Abstract
Social–psychological research has suggested that being imitated changes the way that we experience others: We like someone who imitates us more, and the interaction with this person runs more smoothly. Whether being imitated also affects basic social reactions, such as empathy for pain, is an open question. Empathy for pain refers to the observation that perceiving another person in pain results in pain-related brain activation in the observer. The aim of the present study was to combine the two lines of research, to investigate whether being imitated can influence empathy for pain. To this end, we developed an experimental approach combining an imitation task with a pain perception task. Subjective reports, as well as physiological responses, indicated that being imitated enhances affective responses to seeing someone else in pain. Furthermore, using rubber hand illusion measures, we provided evidence for the role of shared representations in the sensory and motor domains as a core underlying mechanism. In this way, our study integrated social–psychological research on being imitated with cognitive research on empathy for pain. This has broad implications, since imitation plays a crucial role in our daily social interactions, and our study provides insights into a basic cognitive mechanism that might underlie these social situations.
Similar content being viewed by others
Imitation is an important part of the human behavioral repertoire. It sometimes occurs automatically, without intention, and even between complete strangers (Brass, Bekkering, & Prinz, 2001; Brass, Bekkering, Wöhlschläger, & Prinz, 2000; Lakin & Chartrand, 2003). Moreover, an extensive body of social–psychological research has shown that imitation is very important for our social life, by changing the way that we experience others. Research on the so-called chameleon effect has suggested that we like someone who imitates us more, and that interactions with this person correspondingly run more smoothly (Chartrand & Bargh, 1999). Furthermore, several experiments have indicated that being imitated enhances prosocial orientation (positive social behavior toward others; Kühn et al., 2010; Lakin, Chartrand, & Arkin, 2008; Stel, van Baaren, & Vonk, 2008).
Although the positive consequences of being imitated have been demonstrated for relatively complex social behavior, such as liking and prosocial actions, the question arises whether being imitated also influences more automatic and implicit social processes, such as reacting to someone else being in pain. Several studies have demonstrated that perceiving another person in pain activates brain regions involved in the affective–motivational dimensions of pain (Goubert, Vervoort, & Craig, 2012; Jackson, Meltzoff, & Decety, 2005; Lamm, Decety, & Singer, 2011; Singer et al., 2006), especially when one has an affective relationship with the person observed (Cheng, Chen, Lin, Chou, & Decety, 2010; Singer et al., 2004). This finding, called empathy for pain, shows that the observation of pain activates pain-related brain regions in the observer that are also active when directly experiencing pain. Thus, it indicates that first-person experience of pain and the observation of pain in others are based on shared neural circuits, with growing evidence for both affective and sensory sharing in empathy for pain responses (Bufalari, Aprile, Avenanti, Di Russo, & Aglioti, 2007; Cheng, Yang, Lin, Lee, & Decety, 2008; Lamm, Nusbaum, Meltzoff, & Decety, 2007; Loggia, Mogil, & Bushnell, 2008; for a review, see Keysers, Kaas, & Gazzola, 2010). Several studies have indicated that this sharing of representations when observing someone else in pain can be influenced by a wide range of cognitive mechanisms, and is thus modulated by top-down processing (e.g., Cheng et al., 2007; Decety, Echols, & Correll, 2010; de Vignemont & Singer, 2006; Hein & Singer, 2008; Lamm, Meltzoff, & Decety, 2010; Singer et al., 2006). Furthermore, it has been shown that features such as the race (Xu, Zuo, Wang, & Han, 2009) and gender (Han, Fan, & Mao, 2008; Yang, Decety, Lee, Chen, & Cheng, 2009) of the person in pain play crucial roles. Decety and Lamm (2006) therefore suggested a model in which the empathic response can be seen as the intertwined influence of both bottom-up and top-down factors.
The aim of our first experiment was to investigate whether being imitated can also influence affective responses to seeing someone else in pain. In particular, we wanted to investigate whether the functional system that is involved in motor imitation interacts with the system that mediates empathy for pain and to explore the underlying mechanisms. Although both systems are related to different neural structures (e.g., Grèzes & Decety, 2001; Singer et al., 2004), they are based on a similar functional mechanism: Both being imitated and empathy for pain have been related to shared representations of self and other (Bastiaansen, Thioux, & Keysers, 2009; Brass & Heyes, 2005; Heberlein & Atkinson, 2009). Furthermore, the chameleon effect has already been linked to empathy (the ability to share the affective experiences of others; Singer & Lamm, 2009), and an underlying shared representational system has been proposed here as well (Chartrand & van Baaren, 2009). We predicted that being imitated would increase empathy for pain relative to not being imitated. To test this hypothesis, we developed an experimental approach combining a simple imitation task (participants performed finger-lifting movements that were or were not imitated by a hand on screen) with a pain perception task (painful stimulation was applied to the hand on screen) and investigated whether measures of empathy for pain were higher in the imitative condition. To measure whether being imitated would lead to higher empathy for pain, we focused on explicit and implicit responses. First, we used a self-report measure after each pain movie. In addition, since Preston and de Waal (2002) argued that the activation of brain representations can result in somatic and autonomic responses, we tested whether physiological responding was affected. Thus, as a somatic response, we measured the startle blink reflex with electromyography (EMG), a blink reflex of the eye that is part of an automatic reaction to sudden, intense stimuli (Miller, Patrick, & Levenston, 2002). It has been shown that the amplitude of this blink reflex varies according to changes in affective value, with larger amplitudes for negative and smaller amplitudes for positive situations, both relative to neutral situations (Vrana, Spence, & Lang, 1988). If being imitated leads to higher empathy for pain, we expected the startle blink amplitude to be larger in the imitation condition, since observing somebody in pain has a negative affective value.
Furthermore, in a second experiment, we aimed to explore the underlying mechanism(s) in more depth, using a spatial variation of the paradigm described above. More specifically, we wanted to investigate whether self–other confusion might underlie the observed effects, by developing a setup in which this confusion would be thought to be enhanced. If self–other confusion modulates the influence of being imitated on empathy for pain, we expected this new setup (specifically aimed at eliciting stronger confusion) to increase this influence.
Experiment 1
Method
Participants
A group of 20 right-handed volunteers (M age = 19.61 years, SD = 1.56) participated in the study, all of whom had normal or corrected-to-normal vision. To control for possible sex differences (see, e.g., Han, Fan, & Mao, 2008; Yang et al., 2009), only female volunteers were recruited. They were given course credits in exchange for participation and provided written consent at the beginning of the experiment. The study was granted ethical approval by the local ethics committee.
Experimental design
Blocks of trials consisted of two phases: an action phase, in which movements of the participants were imitated (imitation block) or not imitated (nonimitation block), and a pain perception phase that immediately followed the action phase. In the pain perception phase, one of nine pain movies was presented (see Table 1). Each pain movie was combined two times with both an imitation and a nonimitation block, and once after each type of imitation block, a startle probe was presented during the pain movie to elicit the startle blink reflex. As such, the experiment consisted of 36 trials: Each of the nine pain movies was combined with both imitation (imitation and nonimitation) and both startle (startle and no-startle) conditions. The content of the pain movie, the block condition, and the startle condition were completely randomized.
Stimuli and apparatus
The stimulus materials consisted of three types of 720 × 576 video clips, created by professionals: a hand in a resting position, simple finger movements (for the action phase of the task), and pain videos showing a hand receiving pain stimulation (for the pain perception phase).
In the resting-state video clip, a right hand with palm down and fingers slightly spread was shown, in a position similar to that of the participant’s right hand placed on the response box. This video remained on screen between presentation of the other videos, in order to assure continuous observation of a right hand on screen.
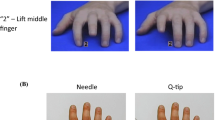
During the action phase of the experimental task, participants carried out simple finger movements of the index, middle, ring, or little finger. These finger movements were recorded with a custom-built response device using light sensors. This device allowed us to use finger-lifting movements of participants as triggers for the presentation of the appropriate finger movement video. As such, participants immediately observed finger movements of the video-taped hand on screen in response to their own lifting movements. For example, in an imitative block, the lifting of an index finger resulted in the presentation of the index-finger-lifting video, whereas the middle-, ring-, or little-finger-lifting video was shown in a nonimitative block. All of the finger movement clips had a total duration of 2,000 ms.
Finally, nine pain movies in which painful stimulation was applied to the hand on screen were recorded for the pain perception part of the task (all with a total duration of 8,000 ms; see Table 1).
Self-report measures
During the experiment, four behavioral questions were presented after each pain movie, to measure explicit reactions to observing the hand in pain: How unpleasant do you think the other person found the pain stimulation?, How intense do you think the other person experienced painful sensations?, How unpleasant did you find the pain stimulation yourself?, How intense did you experience painful sensations yourself?. The first two questions referred to painful experiences of the other person, and the last two questions referred to first-person experiences. Both the affective (unpleasantness) and sensory (intensity) dimensions of pain had to be rated on a scale from −5 (not unpleasant/intense at all) to +5 (very unpleasant/intense), since research has suggested that both dimensions might be activated when observing someone else in pain (Bufalari et al., 2007; Cheng et al., 2008; Keysers et al., 2010; Lamm et al., 2007; Loggia et al., 2008).
After the experiment, participants filled in the Interpersonal Reactivity Index (IRI; Davis, 1980; for the Dutch translation, see De Corte et al., 2007) as a measure of dispositional trait empathy. This questionnaire consists of 28 items that have to be rated on a 5-point Likert scale, and can be divided into four subscales: Perspective Taking, Empathic Concern, Fantasy, and Personal Distress. The internal consistency and construct validity of the Dutch translation suggest that the IRI is a valuable tool for measuring self-report empathic tendencies (De Corte et al., 2007). The Cronbach’s α values in the present study were .62 for perspective taking, .61 for empathic concern, .80 for fantasy, and .80 for personal distress.
Procedure
Participants were seated in front of a standard computer screen at arm’s length and were asked to place the four fingers of their right hand on a custom-made response box. As soon as the video-taped right hand appeared on screen (resting-state movie), participants were instructed to voluntarily and randomly lift one of their four fingers that was placed on the response box. Immediately after movement of one of the participants’ fingers (delay = 0 ms, estimate of intrinsic delay of computer/software = 66.93 ms), a movie was shown in which the hand on screen performed either the same or a different movement, in the imitation or nonimitation blocks, respectively (see Fig. 1). After 20 such movements (all imitative or all nonimitative), one of the pain movies, in which the hand on screen received painful stimulation, was immediately presented. After this pain movie, four behavioral questions appeared on screen, which had to be rated on a scale from −5 to +5.
During the pain clips, a burst of white noise of 95 dB(A) was presented after 4,000 ms via headphones in only 50 % of the cases, in order to avoid predictability of the occurrence of this startle probe (Hawk & Cook, 2000). Prior to the start of the experiment, the startle noise was presented successively five times, in order to control for initial habituation.
Before the start of the experiment, two practice blocks (one of each imitative condition) were presented in order to familiarize the participants with the procedure. The pain movie shown in these practice blocks was not used in the experimental phase. Furthermore, in these practice blocks, we verified whether participants understood the behavioral questions correctly. More specifically, they were explicitly made aware of the distinction between the other- and self-related questions, and of the fact that the question How intense did you experience painful sensations yourself? related to self-experienced painful sensations alone.
Finally, at the end of the experiment, participants filled in the IRI as a measure of trait empathy.
Electrophysiological recording and analyses
Psychophysiological signals were registered with a Biopac MP150 system and digitized using AC1001 (AcqKnowledge Software for Windows, with electronic manual; Biopac Systems, Inc., Goleta CA).
Startle blink reflex
The startle eye blink reflex was measured according to Blumenthal et al.’s (2005) guidelines. Two small Ag/AgCL electrodes (5 mm) were placed over the orbicularis oculi muscle of the left eye, while a ground electrode was placed in the middle of the forehead. The raw EMG signal was amplified with a gain of 5,000, filtered with a hardware band-pass filter of 0.5–500 Hz, and digitally sampled at 1000 Hz, and later rectified and integrated offline using the PSPHA script (De Clercq, Verschuere, De Vlieger, & Crombez, 2006). The magnitude of the eye blink amplitude was computed as the subtraction of the mean rectified baseline value (0–20 ms after probe onset) from the rectified peak value in the 21- to 120-ms interval after probe onset. Trials on which baseline values deviated more than 2.5 SDs from the participant’s mean baseline value were visually inspected, and if necessary (e.g., due to movement artifacts or blink onset before probe onset) eliminated (11.74 %). Finally, the reflex magnitudes were converted to t scores across trials on a within-participants basis, in order to adjust for between-participants differences in the response and baseline EMG magnitudes (Funayama, Grillon, Davis, & Phelps, 2001), as follows: z-score value = (raw magnitude value – mean of all raw values)/(SD of all raw values); t-score value = (z-score value × 10) + 50. The z-score values were trimmed (all scores below −3 and above +3 were put at −3 and +3, respectively) before being converted to t scores.
Additional measures of the autonomic nervous system (ANS)
We measured two additional implicit measures—namely, skin conductance and heart rate changes, both of which are indices of autonomic functioning that have been shown to be responsive to negative emotional stimuli (Bradley, Codispoti, Cuthbert, & Lang, 2001).
Skin conductance was measured using a constant voltage (0.5 V) and two Ag/AgCL electrodes with a diameter of 8 mm. The electrodes were filled with conductive gel and were attached on the thenar and hypothenar eminences of the left hand. Skin conductance was digitized at 10 Hz for the entire duration of the pain movie (8,000 ms). Using PSPHA, skin conductance responses were calculated as the difference between the highest and the lowest value in this 8,000-ms time window. In order to normalize the data, skin conductance amplitudes were square-root transformed prior to analysis (Dawson, Schell, & Fillion, 2000).
Finally, heart rate was measured using three Ag/AgCL electrodes with a diameter of 8 mm filled with conductive gel and placed in the lead II configuration (i.e., by attaching electrodes to both legs and the right arm, heart rate is measures as the voltage drop from the left leg to the right arm). The heart rate was filtered (band pass: 0–40 Hz) and digitized at 500 Hz. For heart rate changes, PSPHA was used to detect R-peaks, so as to calculate the distance between them (i.e., the interbeat interval: IBI), and to correct for artifacts. Prior to analysis, the IBI was converted to heart rate in beats per minute (bpm) for each real-time epoch (1,000 ms). The mean bpm in the first 1,000 ms of the video clip was then compared to the mean bpm of the following 7,000 ms to calculate the mean heart rate change (i.e., mean heart rate in last 7,000 ms – mean heart rate in first 1,000 ms).
Results
A .05 significance level was used in all statistical tests. Due to equipment failure, physiological recording was limited to 15 participants for all physiological measures.
Subjective reports
All four questions followed the expected pattern, resulting in higher scores after imitation than after nonimitation blocks (see Table 2). A 2 (condition: imitation vs. nonimitation) × 4 (item: 1–4) repeated measures analysis, however, showed a significant Condition × Item interaction, F(3, 17) = 3.75, p < .05. Planned comparisons indicated that conditions differed only for the affective–other and self–sensory questions [t(19) = 2.21, p < .05, d = 0.40, and t(19) = 3.25, p < .01, d = 0.44, respectively], but not for the other two questions (both ps > .05).
Blink modulation and ANS activity
A paired t test revealed a significant difference in startle magnitude between the imitation and nonimitation conditions, t(14) = 3.41, p < .01, d = 3.35, with higher scores after imitation than after nonimitation blocks (see Fig. 2).
Interestingly, we found no significant correlations between the behavioral and physiological data, nor any significant correlations with participants’ scores on the subscales of the IRI. Furthermore, the IRI subscale scores were added as covariates in all analyses in order to investigate the moderating effect of dispositional empathic tendencies. The interactions with these subscores were again not significant for both explicit and implicit responses (all ps > .05).
In contrast to the startle blink reflex, the skin conductance and heart rate changes were not sensitive to the imitation manipulation (ps > .05). This was not completely surprising, however, given that the startle blink reflex amplitude has been shown to be most sensitive to changes in valence (Bradley, 2009).
Discussion
The aim of this first experiment was to investigate whether being imitated has an influence on the way that we react to perceiving pain in others. Using behavioral and physiological measures, the present study provides evidence for the hypothesis that being imitated leads to higher affective responses when observing pain. On the behavioral level, participants judged that the other person experienced the pain stimulation as being more unpleasant and that they experienced more intense painful sensations themselves after being imitated. Furthermore, the startle blink magnitude was significantly larger when viewing the pain movie after being imitated, indicating that higher negative affect was elicited in this condition. This indicates a stronger pain-related response after being imitated, as compared with not being imitated. Since Preston and de Waal (2002) argued that observing pain can lead to associated physiological responses as an index of higher activation in pain-related brain areas, and since the startle reflex has previously been related to empathy for pain (Caes et al., 2012), we believe that our results provide evidence for the idea that being imitated increases empathy for pain.
The question remains, however, which mechanism(s) could underlie the increase of empathy for pain when being imitated. First, social–psychological research has suggested that being imitated leads to enhanced liking for the other person (Chartrand & Bargh, 1999). This increased liking could in turn lead to higher empathy for pain, since it has been shown that reactions to observing another person in pain are stronger when we have an affective relationship with the person observed (Cheng et al., 2010; Hein, Silani, Preuschoff, Batson, & Singer, 2010). A second mechanism, however, is strongly based on the idea of shared representations, and suggests that the underlying process combining imitation and observational pain research is more basic in nature. As we suggested above, both motor imitation and empathy for pain rely on shared representations of self and other, possibly eliciting a self–other confusion mechanism (e.g., Brass, Derrfuss, Matthes-von Cramon, & von Cramon, 2003; Liepelt, von Cramon, & Brass, 2008). In the imitative condition, this confusion is thought to be elicited, since the same actions are performed, and these actions are commonly coded. Furthermore, when representations of self and other become difficult to distinguish, pain applied to the other person should have a stronger influence on your own bodily reactions. Note that the present setup (using no delay between the executed and observed movements and a first-person perspective) increased the likelihood that this self–other confusion mechanism would take place. However, since significant effects on the ratings were observed for items, suggesting both an abstract empathy process (affective–other question) and self–other confusion (sensory–self question), it is difficult to distinguish between the two accounts on the basis of the present results. Furthermore, the absence of correlations between the subjective reports and implicit responses suggests that multiple mechanisms may be at work, although our relatively small sample size also might have been responsible for the absence of correlations in this experiment. The finding that being imitated changed empathy for pain on a trial-by-trial basis, however, is more in accordance with a self–other confusion interpretation rather than a trial-by-trial change in liking.
Hence, to further investigate the possible influence of an underlying self–other confusion mechanism based on shared representations between self and other, we conducted a second experiment in which we aimed to explore this process in more depth and to gain better insight into the effects observed in the first experiment. The general logic of the second experiment was thus to link the observed effects of the first experiment to processes related to confusion between self and other, as well as to investigate whether the strength of empathy for pain would be affected by increasing this self–other overlap.
Experiment 2
In the second experiment, we aimed both to replicate and extend the findings of the first study. To do so, we linked our setup to the rubber hand illusion (RHI) paradigm, in which participants feel ownership over a rubber hand when viewing this rubber hand being stroked simultaneously with their own, hidden hand (Botvinick & Cohen, 1998). This illusion, commonly observed with a sensory manipulation, is also thought to reflect sharing of representations between self and other (e.g., Tajadura-Jiménez, Grehl, & Tsakiris, 2012). First, we wanted to investigate whether the setup that we used in Experiment 1, with the hand being presented on a computer screen positioned in front of participants, would elicit a stronger RHI in the imitative than in the nonimitative condition (see Dummer, Picot-Annand, Neal, & Moore, 2009, for a similar action-induced RHI). Second, we also wanted to explore whether we could create a setup that would increase self–other confusion by manipulating the spatial position of the observed hand. Hence, we varied our original setup from Experiment 1 by tilting the screen on which the videotaped right hand was presented and placing the participant’s own right hand under it, covering the latter and thus making it invisible (in contrast to the first setup, in which the screen was placed in front of the participant). Since it was thought that these adjustments would elicit a stronger RHI (as do similar manipulations with a sensory RHI; Lloyd, 2007), and thus enhance confusion between self and other, we expected the effects of being imitated on empathy for pain to be stronger in this newly developed setup than in the original one. In this way, we wanted to investigate whether self–other confusion might (in part) be responsible for the observed effects.
Method
Participants
A group of 21 right-handed volunteers (M age = 21.38 years, SD = 2.36) participated in this second study, all of whom had normal or corrected-to-normal vision. To control for possible sex differences, only female volunteers were recruited. They were given €10 for participation and provided written consent at the beginning of the experiment. The study was granted ethical approval by the local ethics committee.
Experimental design
The second experiment consisted of two parts in a within-subjects design. In one part, participants performed the task with a setup like that of the first experiment (i.e., the hand of the participant lay in front of the screen: “front”-position setup), but a slightly different setup was used in the other part of the experiment (i.e., the hand of the participant lay under the screen: “under”-position setup). The order of these parts was counterbalanced across participants: Odd participants started with the front-position setup, whereas even participants started with the under-position setup. Furthermore, an additional movie was used in this study (in which a knife cuts the hand). In this way, ten movies were available, of which five were randomly allocated to each of the two setups for all participants. Since the experimental design was similar to that of the first experiment in both parts, each part consisted of 20 trials (five pain movies combined with two imitative and two startle conditions).
Stimuli and apparatus
The video materials were those from the first experiment, with the addition of a tenth pain movie (knife movie, see above).
Participants performed the task as explained in Experiment 1 under two setups with different spatial positions: hand in front of or under the screen. In the front-position setup, the screen on which all of the stimulus materials were presented was placed in front of the participants. In this way, a participant’s own hand was still visible. In the under-position setup, on the other hand, the same screen was tilted horizontally, and participants were asked to place their hands under the screen. To ensure that they could only see the hand on the screen, a towel attached to the screen covered the participant’s own hand.
Self-report measures
In contrast to the first experiment, 11 explicit questions were presented after each pain movie, in order to get a better understanding of any possible underlying self–other confusion and RHI processes. The first three questions, to be rated on a scale from −5 to +5, were used to examine whether an RHI was elicited, on the basis of three different aspects of this illusion (Longo, Schuur, Kammers, Tsakiris, & Haggard, 2008; Tsakiris, Longo, & Haggard, 2010): It felt as if I could control the hand on screen (agency), It felt as if my own hand was at the location of the hand on screen (location), and It felt as if the hand on screen was my own hand (ownership). Furthermore, a fourth question, referring to empathy for pain, had to be answered: I felt pain on my own hand when I saw the hand on screen being injured. Finally, a Dutch translation of the scale of Batson, Fultz, and Schoenrade (1987) was used, presenting seven items measuring two types of emotional responses. These items measured the subjective feelings of participants while viewing painful stimulation, with four of the items referring to self-oriented feelings (i.e., personal distress: While viewing the painful stimulation of the other person I felt worried/distressed/anxious/sad), and three of the items referring to other-oriented feelings (i.e., concern: While viewing the painful stimulation of the other person I felt understanding/empathetic/compassionate). As such, the questions referring to the observed painful situations could be divided into two categories: self (specific questions referring to pain + self-items from the Batson scale; Cronbach’s α > .90) versus other (other-items from the Batson scale; Cronbach’s α > .90).
After the experiment, participants again filled in the IRI (Cronbach’s α: perspective taking = .82, empathic concern = .42, fantasy = .85, personal distress = .84).
Procedure
The procedure was similar to that of Experiment 1, with the exception that participants performed the task under both the front- and the under-position setups. Furthermore, participants now performed a random number of movements between 15 and 20, to decrease the length of the experiment.
Results
A .05 significance level was used in all statistical tests. Behavioral outliers were defined as participants deviating more than 2.5 SDs on their general RHI score from the general mean of this score (n = 1; this participant was discarded from all further analyses). Due to equipment failure, physiological recording was limited to 19 of the participants for all physiological measures.
Subjective reports
First, the behavioral questions referring to aspects of the RHI were inspected to verify which dimensions of the illusion were successfully elicited. However, since all items were strongly correlated (all ps < .05) and all items showed the same pattern, a general RHI score was calculated as the mean of the scores on the three items taken together, allowing us to use this single score in all further analyses (Cronbach’s α > .81). A 2 (position: front vs. under) × 2 (condition: imitation vs. nonimitation) repeated measures analysis showed a significant main effect of position, F(1, 19) = 4.25, p < .05, d = 0.60: The general RHI scores were higher in the under-position than in the front-position setup. Furthermore, the main effect of condition, F(1, 19) = 68.27, p < .001, d = 4.25, was also significant, but its interaction with position was not, F(1, 19) < 1. Scores were significantly higher in the imitative than in the nonimitative condition for both positions (see Fig. 3).
Second, all items probing empathy for pain were divided into self- and other-related questions (see above) and analyzed by means of a 2 (position: front vs. under) × 2 (condition: imitation vs. nonimitation) × 2 (perspective: self vs. other) repeated measures analysis. Only main effects of condition, F(1, 19) = 15.57, p = .001, d = 0.60, and perspective, F(1, 19) = 12.00, p < .01, d = 1.00, were observed (all other ps > .05). Higher scores were found in the imitative than in the nonimitative condition (see Fig. 4) and for the other- as compared to the self-related items.
However, in order to test whether the imitation effect could be explained by self–other confusion, we performed a secondary mediation analysis investigating whether the effect of condition on our dependent variables (other- and self-related behavioral scores) could be explained by RHI measures. For each participant, an RHI effect was calculated as the difference between the general RHI scores in the imitative and nonimitative conditions, irrespective of positional setup (RHI effect = general RHI score in the imitation condition – general RHI score in the non-imitation condition). For this mediation analysis, we used a bootstrapping method following the procedure described by Preacher and Hayes (Hayes, 2009; Preacher & Hayes, 2004), a nonparametric resampling procedure. Figure 5 represents the effects and their corresponding weights that would have to be distinguished to perform the mediation analysis (only the “other-related items” outcome is mentioned in the figure; however, the figure is applicable for all outcomes). The direct effect of condition on other-related scores has the weight c', whereas the indirect effect, by means of the proposed mediator “RHI effect,” has the weight ab. The effect of condition on RHI effect is represented by weight a, and weight b is the effect of RHI effect on other-related items, partialing out the effect of condition. The total effect c of condition on other-related items consists of both the direct (c') and the indirect (ab) effects. In the bootstrap analyses, the indirect effect ab would be found to be significant if the bootstrap confidence interval (CI) excluded zero. Overall, mediation would be assumed if (1) the total effect c was significant in addition to the indirect effect ab, and (2) the total effect c was significantly reduced when controlling for the indirect effect ab (i.e., c' was nonsignificant).
Bootstrap analyses (with 5,000 resamples) for the RHI effect as a mediator in the relation between condition and other- and self-related items resulted in a significant total effect of condition upon these items (c = 0.39, SE = 0.27, p < .05, and c = 0.47, SE = 0.35, p < .05, respectively), but no direct effect of condition (c' = 0.51, SE = 0.34, p > .05, and c' = 0.66, SE = 0.45, p > .05). Furthermore, a direct effect of condition upon RHI effect was found (a = 3.65, SE = 0.39, p < .001, and a = 3.65, SE = 0.39, p < .001, respectively, for other- and self-related items), indicating that a higher RHI effect was found in the imitation than in the nonimitation condition. A direct effect of RHI effect on other- and self-related items was also found (b = 0.25, SE = 0.06, p < .001, and b = 0.31, SE = 0.08, p < .001, respectively), showing that a higher RHI effect resulted in higher self-report scores. Furthermore, the indirect effect of condition on the dependent variable through the RHI effect was significant (ab = 0.90, SE = 0.23, and ab = 1.12, SE = 0.31, respectively, for the other- and self-related items), as the bootstrapped confidence interval excluded zero (90 % CI = 0.58 to 1.35 and 90 % CI = 0.65 to 1.70, respectively). Finally, significant positive correlations emerged between the RHI effect and effects on the other- and self-related scores: r = .44, p < .05, and r = .38, p < .05, respectively.
Blink modulation and ANS activity
A 2 (position: front vs. under) × 2 (condition: imitation vs. nonimitation) repeated measures analysis of the eye blink data revealed a significant main effect of condition, F(1, 18) = 11.53, p < .01, d = 2.72. The main effect of position and its interaction with condition, however, were nonsignificant (both Fs < 1; see Fig. 6). Performing the same mediation analysis mentioned above for self-report measures with the blink data as the dependent variable resulted in similar results. Bootstrap analyses resulted in a significant total effect of condition upon the eye blink data (c = 3.78, SE = 0.79, p < .001), but no direct effect of condition (c' = 2.68, SE = 1.10, p > .05). Furthermore, a direct effect of condition upon RHI effect was found (a = 3.60, SE = 0.43, p < .001), and a direct effect of RHI effect on the eye blink data was also found (b = 0.31, SE = 0.22, p < .05). Finally, the indirect effect of condition on the dependent variable through the RHI effect was significant (ab = 1.10, SE = 0.87), as the bootstrapped confidence interval excluded zero (90 % CI = 0.28 to 2.61).Performing the same analysis with the other behavioral effects (effects of other- and self-related items) as mediators did not lead to a mediation pattern.
The heart rate data were again insensitive to our manipulation (ps > .05), but the skin conductance data did show effects similar to those for the startle blink data in this experiment. The same 2 (position: front vs. under) × 2 (condition: imitation vs. nonimitation) repeated measures analysis showed a significant main effect only of condition, F(1, 18) = 4.56, p < .05, d = 0.61 (see Fig. 7). However, a mediation analysis with the behavioral RHI effect (but not with the other behavioral effects) revealed a significant total effect of condition upon skin conductance responses (c = 0.19, SE = 0.12, p < .001), but no direct effect of condition (c' = 0.42, SE = 0.15, p > .05). Furthermore, a direct effect of condition upon RHI effect was found (a = 3.34, SE = 0.41, p < .001). A direct effect of RHI effect on the skin conductance data was also found (b = 0.07, SE = 0.03, p < .05). Finally, the indirect effect of condition on the dependent variable through the RHI effect was significant (ab = 0.23, SE = 0.11), as the bootstrapped confidence interval excluded zero (90 % CI = 0.06 to 0.44).
Mean skin conductance in the imitation and nonimitation conditions in the front- and under-position setups in the second experiment. Skin conductance responses are expressed as the difference between the highest and the lowest values in a specified time window, and error bars represent standard errors of the means
Interestingly, again we found no significant correlations between the behavioral and physiological data, nor any correlations or interactions with participants’ scores on the subscales of the IRI (all ps > .05). Furthermore, no correlations were observed between these IRI scales and the behavioral RHI effect (all ps > .05).
Discussion
In this second experiment, we replicated and extended the findings of the first experiment. First, the behavioral and physiological measures in two setups both confirmed the hypothesis that being imitated enhances affective responses when seeing someone else in pain. On the behavioral side, ratings on other- and self-related items after viewing the pain movies were higher when participants were being imitated during the action phase. Furthermore, the mean blink magnitude and mean skin conductance responses were also higher in the imitation condition, indicating higher physiological affective responding when viewing someone else in pain after being imitated. However, we did not find a difference between the two setups that we used in this experiment: Both the setup in which the hands of participants were placed in front of the screen and the setup in which their hands were placed under this screen showed stronger responses in the imitation condition, without an interaction between the two setups. This was somewhat unexpected, since research using sensory RHI paradigms has shown that a more congruent spatial position between one’s own hand and a rubber hand elicits the strongest RHI (Lloyd, 2007), and we expected this to result in higher empathy for pain due to increased self–other confusion. In this experiment, the behavioral results indicated that both the front- and the under-position setups elicited a very strong RHI in the imitation condition, and that this illusion was indeed slightly stronger in the setup with the most congruent spatial position. Although this suggests that our spatial-position manipulation was successful, it seems that this stronger RHI in the under-position setup failed to elicit stronger affective responding when viewing someone else in pain.
A more in-depth analysis of the results, however, revealed that the imitation effect on affective responding when viewing someone else in pain disappeared when taking the behavioral RHI effect into account. Since similar analyses with the other behavioral effects did not result in this disappearance, this suggests that the differences in affective responses between the imitative and nonimitative conditions could—at least in part—be accounted for by an RHI being very strongly elicited when a person is imitated. It thus seems that the RHI (or self–other confusion) elicited in the imitation condition might have been responsible for the effect of imitation on empathy for pain, but that this effect could not be enhanced by a spatial-position manipulation that increased the illusion. We believe that this was due to the fact that the imitation condition elicited a very strong RHI in both the front- and under-position setups, indicating that this effect was still robust under a spatially incongruent position, and might have already reached its limit in this condition. In a recent article, Kalckert and Ehrsson (2012) used a similar motor-induced RHI and measured both agency and ownership over the rubber hand. When varying the spatial position with a 180-deg rotation, ownership over the rubber hand diminished, whereas agency remained very strong. Our results were not able to distinguish between ownership and agency, since both aspects of the RHI seemed to be elicited in the imitation condition in both spatial positions. However, since the hand in the front-position setup was not completely incongruent with the position of the own hand, our spatial manipulation was not as strong as the one used by Kalckert and Ehrsson. Nevertheless, both our study and the research by Kalckert and Ehrsson suggest that (some) measures of the RHI are invariant to spatial manipulations.
General discussion
In this study, we have shown in two different experiments that being imitated leads to higher affective responding when seeing someone else in pain, on both an explicit behavioral and an implicit physiological level. Behavioral scores of empathy for pain were higher when participants were being imitated in both experiments. Furthermore, we replicated the finding that startle blink magnitude when viewing a painful movie was higher after being imitated, indicating stronger negative affect in this condition. Finally, the second experiment suggested that skin conductance (as an index of autonomic nervous system activity) was also higher in the imitative situation, again providing evidence for stronger affective responding when viewing someone in pain after being imitated.
In the second experiment, we related our setup to rubber hand illusion paradigms in order to investigate a self–other confusion mechanism. We measured the RHI in our paradigm and induced a position manipulation that was thought to increase this illusion. Furthermore, we expected a stronger RHI to be related to stronger empathy for pain. In this experiment, we found an influence of both the position of the setup and the imitative condition on the RHI, but not the predicted interaction between position and condition that we expected from a self–other confusion account. Furthermore, we found a strong effect of imitative condition on our measures of empathy for pain, whereas we did not find an interaction between condition and the position of the setup. However, although we did not find evidence for this interaction, secondary analyses in which it was shown that imitation effects were mediated by RHI measures suggested that the sharing of representations between self and other might nevertheless be responsible for the observed results. These results indicated that being imitated elicited a very strong RHI, and that this effect accounted for the higher affective responding in this imitative situation. Although it has already been shown that an RHI can increase affective reactions when seeing a rubber hand in pain (Armel & Ramachandran, 2003; Ehrsson, 2007; Farmer, Tajadura-Jiménez, & Tsakiris, 2012), this has never been shown with an action-induced illusion like the one used in the present study. Furthermore, it was shown that our action-induced RHI was strongly influenced by the imitation manipulation in our paradigm, whereas the spatial manipulation was less important, and did not seem to influence affective responding to painful stimuli. It seems that being imitated elicited a strong RHI that influenced empathy for pain, and that this manipulation worked with both a spatially congruent and a spatially incongruent position.
It remains an important open question, however, whether a self–other confusion mechanism might also be important in more complex social situations, in which the link with the RHI might not be as evident as it was in our imitative setup. Furthermore, whereas social–psychological research has indicated that complex prosocial effects of being imitated only take place when participants are unaware of the imitative situation (Chartrand & Bargh, 1999; van Baaren, Holland, Kawakami, & van Knippenberg, 2004), the imitation-versus-nonimitation situation was completely transparent in the present study. Due to the absence of a delay between the executed and observed movements, the basic simplicity of these movements, and the use of a first-person perspective, it was immediately clear to participants whether or not they were being imitated. However, a study by Singer et al. (2008) indicated that prosocial behavior and empathy are not necessarily positively correlated. As such, whereas lack of awareness of imitation seems necessary to elicit prosocial behavior, our study indicates that empathy for pain is more immune to such a top-down modulation, and can be influenced by transparent imitative situations. This might suggest that the shared representational system acts differently under different social situations, and it remains to be investigated why and how this may be the case. One possibility is that in social–psychological research, awareness of being imitated induces reactance because people get the impression of being mocked by the imitator. This was obviously not true in the present setup. Thus, it seems that the influence of being imitated on basic processes such as empathy for pain remains strong despite awareness, in a setup in which self–other confusion is thought to underlie the effects. It has to be noted, however, that we might not be able to generalize the results found in our particular setup to more ecologically valid situations. As was mentioned above, several choices were made in order to increase self–other confusion that might have resulted in an important discrepancy between our setup and complex imitative situations. Furthermore, whereas social–psychological research usually employs real-life social interactions, the effects observed in the present study were based on the interaction with a previously videotaped hand on screen (in both the action and pain perception phases), without providing any social context. Importantly, pain perception research has indicated that online social interactions and movie- or picture-based interactions activate both converging and diverging brain areas, with a core network including the anterior cingulate cortex (ACC) and bilateral anterior insula (AI) being activated most consistently over all situations (Lamm et al., 2011; Zaki & Ochsner, 2012). Furthermore, Perani et al. (2001) have also shown that virtual social interactions (e.g., using video clips) are associated with only part of the action observation network activated in natural social interactions. However, research on automatic imitation has consistently shown that these effects are very strong with videotaped hands (e.g., Brass et al., 2001; Brass et al., 2000). Furthermore, it has been demonstrated that participants attribute intentionality to these videotaped hands, since manipulating this belief leads to a reduction of automatic imitation effects (Liepelt, von Cramon, & Brass, 2008). Research by Hogeveen and Obhi (2012) has additionally shown that naturalistic social interaction and action observation of human actions involve common motor resonance mechanisms. Finally, Kalckert and Ehrsson (2012) have shown that only the feeling of ownership, not that of agency, is preserved when changing a rubber hand from a first- to a third-person perspective, suggesting that the mechanisms in both perspectives are at least partially different. Since we used a first-person perspective setup, this suggests that our paradigm might not be entirely suitable to explain common third-person imitative experiences. Thus, it is clear that our operationalization of being imitated shows discrepancies with those used in social–psychological research on related phenomena in naturalistic environments. Furthermore, the temporal contingency that was present in both experimental conditions in our setup (being imitated and not being imitated) also forces us to consider future research exploring what would happen if a control condition were included in which no contingent reaction of the hand on screen were present (e.g., seeing a hand that never moves, or without any systematic relationship to the participants’ movements), in order to distinguish the positive effects of being imitated from the negative effects of not being imitated. Although we recognize all of these setup-related differences from classical social–psychological paradigms, we nevertheless believe that our study might be important in understanding social (imitative) interactions, especially since all of the studies mentioned above showed—next to diverging activations—partially overlapping areas being activated when real-life and experimentally induced interactions were compared as well. However, we also believe that it will be important to further investigate all of these aspects in more depth and to explore in which social interactions a mechanism assuming overlapping self–other representations might be relevant.
Another important limitation of the study is the indirect way in which empathy for pain is measured. Therefore, future research will need to directly investigate whether being imitated leads to stronger activation of pain-related brain areas by using brain-imaging techniques. Research has indicated that areas associated with the affective–motivational dimensions of pain, including the bilateral AI and the dorsal ACC, are most consistently activated when seeing someone else in pain (Lamm et al., 2011; Singer et al., 2004). However, some studies have suggested that the sensory dimensions of pain are affected in these situations, as well (Keysers et al., 2010; Loggia et al., 2008). On the basis of present results, we would expect affective (and sensory) parts of the pain matrix to show stronger activation when one sees someone else in pain after being imitated, as compared to after not being imitated. Furthermore, regions such as the temporal parietal junction and the medial prefrontal cortex have been related to shared representational processes such as self–other distinction (Decety, Chaminade, Grèzes, & Meltzoff, 2002; Farrer & Frith, 2002; Spengler, von Cramon, & Brass, 2010). Since there is an obvious link between these mechanisms and the account used to explain the results in the present article, we think it will be warranted to explore the involvement of these regions in our paradigm, as well.
As a final comment, we would point out that it is somewhat disputable whether the term empathy for pain is adequate to describe the results in our study, since empathy requires the ability to experience the feelings of another person while at the same time being able to recognize this person as another entity. A self–other confusion process, which we believe to be the underlying mechanism of the effects in our study, would suggest a different situation. However, although our autonomic results suggest that there is self–other confusion at an implicit level, our behavioral results in both experiments indicate that people still consciously seem to distinguish self from other. The responses to other- and self-related questions were differently rated, with higher scores on other-related than on self-related subjective judgments. This suggests that self–other confusion was not complete, and we therefore opted to use the term empathy for pain nevertheless. However, our results suggest that this term might not encompass the whole process.
To summarize, our data suggest that being imitated leads to a stronger affective response when seeing someone else in pain. This suggests a tight link between shared representations on the motor and sensory levels.
References
Armel, K. C., & Ramachandran, V. S. (2003). Projecting sensations to external objects: Evidence from skin conductance response. Proceedings of the Royal Society of London B, 270, 1499–1506. doi:10.1098/rspb.2003.2364
Bastiaansen, J. A. C. J., Thioux, M., & Keysers, C. (2009). Evidence for mirror systems in emotions. Philosophical Transactions of the Royal Society B, 364, 2391–2404. doi:10.1098/rstb.2009.0058
Batson, C. D., Fultz, J., & Schoenrade, P. A. (1987). Distress and empathy: Two qualitatively distinct vicarious emotions with different motivational consequences. Journal of Personality, 55, 19–39. doi:10.1111/j.1467-6494.1987.tb00426.x
Blumenthal, T. D., Cuthbert, B. N., Filion, D. L., Hackley, S., Lipp, O. V., & Van Boxtel, A. (2005). Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology, 42, 1–15. doi:10.1111/ j.1469-8986.2005.00271.x
Botvinick, M., & Cohen, J. (1998). Rubber hands “feel” touch that eyes see. Nature, 391, 756. doi:10.1038/35784
Bradley, M. M. (2009). Natural selective attention: Orienting and emotion. Psychophysiology, 46, 1–11. doi:10.1111/j.1469-8986.2008.00702.x
Bradley, M. M., Codispoti, M., Cuthbert, B. N., & Lang, P. J. (2001). Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion, 1, 276–298. doi:10.1037/1528-3542.1.3.276
Brass, M., Bekkering, H., & Prinz, W. (2001). Movement observation affects movement execution in a simple response task. Acta Psychologica, 106, 3–22. doi:10.1016/S0001-6918(00)00024-X
Brass, M., Bekkering, H., Wohlschläger, A., & Prinz, W. (2000). Compatibility between observed and executed finger movements: Comparing symbolic, spatial, and imitative cues. Brain and Cognition, 44, 124–143. doi:10.1006/brcg.2000.1225
Brass, M., Derrfuss, J., Matthes-von Cramon, G., & von Cramon, D. Y. (2003). Imitative response tendencies in patients with frontal brain lesions. Neuropsychology, 17, 265–271. doi:10.1037/0894-4105.17.2.265
Brass, M., & Heyes, C. M. (2005). Imitation: Is cognitive neuroscience solving the correspondence problem? Trends in Cognitive Sciences, 9, 489–495. doi:10.1016/j.tics.2005.08.007
Bufalari, I., Aprile, T., Avenanti, A., Di Russo, F., & Aglioti, S. M. (2007). Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex, 17, 2553–2561. doi:10.1093/cercor/bh1161
Caes, L., Uzieblo, K., Crombez, G., De Ruddere, L., Vervoort, T., & Goubert, L. (2012). Negative emotional responses elicited by the anticipation of pain in others: Psychophysiological evidence. The Journal of Pain, 13, 467–476. doi:10.1016/j.jpain.2012.02.003
Chartrand, T. L., & Bargh, J. A. (1999). The chameleon effect: The perception–behaviour link and social interaction. Journal of Personality and Social Psychology, 76, 893–910. doi:10.1037/0022-3514.76.6.893
Chartrand, T. L., & van Baaren, R. (2009). Human mimcry. Advances in Experimental Social Psychology, 41, 219–274. doi:10.1016/S0065-2601(08)00405-X
Cheng, Y. W., Chen, C. Y., Lin, C. P., Chou, K. H., & Decety, J. (2010). Love hurts: An fMRI study. NeuroImage, 51, 923–929. doi:10.1016/j.neuroimage.2010.02.047
Cheng, Y., Lin, C.-P., Liu, H.-L., Hsu, Y.-Y., Lim, K.-E., Hung, D., & Decety, J. (2007). Expertise modulates the perception of pain in others. Current Biology, 17, 1708–1713. doi:10.1016/j.cub.2007/09/020
Cheng, Y., Yang, C.-Y., Lin, C.-P., Lee, P.-L., & Decety, J. (2008). The perception of pain in others suppresses somatosensory oscillations: A magnetoencephalography study. NeuroImage, 40, 1833–1840. doi:10.1016/j.neuroimage.2008.01.064
Davis, M. H. (1980). A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, 10, 85.
Dawson, M. E., Schell, A. M., & Fillion, D. L. (2000). The electrodermal system. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), Handbook of psychophysiology (pp. 200–224). Cambridge: Cambridge University Press.
De Clercq, A., Verschuere, B., De Vlieger, P., & Crombez, G. (2006). Psychophysiological analysis (PSPHA): A modular script-based program for analyzing psychophysiological data. Behavior Research Methods, 38, 504–510. doi:10.3758/BF03192805
De Corte, K., Buysse, A., Verhofstadt, L. L., Roeyers, H., Ponnet, K., & Davis, M. H. (2007). Measuring empathic tendencies: Reliability and validity of the Dutch version of the Interpersonal Reactivity Index. Psychologica Belgica, 47, 235–260.
de Vignemont, F., & Singer, T. (2006). The empathic brain: How, when, and why? Trends in Cognitive Sciences, 10, 435–441. doi:10.1016/j.tics.2006.08.008
Decety, J., Chaminade, T., Grèzes, J., & Meltzoff, A. N. (2002). A PET exploration of the neural mechanisms involved in reciprocal imitation. NeuroImage, 15, 265–572. doi:10.1006/nimg.2001.0938
Decety, J., Echols, S., & Correll, J. (2010). The blame game: The effect of responsibility and social stigma on empathy for pain. Journal of Cognitive Neuroscience, 22, 985–997. doi:10.1162/jocn.2009.21266
Decety, J., & Lamm, C. (2006). Human empathy through the lens of social neuroscience. The Scientific World Journal, 6, 1146–1163. doi:10.1100/tsw.2006.221
Dummer, T., Picot-Annand, A., Neal, T., & Moore, C. (2009). Movement and the rubber hand illusion. Perception, 38, 271–280. doi:10.1068/p5921
Ehrsson, H. H. (2007). The experimental induction of out-of-body experiences. Science, 317, 1048. doi:10.1126/science.1142175
Farmer, H., Tajadura-Jiménez, A., & Tsakiris, M. (2012). Beyond the colour of my skin: How skin colour affects the sense of body-ownership. Consciousness and Cognition, 21, 1242–1256. doi:10.1016/j.concog.2012.04.011
Farrer, C., & Frith, C. D. (2002). Experiencing oneself vs. Another person as being the cause of an action: The neural correlates of the experience of agency. NeuroImage, 15, 596–603. doi:10.1006/nimg.2001.1009
Funayama, E. S., Grillon, C., Davis, M., & Phelps, E. A. (2001). A double dissociation in the affective modulation of startle in humans: Effects of unilateral temporal lobectomy. Journal of Cognitive Neuroscience, 13, 721–729. doi:10.1162/08989290152541395
Goubert, L., Vervoort, T., & Craig, K. D. (2012). Empathy and pain. In R. F. Schmidt & G. F. Gebhart (Eds.), Encyclopedia of pain (2nd ed.). Heidelberg, Germany: Springer.
Grèzes, J., & Decety, J. (2001). Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Human Brain Mapping, 12, 1–19. doi:10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V
Han, S. H., Fan, Y., & Mao, L. (2008). Gender difference in empathy for pain: An electrophysiological investigation. Brain Research, 1196, 85–93. doi:10.1016/jbrainres.2007.12.062
Hawk, L. W., & Cook, E. W. (2000). Independence of valence modulation and prepulse inhibition of startle. Psychophysiology, 37, 5–12. doi:10.1111/1469-8986.3710005
Hayes, F. (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76, 408–420. doi:10.1080/03637750903310360
Heberlein, A. S., & Atkinson, A. P. (2009). Neuroscientific evidence for simulation and shared substrates in emotion recognition. Emotion Review, 1, 162–177. doi:10.1177/1754073908100441
Hein, G., Silani, G., Preuschoff, K., Batson, C. D., & Singer, T. (2010). Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron, 68, 149–160. doi:10.1016/j.neuron.2010.09.003
Hein, G., & Singer, T. (2008). I feel how you feel but not always: The empathic brain and its modulation. Current Opinion in Neurobiology, 18, 153–158. doi:10.1016/j.conb.2008.07.012
Hogeveen, J., & Obhi, S. S. (2012). Social interaction enhances motor resonance for observed human actions. Journal of Neuroscience, 32, 5984–5989.
Jackson, P. L., Meltzoff, A. N., & Decety, J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage, 24, 771–779. doi:10.1016/j.neuroimage.2004.09.006
Kalckert, A., & Ehrsson, H. H. (2012). Moving a rubber hand that feels like your own: A dissociation of ownership and agency. Frontiers in Human Neuroscience, 6, 40. doi:10.3389/fnhum.2012.00040
Keysers, C., Kaas, J. H., & Gazzola, V. (2010). Somatosensation in social perception. Nature Reviews Neuroscience, 11, 417–428. doi:10.1038/nrn2833
Kühn, S., Muller, B. C., van Baaren, R. B., Wietzker, A., Dijksterhuis, A., & Brass, M. (2010). Why do I like you when you behave like me? Neural mechanisms mediating positive consequences of observing someone being imitated. Social Neuroscience, 5, 384–392. doi:10.1080/17470911003633750
Lakin, J. L., & Chartrand, T. L. (2003). Using nonconscious behavioral mimicry to create affiliation and rapport. Psychological Science, 14, 334–339. doi:10.1111/1467-9280.14481
Lakin, J. L., Chartrand, T. L., & Arkin, R. M. (2008). I am too just like you—Nonconscious mimicry as an automatic behavioral response to social exclusion. Psychological Science, 19, 816–822. doi:10.1111/j.1467-9280.2008.02162.x
Lamm, C., Decety, J., & Singer, T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54, 2492–2502. doi:10.1016/j.neuroimage.2010.10.014
Lamm, C., Meltzoff, A. N., & Decety, J. (2010). How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience, 22, 362–376. doi:10.1162/jocn.2009.21186
Lamm, C., Nusbaum, H. C., Meltzoff, A. N., & Decety, J. (2007). What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE, 12, e1292. doi:10.1371/journal.pone.0001292
Liepelt, R., von Cramon, D. Y., & Brass, M. (2008). What Is matched in direct matching? Intention attribution modulates motor priming. Journal of Experimental Psychology. Human Perception and Performance, 34, 578–591. doi:10.1037/0096-1523.34.3.578
Lloyd, D. M. (2007). Spatial limits on referred touch to an alien limb may reflect boundaries of visuo-tactile peripersonal space surrounding the hand. Brain and Cognition, 64, 104–109. doi:10.1016/j.bandc.2006.09.013
Loggia, M. L., Mogil, J. S., & Bushnell, M. C. (2008). Empathy hurts: Compassion for another increases both sensory and affective components of pain perception. Pain, 136, 168–176. doi:10.1016/j.pain.2007.07.017
Longo, M. R., Schuur, F., Kammers, M. P. M., Tsakiris, M., & Haggard, P. (2008). What is embodiment? A psychometrix approach. Cognition, 107, 978–998. doi:10.1016/j.cognition.2007.12.004
Miller, M. W., Patrick, C. J., & Levenston, G. K. (2002). Affective imagery and the startle response: Probing mechanisms of modulation during pleasant scenes, personal experiences, and discrete negative emotions. Psychophysiology, 39, 519–529. doi:10.1017/S0048577202394095
Perani, D., Fazio, F., Borghese, N. A., Tettamanti, M., Ferrari, S., Decety, J., & Gilardi, M. C. (2001). Different brain correlates for watching real and virtual hand actions. NeuroImage, 14, 749–758. doi:10.1006/nimg.2001.0872
Preacher, K. J., & Hayes, A. F. (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, 36, 717–731. doi:10.3758/BF03206553
Preston, S. D., & de Waal, F. B. M. (2002). Empathy: Its ultimate and proximate bases. The Behavioral and Brain Sciences, 25, 1–20. doi:10.1017/S0140525X02000018. disc. 20–71.
Singer, T., & Lamm, C. (2009). The social neuroscience of empathy. Annals of the New York Academy of Sciences, 1156, 81–96. doi:10.1111/j.1749-6632.2009.04418.x
Singer, T., Seymour, B., O’Doherty, J., Kaube, H., Dolan, R. J., & Frith, C. D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303, 1157–1162. doi:10.1126/science.1093535
Singer, T., Seymour, B., O’Doherty, J. P., Stephan, K. E., Dolan, R. J., & Frith, C. D. (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature, 439, 466–469. doi:10.1038/nature04271
Singer, T., Snozzi, R., Bird, G., Petrovic, P., Silani, G., Heinrichs, M., & Dolan, R. J. (2008). Effects of oxytocin and prosocial behaviour on brain responses to direct and vicariously experienced pain. Emotion, 8, 781–791. doi:10.1037/a0014195
Spengler, S., von Cramon, D. Y., & Brass, M. (2010). Resisting motor mimicry: Control of imitation involves processes central to social cognition in patients with frontal and temporo-parietal lesions. Social Neuroscience, 5, 401–416. doi:10.1080/17470911003687905
Stel, M., van Baaren, R. B., & Vonk, R. (2008). Effects of mimicking: Acting prosocially by being emotionally moved. European Journal of Social Psychology, 38, 965–976. doi:10.1002/ejsp.472
Tajadura-Jiménez, A., Grehl, S., & Tsakiris, M. (2012). The other in me: Interpersonal multisensory stimulation changes the mental representation of the self. PLoS One, 7, e40682. doi:10.1371/journal.pone.0040682
Tsakiris, M., Longo, M. R., & Haggard, P. (2010). Having a body versus moving your body: Neural signatures of body-ownership. Neuropsychologia, 48, 2740–2749. doi:10.1016/j.neuropsychologia.2010.05.021
van Baaren, R. B., Holland, R. W., Kawakami, K., & van Knippenberg, A. (2004). Mimicry and prosocial behavior. Psychological Science, 15, 71–74. doi:10.1111/j.0963-7214.2004.01501012.x
Vrana, S. R., Spence, E. L., & Lang, P. J. (1988). The startle probe response: A new measure of emotion? Journal of Abnormal Psychology, 97, 487–491. doi:10.1037/0021-843X.97.4.487
Xu, X. J., Zuo, X. Y., Wang, X. Y., & Han, S. H. (2009). Do you feel my pain? Racial group membership modulates empathic neural responses. Journal of Neuroscience, 29, 8525–8529. doi:10.1523/JNEUROSC.2418-09.2009
Yang, C. Y., Decety, J., Lee, S. Y., Chen, C. Y., & Cheng, Y. W. (2009). Gender differences in the mu rhythm during empathy for pain: An electroencephalographic study. Brain Research, 1252, 176–184. doi:10.1016/j.brainres.2008.11.062
Zaki, J., & Ochsner, K. (2012). The neuroscience of empathy: Progress, pitfalls, and promise. Nature Neuroscience, 15, 675–680. doi:10.1038/nn.3085
Author note
This research was supported by Research Foundation Flanders Grant No. FWO10/ASP/321 to the first author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Coster, L., Verschuere, B., Goubert, L. et al. I suffer more from your pain when you act like me: Being imitated enhances affective responses to seeing someone else in pain. Cogn Affect Behav Neurosci 13, 519–532 (2013). https://doi.org/10.3758/s13415-013-0168-4
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-013-0168-4