERP correlates of threat-related attentional bias. As we noted earlier, the N2pc has a notable advantage of providing an objective neural index of early shifts in visuospatial attention, which has high temporal sensitivity. A consistent finding across previous studies is that attentional shifts toward threatening faces arise rapidly, with the emergence of an N2pc as early as ~180–250 ms poststimulus onset (e.g., Eimer & Kiss, 2007; Fox, Derakshan, & Shoker, 2008; Holmes et al., 2009). Various mechanisms have been proposed to underlie rapid attentional orienting toward sources of threat, including the facilitation of sensory processing by the amygdala (e.g., Pessoa, 2009; Pessoa & Adolphs, 2010; Pourtois et al., 2013) and attentional networks in frontoparietal cortex (e.g., Armony & Dolan, 2002). Crucially, the present findings of early N2pc reveal that this initial stage of visuospatial selection (i.e., 180–252 postcue onset) is modulated by concurrent demands on processing: When top-down cognitive control processes were depleted by a concurrent WM task, task-irrelevant threat was more likely to capture attention. These findings relate to growing evidence that the attentional processes typically considered to be primarily reflexive or bottom-up driven (e.g., those involved in initial attention capture), can be influenced by executive control mechanisms (Kiefer, 2012).
High WM load was also associated with subsequent enhanced processing of threat cues, as reflected by the late N2pc and SPCN (i.e., 252–320 ms; and 320–500 ms, respectively). Holmes et al. (2009) noted that enhanced processing of angry faces across both the N2pc and SPCN is consistent with proposals that threatening stimuli not only rapidly capture attention (e.g., Öhman & Mineka, 2003), but also hold it (at least over the relatively short time intervals assessed here; Fox, Russo, Bowles, & Dutton, 2001). From an evolutionary perspective, such holding of attention may allow novel or potentially significant events to be monitored. Holding attention on threat cues may relate to maintenance of such information in visual short-term memory, which contributes to the SPCN (e.g., Dell’Acqua et al., 2006; Jolicœur et al., 2006; Vogel & Machizawa, 2004). It should be noted, however, that the SPCN was measured whilst face stimuli were still displayed on the screen. It is therefore hard to disentangle the extent to which the SPCN findings reflect maintained processing of threat information in visual short-term memory versus maintained attention focusing on external threat stimuli. However, as noted earlier, working memory and selective attention are increasingly conceptualized as closely related, overlapping constructs, which are supported by common neural mechanisms (Chun et al., 2011; Gazzaley & Nobre, 2012). Thus, the present SPCN results may reflect activation in neural mechanisms associated with maintaining attention on both internal and external stimulus representations of threat.
The primary results from this study indicate that, when executive control resources are depleted by additional cognitive demands (i.e., in this case, high WM load), the capture and holding of attention by task-irrelevant threat cues is enhanced. One explanation for this is that, under conditions of depleted cognitive control, task-irrelevant threat is less efficiently inhibited and therefore more likely to intrude into the focus of attention. Conversely, under conditions in which cognitive control resources have not been depleted, such as in the present low-WM-load condition, task-irrelevant threat can be inhibited and prevented from entering the focus of attention. However, mechanisms of cognitive control and inhibition may require a trigger, such as the detection or awareness of concurrent task demands that are judged to be potential sources of interference during task performance. This may explain why attenuation of attentional orienting to task-irrelevant threat was not previously observed in a visual probe task that did not involve secondary tasks or other demands on cognitive resources (Holmes et al., 2009). The present findings are novel because, to our knowledge, no previous study has demonstrated an effect of cognitive (WM) load on attention allocation to threat. Notably, this effect extends across distinct attentional operations relating to initial orienting and subsequent maintenance of attention.
Additional notable findings are the significant positive relationships between the behavioral and ERP measures, which support the view that they reflect common mechanisms underlying attentional threat-prioritization. Specifically, greater N2pc and SPCN responses to threat, relative to neutral, cues were associated with faster RTs to probes that subsequently replaced the threat cues, which is a widely used behavioral index of attentional bias to threat. Despite these positive inter-correlations between the RT and ERP measures, only the ERP measures confirmed the hypothesized effect of cognitive load on increasing attention to task-irrelevant threat. A recent MEG study also reported that N2pc and RT data showed different effects of processing demands on attention to threat (Fenker et al., 2010). However, this study examined the effect of varying perceptual load, which, as noted earlier, is argued to have the opposite effect on distractor processing than cognitive (WM) load (Lavie, 2010). Results indicated that perceptual load did not significantly influence N2pc that was elicited by task-irrelevant fearful faces; whereas RT data showed an attentional bias for fearful faces only when perceptual load was low. Comparison of results with the present ones is complicated by substantial methodological differences, such as different types of load manipulation (perceptual versus WM load) and attentional tasks (visual search versus visual probe). These findings highlight the need to clarify the effects of cognitive (WM) versus perceptual load manipulations on threat processing, to assess prioritization of threat processing in the context of Lavie’s (2010) load theory of attention. Further work is also required in order to determine the extent to which cognitive (WM) load influences the capture of attention by task-irrelevant cues when these cues are more generally negative or positive in terms of emotional valence, as opposed to being specifically threat-related.
Regarding the lack of effect of WM load on the behavioral RT index of attentional bias in this study, it should be noted that the RT index is less direct than the ERP measures and is obtained after the offset of the threat stimuli (i.e., the RT measure reflects attention allocation to probes that replace threat cues, rather than attention allocation to the cues per se). These results highlight the importance of using temporally sensitive measures such as ERPs, as they can provide a more detailed account of the temporal dynamics of attentive processing than RTs.
In conclusion, to our knowledge, this is the first study to demonstrate a modulatory role of cognitive control resources on neural processes underlying visuospatial attentional bias to task-irrelevant threat. Angry face stimuli attracted greater attention when cognitive control resources were depleted (i.e., under high, relative to low, concurrent WM load). The present findings indicate the importance of executive processes for the resistance of interference from distracting threat cues.
Notes
The grand averaged waveforms suggested the presence of differential contralaterality effects as a function of WM load within a time range overlapping with the P1 component (80–120 ms). The ERPs indicated the presence of an enhanced contralateral negativity to angry face cues under high WM load (indicating a possible attentional bias toward angry faces), relative to an ipsilateral negativity to angry face cues under low WM load (indicating a possible attentional bias away from angry faces and toward neutral faces). However, the interaction just failed to reach significance, F(1, 21) = 3.97, p = .06, η p 2 = .16. Since this effect was not predicted by any of our hypotheses, caution should be exercised in the interpretation of this marginal result. Nonetheless, it might be of value to follow up in future research the possibility of an early attentional bias effect within this time range.
References
Armony, J. L., & Dolan, R. J. (2002). Modulation of spatial attention by fear-conditioned stimuli: An event-related fMRI study. Neuropsychologia, 40, 817–826.
Berggren, N., Koster, E. H. W., & Derakshan, N. (2012). The effect of cognitive load in emotional attention and trait anxiety: An eye movement study. Journal of Cognitive Psychology, 24, 79–91.
Bishop, S. J., Jenkins, R., & Lawrence, A. D. (2007). Neural processing of fearful faces: Effects of anxiety are gated by perceptual capacity limitations. Cerebral Cortex, 17, 1595–1603.
Calvo, M. G., Nummenmaa, L., & Hyönä, J. (2007). Emotional and neutral scenes in competition: Orienting, efficiency, and identification. Quarterly Journal of Experimental Psychology, 60, 1585–1593.
Chun, M. M. (2011). Visual working memory as visual attention sustained internally over time. Neuropsychologia, 49, 1407–1409.
Chun, M. M., Golomb, J. D., & Turk-Browne, N. B. (2011). A taxonomy of external and internal attention. Annual Review of Psychology, 62, 73–101. doi:10.1146/annurev.psych.093008.100427
Corbetta, M., Patel, G., & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58, 306–324.
Davis, M., & Whalen, P. J. (2001). The amygdala: Vigilance and emotion. Molecular Psychiatry, 6, 13–34.
de Fockert, J. W., Rees, G., Frith, C. D., & Lavie, N. (2001). The role of working memory in visual selective attention. Science, 291, 1803–1806. doi:10.1126/science.1056496
Dell’Acqua, R., Sessa, P., Jolicœur, P., & Robitaille, N. (2006). Spatial attention freezes during the attention blink. Psychophysiology, 43, 394–400. doi:10.1111/j.1469-8986.2006.00411.x
Desimone, R., & Duncan, J. (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. doi:10.1146/annurev.ne.18.030195.001205
Eimer, M., & Kiss, M. (2007). Attentional capture by task-irrelevant fearful faces is revealed by the N2pc component. Biological Psychology, 74, 108–112.
Feldmann-Wüstefeld, T., Schmidt-Daffy, M., & Schubö, A. (2011). Neural evidence for the threat detection advantage: Differential attention allocation to angry and happy faces. Psychophysiology, 48, 697–707.
Fenker, D. B., Heipertz, D., Boehler, C. N., Schoenfeld, M. A., Noesselt, T., Heinze, H.-J., … Hopf, J. M. (2010). Mandatory processing of irrelevant fearful face features in visual search. Journal of Cognitive Neuroscience, 22, 2926–2938. doi:10.1162/jocn.2009.21340
Fox, E., Derakshan, N., & Shoker, L. (2008). Trait anxiety modulates the electrophysiological indices of rapid spatial orienting towards angry faces. NeuroReport, 19, 259–263.
Fox, E., Russo, R., Bowles, R., & Dutton, K. (2001). Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General, 130, 681–700. doi:10.1037/0096-3445.130.4.681
Gazzaley, A., & Nobre, A. C. (2012). Top-down modulation: Bridging selective attention and working memory. Trends in Cognitive Sciences, 16, 129–135. doi:10.1016/j.tics.2011.11.014
Gray, J. A. (1982). The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford, UK: Oxford University Press.
Holmes, A., Bradley, B. P., Kragh Nielsen, M., & Mogg, K. (2009). Attentional selectivity for emotional faces: Evidence from human electrophysiology. Psychophysiology, 46, 62–68.
Hopf, J.-M., Luck, S. J., Girelli, M., Hagner, T., Mangun, G. R., Scheich, H., & Heinze, H.-J. (2000). Neural sources of focused attention in visual search. Cerebral Cortex, 10, 1233–1241.
Jolicœur, P., Sessa, P., Dell’Acqua, R., & Robitaille, N. (2006). On the control of visual spatial attention: Evidence from human electrophysiology. Psychological Research, 70, 414–424.
Jonides, J., Lewis, R. L., Nee, D. E., Lustig, C. A., Berman, M. G., & Moore, K. S. (2008). The mind and brain of short-term memory. Annual Review of Psychology, 59, 193–224. doi:10.1146/annurev.psych.59.103006.093615
Kiefer, M. (2012). Executive control over unconscious cognition: Attentional sensitization of unconscious information processing. Frontiers in Human Neuroscience, 6, 1–12.
Lavie, N. (2005). Distracted and confused? Selective attention under load. Trends in Cognitive Sciences, 9, 75–82. doi:10.1016/j.tics.2004.12.004
Lavie, N. (2010). Attention, distraction, and cognitive control under load. Current Directions in Psychological Science, 19, 143–148. doi:10.1177/0963721410370295
Lavie, N., & de Fockert, J. W. (2005). The role of working memory in attentional capture. Psychonomic Bulletin & Review, 12, 669–674.
Lavie, N., Hirst, A., de Fockert, J. W., & Viding, E. (2004). Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General, 133, 339–354. doi:10.1037/0096-3445.133.3.339
LeDoux, J. E. (1996). The emotional brain: The mysterious underpinnings of emotional life. New York, NY: Simon & Schuster.
Luck, S. J., & Hillyard, S. A. (1994). Spatial filtering during visual search: Evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception and Performance, 20, 1000–1014. doi:10.1037/0096-1523.20.5.1000
McNaughton, N., & Gray, J. A. (2000). Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. Journal of Affective Disorders, 61, 161–176.
Mogg, K., & Bradley, B. P. (1998). A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy, 36, 809–848.
Mogg, K., & Bradley, B. P. (1999). Orienting of attention to threatening facial expressions presented under conditions of restricted awareness. Cognition and Emotion, 13, 713–740.
Nummenmaa, L., Hyönä, J., & Calvo, M. G. (2009). Emotional scene content drives the saccade generation system reflexively. Journal of Experimental Psychology: Human Perception and Performance, 35, 305–323.
Öhman, A., Flykt, A., & Esteves, F. (2001). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General, 130, 466–478. doi:10.1037/0096-3445.130.3.466
Öhman, A., Flykt, A., & Lundqvist, D. (2000). Unconscious emotion: Evolutionary perspectives, psychophysiological data, and neuropsychological mechanisms. In R. Lane & L. Nadel (Eds.), The cognitive neuroscience of emotion (pp. 296–327). New York, NY: Oxford University Press.
Öhman, A., & Mineka, S. (2003). The malicious serpent: Snakes as a prototypical stimulus for an evolved module for fear. Current Directions in Psychological Science, 12, 5–9.
Okon-Singer, H., Tzelgov, J., & Henik, A. (2007). Distinguishing between automaticity and attention in the processing of emotionally-significant stimuli. Emotion, 7, 147–157.
Pecchinenda, A., & Heil, M. (2007). Role of working memory load on selective attention to affectively valent information. European Journal of Cognitive Psychology, 19, 898–909.
Pessoa, L. (2009). How do emotion and motivation direct executive function? Trends in Cognitive Science, 13, 160–166. doi:10.1016/j.tics.2009.01.006
Pessoa, L., & Adolphs, R. (2010). Emotion processing and the amygdala: From a “low road” to “many roads” of evaluating biological significance. Nature Reviews Neuroscience, 11, 773–783.
Phillips, L. H., Channon, S., Tunstall, M., Hedenstrom, A., & Lyons, K. (2008). The role of working memory in decoding emotions. Emotion, 8, 184–191.
Pourtois, G., Schettino, A., & Vuilleumier, P. (2013). Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biological Psychology, 92, 492–512.
Richards, H. J., Hadwin, J. A., Benson, V., Wenger, M. J., & Donnelly, N. (2011). The influence of anxiety on processing capacity for threat detection. Psychonomic Bulletin & Review, 18, 883–889.
Tottenham, N., Tanaka, J., Leon, A. C., McCarry, T., Nurse, M., & Hare, T. A. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168, 242–249.
Van Dillen, L. F., Heslenfeld, D. J., & Koole, S. L. (2009). Tuning down the emotional brain: An fMRI study of the effects of cognitive load on the processing of affective images. NeuroImage, 45, 1212–1219.
Van Dillen, L. F., & Koole, S. L. (2009). How automatic is “automatic vigilance”? The role of working memory in attentional interference of negative information. Cognition and Emotion, 23, 1106–1117.
Vogel, E. K., & Machizawa, M. G. (2004). Neural activity predicts individual differences in visual working memory capacity. Nature, 428, 748–751. doi:10.1038/nature02447
Vuilleumier, P. (2005). How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585–594. doi:10.1016/j.tics.2005.10.011
Vuilleumier, P., & Huang, Y. M. (2009). Emotional attention: Uncovering the mechanisms of affective biases in perception. Current Directions in Psychological Science, 18, 148–152.
Woodman, G. F., & Luck, S. J. (1999). Electrophysiological measurement of rapid shifts of attention during visual search. Nature, 400, 867–869.
Author note
This research has been supported by an internal grant from the University of Roehampton. The authors thank Paul Bretherton for his technical assistance. We thank Professor Deanna Barch and two anonymous reviewers for comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holmes, A., Mogg, K., de Fockert, J. et al. Electrophysiological evidence for greater attention to threat when cognitive control resources are depleted. Cogn Affect Behav Neurosci 14, 827–835 (2014). https://doi.org/10.3758/s13415-013-0212-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-013-0212-4