Abstract
The amygdala is a key region in emotion processing. In particular, fMRI studies have demonstrated that the amygdala is active during the viewing of emotional faces. Previous research has consistently found greater amygdala responses to fearful than to neutral faces in adults, convergent with a focus in the animal literature on the amygdala’s role in fear processing. Studies have shown that the amygdala also responds differentially to other facial emotion types in adults. Yet the literature regarding when this differential amygdala responsivity develops is limited and mixed. Thus, the goal of the present study was to examine amygdala responses to emotional and neutral faces in a relatively large sample of healthy school-age children (N = 52). Although the amygdala was active in response to emotional and neutral faces, the results did not support the hypothesis that the amygdala responds differentially to emotional faces in 7- to 12-year-old children. Nonetheless, amygdala activity was correlated with the severity of subclinical depression symptoms and with emotional regulation skills. Additionally, sex differences were observed in frontal, temporal, and visual regions, as well as effects of pubertal development in visual regions. These findings suggest important differences in amygdala reactivity in childhood.
Similar content being viewed by others
Early investigations of the amygdala’s functional role in humans consistently noted that the amygdala is significantly involved in the processing of fear-related stimuli (e.g., Adolphs, Tranel, Damasio, & Damasio, 1995; Bishop, Duncan, & Lawrence, 2004; Broks et al., 1998; Calder, 1996; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998; LeDoux, 2003; Morris et al., 1998; Phillips et al., 1997). More recent studies have indicated that the amygdala is responsive to other emotions, as well. For example, meta-analytic work has indicated that emotional stimuli are significantly more likely to elicit amygdala activity than are neutral stimuli, for a variety of both negative and positive emotions (e.g., fear, disgust, sadness, humor, and happiness; Costafreda, Brammer, David, & Fu, 2008). However, whereas amygdala responsivity to emotional stimuli in adults has been well studied, relatively little is known about when differential amygdala responsivity to different emotionally evocative stimuli arises during development. The existing studies in the developmental literature (looking at healthy samples or investigating healthy groups within studies of psychopathology) have provided mixed results, with some studies finding increased amygdala activity to emotional as compared to neutral faces (e.g., Britton et al., 2010; Guyer et al., 2008; Monk et al., 2003), and others failing to detect differential responses (e.g., Thomas, Drevets, Dahl, et al., 2001a; Tottenham et al., 2011; Viding et al., 2012). Many of these studies employed relatively small sample sizes across a wide age range, which may have contributed to the conflicting findings. Thus, the goal of this study was to examine whether the amygdala shows differential activity to emotional and neutral faces in a relatively large sample of healthy children between the ages of 7 and 12.
Differential amygdala responses to emotional faces in adults
There is clear evidence that the amygdala is more active in response to fearful than to neutral faces in adults (Breiter et al., 1996; Bryant et al., 2008; Pessoa, Kastner, & Ungerleider, 2002; Surguladze et al., 2003; Wright et al., 2001), but evidence is mixed as to whether amygdala responses to fearful faces are stronger than its responses to other types of emotional faces. Some studies have found greater amygdala activity to fearful than to either happy (Morris et al., 1998; Whalen et al., 1998) or disgusted (Phillips et al., 2004) faces, and a meta-analysis found that amygdala activity to fear stimuli was significantly more likely than activity to sad and happy stimuli (Phan, Wager, Taylor, & Liberzon, 2002). However, other studies have shown amygdala responsivity to a wide variety of emotional stimuli in healthy adults (Breiter et al., 1996; Iidaka et al., 2001; Pessoa et al., 2002; Surguladze et al., 2003; Wright et al., 2001), and a more recent meta-analysis showed consistent amygdala activity in response to happy, sad, and fearful faces (Fusar-Poli et al., 2009), although this meta-analysis also provided some evidence for greater amygdala responsivity to fearful faces relative to either happy or sad faces. Thus, the literature has provided robust evidence for greater amygdala activity to emotional than to neutral faces in adults, with somewhat more mixed evidence with regard to whether this activity is greater for fearful than for others types of emotional faces.
Differential amygdala responses to emotional faces across development
The ability to discriminate facial emotions develops in early childhood and improves at differential rates for different emotion types through school age into adulthood (De Sonneville et al., 2002; Herba, Landau, Russell, Ecker, & Phillips, 2006; Thomas, De Bellis, Graham, & LaBar, 2007; Vicari, Reilly, Pasqualetti, Vizzotto, & Caltagirone, 2007). Similarly, the neural circuitry for emotional processing develops over early childhood and adolescence. It has been suggested that amygdala responses to emotional stimuli, including faces, follow an inverted U-shaped developmental trajectory, where adolescents show stronger emotional responses than do children or adults (Moore et al., 2012). This may be due to the differential developmental trajectories of the amygdala and prefrontal cortex (PFC) control regions, in that the amygdala and other subcortical regions reach maturity earlier than do the cortical regions that contribute to their regulation (Casey, Duhoux, & Malter Cohen, 2010; Somerville & Casey, 2010). Several studies using fMRI emotional processing tasks have provided support for heightened emotional reactivity during childhood and adolescence as compared to adulthood (Guyer et al., 2008; Monk et al., 2003) and for greater reactivity in adolescence than in either adulthood or childhood (Hare et al., 2008). However, other researchers have found different relationships between amygdala activity and age. For example, in a study of children and adolescents (9–17 years old), age correlated negatively with left amygdala response to fearful faces, particularly in females (Killgore, Oki, & Yurgelun-Todd, 2001).
Furthermore, the age at which differential amygdala responsivity to emotional and neutral facial expressions emerges is unclear. Examining the few studies that have focused on amygdala reactivity in healthy children and adolescents, in addition to studies on psychopathology that have reported results from healthy control samples, the data on when differential amygdala reactivity to emotional and neutral faces arises are very mixed. Two studies of children/adolescents that included a wide age range (9–17 years old, Ns = 31 and 17) showed significantly greater amygdala responses to fearful than to neutral faces (Guyer et al., 2008; Monk et al., 2003). Similarly, the healthy controls (mean age 13 years old, N = 17) in a study of pediatric obsessive–compulsive disorder showed significant right amygdala activity for fearful versus neutral faces and significant bilateral amygdala responses to the combined contrast of fearful, happy, and disgusted faces versus neutral faces (Britton et al., 2010). On the other hand, studies that have included or focused on younger children have not consistently shown greater amygdala responses to fearful or emotional than to neutral faces. Two such studies examining emotion processing in late school-age children found the amygdala showed deactivation to fearful faces or significantly less activity to fearful than to neutral faces (Lobaugh, Gibson, & Taylor, 2006 [10- to 12-year-olds, N = 10]; Thomas, Drevets, Whalen, et al., 2001b [healthy controls: mean age 11 years old, N = 12]). The healthy control children in three other studies (8- to 15-year-olds, N = 15; mean age 10 years old, N = 27; and 10- to 16-year-old boys, N = 16), exhibited no difference in amygdala responses to fearful as compared to neutral/calm faces (Tottenham et al., 2011). Yet one study using magnetoencephalography did find differential activity in the left amygdala for fearful/happy faces versus neutral faces in younger children (7- to 10-year-olds, N = 10), but it did not show this differential activity in older children and adults, when it is typically observed (Hung, Smith, & Taylor, 2012). Thus, the previous literature exploring emotion processing in school-age children has not provided clear developmental norms. This may be due to the differing age ranges of the participants included in the previous studies (7–17 years old). Studies including older children more often find adult-like differentiation in amygdala responses to different emotions, whereas studies including mostly younger children find more mixed evidence for this. The inconsistencies in the literature may also be due in part to the small sample sizes of healthy participants used in some studies (range 10–31, median of 16 participants). As such, a study with a relatively large sample size and a relatively restricted age range is needed to clarify whether or not differential amygdala activity occurs to different emotional faces in healthy school-age children, in order to inform developmental norms.
Sex and pubertal influences on amygdala response to emotional faces
A number of researchers have also examined sex differences in amygdala responsivity during emotional processing. In adult males, the amygdala is larger than it is in adult females, even when adjusting for whole-brain volume (Hamann, 2005). In addition, males and females show different patterns of laterality in the amygdala responses that predict subsequent memory for emotionally evocative stimuli (Cahill, 2004, 2010), with right amygdala activity predicting subsequent memory in males, but left amygdala activity doing so in females. Yet, sex differences in amygdala responsivity to emotional faces have been less clear. Early meta-analytic work did not find clear effects of sex on amygdala activity (Wager, Phan, Liberzon, & Taylor, 2003). However, a more recent meta-analysis by Fusar-Poli et al. (2009) found significantly greater right amygdala activity in adult males than females, with a recent large-scale study confirming these findings in adolescents (Schneider et al., 2011). Little work has focused on sex differences in amygdala responsivity to emotional faces in school-age children.
Puberty may play a role in the inverted U-shaped trajectory of amygdala responses described above. It has been noted that pubertal development positively correlates with a higher magnitude of responses in the amygdala (and in thalamus and extrastriate visual cortex) to emotional faces during passive viewing. This relationship was observed for viewing angry, fearful, happy, sad, and neutral faces in 13-year-old children, but the same positive relationship between pubertal development and amygdala responsivity was only observed for viewing neutral faces in children 10 years of age (Moore et al., 2012). Greater left amygdala activation has also been found for neutral faces than for nonface control stimuli in pre/early-pubertal as compared to mid/late-pubertal adolescents (Forbes, Phillips, Silk, Ryan, & Dahl, 2011). On the basis of these findings, puberty has been characterized as a developmental period with heightened amygdala activation to emotions.
Amygdala hyperactivity in depression
The amygdala is also hypothesized to be a key structure in the pathology of depression. Elevated amygdala activation during emotion-processing tasks has been clearly shown in depressed adults, adolescents, and children (e.g., Barch, Gaffrey, Botteron, Belden, & Luby, 2012; Beesdo et al., 2009; Gaffrey et al., 2011; Surguladze et al., 2005; Yang et al., 2010). Amygdala hyperactivity has also been shown to be reduced after antidepressant treatment in adults (Sheline et al., 2001), and this reduction predicts better treatment outcomes among adult depressed patients (Canli et al., 2005; Siegle, Carter, & Thase, 2006), potentially suggesting that amygdala response to faces may be a correlate of an underlying disruption or bias in emotion processing and is impacted by treatment of depression. Interestingly, amygdala hyperactivity to emotional faces is also observed in individuals at risk for developing depression (Monk et al., 2008; Zhong et al., 2011). Importantly, even subclinical levels of depression in otherwise psychiatrically healthy adults have been shown to correlate positively with amygdala reactivity both to negative versus neutral words (Laeger et al., 2012) and to the expectation of negative pictures (Abler et al., 2010). Yet, it is unclear whether subclinical levels of depression (or normative depressive emotions) may relate to amygdala reactivity in psychiatrically healthy children. Thus, another goal of the present study was to examine the relationship between self-reports and parent reports of subclinical depressive symptoms in school-age children and amygdala reactivity to emotionally valenced faces.
Present study
In summary, it is unclear from the developmental literature whether children exhibit differential amygdala responses to different emotionally evocative and neutral faces. The discrepant findings in the literature may be due to the small sample sizes of healthy children and to the wide age ranges of participants in some studies. As such, the goal of the present study was to assess amygdala responsivity during facial emotion processing in a relatively large sample of healthy children (7–12 years old) using fMRI in an attempt to inform developmental norms. We tested the hypothesis that children show greater activity for emotional than neutral faces and differential amygdala activity to different facial emotion expressions (happy, angry, sad, fearful, and neutral) during a gender judgment fMRI task. Additionally, we tested the hypotheses that amygdala reactivity to emotional faces would increase as a function of sex (greater amygdala activity in males), age (greater amygdala activity as children approach adolescence), and pubertal development (greater amygdala activity as children progress through puberty). Furthermore, given the link between depression and elevated amygdala reactivity to emotional versus neutral faces, we tested the hypotheses that amygdala reactivity would increase as a function of subclinical depression symptom severity and emotional regulation impairments in healthy children. To better characterize normative emotion processing in this sample of healthy school-age children, we also followed the current emphasis in the field on providing whole-brain results in addition to focused a priori region-of-interest analyses, and tested for effects of emotion type, sex, and puberty, as well as for a relationship with participant age, at a whole-brain level. Because we employed a relatively large sample of school-age children who had been thoroughly screened for any psychiatric diagnoses for several years prior to scanning, the results of the study can provide key insights into normative emotion processing in 7- to 12-year-old children. Particularly, our findings can clarify discrepancies in the literature by testing whether children in this age range show adult-like differentiation in amygdala reactivity to emotional faces. Additionally, it will be pivotal to understand amygdala reactivity to emotional faces in a psychiatrically healthy population, to serve as a foundation for understanding findings in childhood psychopathology.
Materials and method
Participants
The participants represented a subsample of children enrolled in the Preschool Depression Study (PDS), a prospective longitudinal study of preschool-age children and their families conducted at the Washington University School of Medicine Early Emotional Development Program (WUSM EEDP) in St. Louis. The broad goals of the PDS were to explore clinical and neural outcomes relating to preschool-onset major depression. As such, 3- to 5-year-old children and their primary caregivers were recruited from local daycares, preschools, and primary care sites in the St. Louis metropolitan area oversampling for children expressing symptoms of depression while also recruiting healthy children who did not meet diagnostic criteria for any psychiatric conditions (see Luby, Si, Belden, Tandon, & Spitznagel, 2009, for further recruitment details). The children were assessed annually and then participated in a neuroimaging session between the ages of 7 and 12. An additional 40 psychiatrically healthy children were recruited into the PDS when they were between the ages of 7 and 12, at the time of scanning. The in-depth clinical assessments involved in the PDS allowed us to identify a normative and psychiatrically healthy population of school-age children to include in the present analyses. Thus, the present subsample represents 52 children who did not meet criteria for any psychiatric diagnosis at the initial or any subsequent assessment time points up to the time of scan (N = 22) and psychiatrically healthy participants recruited into the PDS at the time of scan (N = 30). All participants in this subsample participated in the neuroimaging phase of the PDS (see Table 1 for their demographic details). A history of head trauma, neurological disease, developmental delay, and use of psychoactive medications were exclusionary for the present analyses. Additionally, only participants who met stringent criteria for neuroimaging data quality were included in this analysis (of the 67 psychiatrically healthy children who participated in the neuroimaging session, 52 met the data quality criteria; further details can be found in the Movement Correction section). Parental written consent and child assent were obtained prior to study participation and the Institutional Review Board at Washington University approved all experimental procedures.
Diagnostic assessment
Trained staff from the WUSM EEDP conducted up to four in-person assessment interviews with participants and their parents/guardians between the baseline assessment and the time of scan. Before the age of 8, children were assessed using the Preschool-Age Psychiatric Assessment (PAPA), a reliable and age-appropriate semistructured parent-report diagnostic interview (based on the DSM-IV) that is widely used in research on preschool-age psychopathology (Egger, Ascher, & Angold, 2003) and has been validated for children up to the age of 8. Once study participants reached the age of 8, the Childhood and Adolescent Psychiatric Assessment (CAPA) was used. The CAPA also examines symptoms of various psychiatric disorders but, unlike the PAPA, also makes use of report from the child or adolescent in addition to report from the primary caregiver (Angold & Costello, 2000). These interviews were audiotaped, reviewed for reliability, and calibrated for accuracy, as has been previously reported (Luby et al., 2009). These assessments were used to select a subset of participants from the PDS who did not meet criteria for any psychiatric disorder at any time point from baseline to the time of scan to assure examination of a psychiatrically healthy population.
Additional measures
Several other factors were assessed at the time of scan (see Table 1 for details). The severity of depression symptoms at the time of scan was assessed using the child- and parent-report versions of the Children’s Depression Inventory (CDI-C and CDI-P, respectively; Kovacs, 1985). The scores reported here represent standardized t scores totaled across the subscales of the CDI. A t score of 50 represents an average score, whereas a score above 65 may identify a potentially depressed individual (consistent with the fact that no children met criteria for MDD during any PAPA/CAPA interview, all children scored below 65 on the CDI).
The Children’s Emotion Management Scale for Sadness (CEMS-S; Zeman, Shipman, & Penza-Clyve, 2001) was used to assess the participants’ behavioral responses to sadness. This consists of 15 statements, each assessed on a 1–3 Likert scale. These questions have been shown to probe three dimensions of sadness management: inhibition, dysregulation, and coping. Higher scores on the inhibition and dysregulation subscales are endorsements of more frequent use of ineffective management strategies (e.g., “I do things like mope when I am sad”; previous results showed positive correlations between the dysregulation and inhibition subscales and CDI scores: Zeman et al., 2001). The coping subscale was reverse-scored, so that higher scores also indicate less effective emotion management. A total score across these three subscales was used for the present analyses, with higher scores indicating less effective sadness management skills.
Parental report on the Tanner Staging Questionnaire (Tanner, 1955) was used to assess pubertal status. Children under 10 years old were not assessed using the Tanner Staging Questionnaire due to the sensitive nature of the questions. All children under 10 years old were assigned to the Stage I group to represent presumed prepubertal status. Due to the young age of the sample, few participants were identified as being in the later (IV and V) stages of puberty and thus Stages III, IV, and V were collapsed into one late-puberty group. Pubertal status was assessed as a three-level factor, with pre (I), early (II), and late (III–V) puberty groups represented.
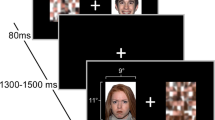
Facial emotion-processing task
The facial emotion-processing task represents one segment of a longer scanning session that the children in the PDS completed, which included high resolution structural imaging, diffusion imaging, and resting state functional connectivity. This task is the only aspect of the scanning session used for the present analysis. During the task, children were shown a series of faces and were asked to judge the gender of the face, responding by button press to indicate whether the face was male or female. This task was chosen as previous research has indicated that the heightened amygdala responses to emotional faces associated with MDD may become more apparent with an implicit processing task rather than one requiring explicit emotion processing (Fales et al., 2008; Monk et al., 2008). This task was also preferable to a passive viewing task, as it helps to ensure engagement with the visual stimuli. The face stimuli were drawn from the MacArthur Network Face Stimuli Set, a validated stimulus set containing images of 43 different actors from different ethnic backgrounds (Tottenham et al., 2009). Children saw faces with neutral, sad, angry, happy, and fearful expression from 10 of the individuals in this stimulus set. One goal of the PDS was to probe potential emotional biases relating to preschool-onset depression apparent with varying intensity of emotional facial expressions. To this end, we created intermediate sad, angry, happy, and fearful expressions by morphing the neutral expression for each individual with each emotional expression resulting in a face halfway between the neutral and each emotional face (MorphAge software). Yet, as this was not of direct interest in the present analysis, we collapsed across the full and half intensity faces for each emotion type to increase our power to find effects of emotion type. Preceding this task, all children underwent a mood induction paradigm in the scanner on the basis of prior work (Gotlib et al., 2005). Previous work has shown that negative mood induction can reactivate affective processing biases (Scher, Ingram, & Segal, 2005) and neural changes in affective processing (Ramel et al., 2007) specifically in patients with a history of depression while not introducing any affective biases in individuals who have not experienced a history of depression. Given that the goals of the PDS were to explore the effects of a history of preschool-onset major depression on the brain, the mood induction was included for all children to reactivate any affective processing biases in children with a history of preschool-onset major depression who were depressed at the time of scan. The mood induction was a short clip from the film My Girl (Gazer & Zieff, 1991) that portrays a child’s loss of a close friend, followed by verbal instructions to elaborate on any negative affect induced. A positive mood repair clip was shown at the end of the MRI session. Because we are only examining children in this subsample who were psychiatrically healthy at all assessments through the time of scan, we did not expect this mood induction to introduce any bias in the present results. To confirm this, we used the children’s reports of their mood before and after the induction (on a 5-point Likert-type scale; 1 = very sad to 5 = very happy) to test whether postinduction mood or change in reported mood correlated with amygdala activity to rule out any induced bias.
Participants performed two runs, each with 45 stimuli, totaling 90 trials for each participant (18 from each of the five emotion types). Each stimulus was presented for 2,250 ms, followed by an intertrial interval of 250, 2,750, or 5,250 ms (each occurring with equal frequencies). Gender judgment button responses were acquired during the stimulus presentation period via a fiber optic button box. Images were projected onto a computer screen behind the participant’s head within the imaging chamber viewed by a mirror attached above the participant’s face.
fMRI scanning
Structural and functional imaging data were collecting using a 3.0-T TIM TRIO Siemens whole-body system at Washington University in St. Louis. T1-weighted structural images were acquired in the sagittal plane using an MPRAGE 3-D sequence (TR = 2,400 ms, TE = 3.16 ms, flip angle = 8°, slab = 176 mm, 176 slices, matrix size = 256 × 256, field of view [FOV] = 256 mm, voxel size = 1 × 1 × 1 mm). Functional images were collected during the face-processing task with a 12-channel head coil using a T2*-weighted gradient-echo echo-planar sequence in the axial plane (TR = 3,000 ms, TE = 27 ms, flip angle = 90°, FOV = 256 mm, voxel size = 4 × 4 × 4 mm). T2-weighted images were collected for registration purposes using a 3-D SPACE acquisition (TR = 3,200 ms, TE = 497 ms, 160 slices, FOV = 256, voxel size = 1 × 1 × 1 mm).
fMRI preprocessing
Imaging data were preprocessed using the following steps: (1) correction for slice-dependent time shifts; (2) removal of the first four images of each run, to allow the BOLD signal to reach a steady state; (3) elimination of odd/even slice intensity differences due to interpolated acquisition; (4) realignment of the data acquired from each participant within and across runs to compensate for rigid body motion (Ojemann et al., 1997); (5) image intensity normalization to a whole-brain mode value of 1,000; (6) registration of the 3-D structural volume (T1) to the atlas template in the Talairach coordinate system (Talairach & Tournoux, 1988), using a 12-parameter affine transform and resampling to a 1-mm cubic representation (Buckner et al., 2004; Ojemann et al., 1997); (7) coregistration of the 3-D fMRI volume to the T2, and the T2 to the participant’s structural image; (8) transformation of the fMRI data to a 3 × 3 × 3 mm voxel atlas space using a single affine 12-parameter transform; and (9) spatial smoothing using a 6-mm full-width at half-maximum Gaussian filter.
Movement correction
Stringent data quality criteria were used for participant inclusion in the present analyses. The signal-to-noise ratio (SNR; i.e., the mean signal/standard deviation across each BOLD run, computed for each slice and then averaged across all slices) for each of the two task runs was calculated using in-house software following preprocessing. Only participants with an SNR for each of the task runs above 200 were included in the present analyses (mean SNR for first run: 579.83 ± 196.35, minimum = 223; mean SNR for second run: 510.35 ± 185.15, minimum = 230). Out of 67 psychiatrically healthy children who met all other inclusion criteria, 52 met these further criteria (i.e., 15 were excluded for not completing both runs of the task or for having an SNR > 200 on at least one run). After the preprocessing steps described above, we applied previously validated corrections for head motion, termed “motion scrubbing,” adapted for fMRI data (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). The motion-scrubbing procedure assesses frame-wise displacement on the basis of the movement parameters used in Preprocessing Step 4. For any given frame (i.e., time point), this represents the differential head motion from the previous frame summing across linear (x, y, z) and rotational displacements (yaw, pitch, and roll, where degrees of rotation are converted to millimeters of movement by calculating displacement on the surface of a sphere with a radius of 50 mm). A temporal mask was created to remove any frame with a sum displacement greater than 0.5 mm. This threshold was selected to be stringent and remove any spikes in head motion while still maintaining the majority of the data. Details on the validity and efficacy of this procedure and on the number of frames removed by the motion scrubbing are presented in the supplementary materials.
fMRI analysis
Analysis of the fMRI data was performed using in-house software (FIDL analysis package, www.nil.wustl.edu/~fidl/). A voxel-wise general linear model (GLM) approach was used, which incorporated regressors for linear trend and baseline shifts. We did not assume a hemodynamic response shape for this analysis because of concerns about potential age-related differences in the shape or timing of this response (e.g., Huettel, Singerman, & McCarthy, 2001; Jacobs et al., 2008; Richter & Richter, 2003). Instead, a finite impulse response approach was used in which seven frames (seven 2-s TRs per trial) were modeled for each trial. Only those trials on which the participant made a correct gender judgment were included in the analysis, though there were overall very few incorrect trials (mean error rate = 4.5 %, between zero and seven errors/emotion type across subjects, mean of 1.7 errors per emotion type). We performed region-of-interest (ROI) analyses focusing on the amygdala and then exploring effects at the whole-brain level. Both the primary ROI-based and whole-brain analyses consisted of a repeated measures analysis of variance (ANOVA) with Time (seven time points per trial) as the repeated measure, Emotion (five facial expression types: neutral, sad, angry, happy, and fearful), as a within-subjects factor, and Sex and Pubertal Status as between-subjects factors. With this analysis, a significant main effect of time indicates that there are differences in activity across time points (testing the null hypothesis that activity is equal at all time points). We did not focus on main effects of emotion, sex, or puberty (which test for mean differences in these factors averaged across time points) as these often can indicate baseline shifts or poor modeling by the GLM in certain regions and are less meaningful than interactions with time. Any interactions with time indicate a significant difference in activity across conditions over at least some time points (e.g., a difference in peak amplitude or in shape). We did not attempt to interpret any four-way interactions (Time × Emotion × Sex × Pubertal Status) because of the complexity of such effect and their likely lack of robustness. However, we did examine up to the three-way interactions.
Amygdala ROI analyses
Amygdala ROIs were defined in two ways: functionally and anatomically. The functionally defined ROIs were isolated from the whole-brain main effect of time from the ANOVA described above and in the Whole-Brain Analyses section. These ROIs selected voxels that showed significant activation to the task at the group level, but may not clearly represent the anatomical boundaries of the amygdala for individual participants. Thus, as an alternative approach, to define amygdala ROIs with greater anatomical specificity, we used FreeSurfer version 4.5.0 (Fischl et al., 2002; Fischl et al., 2004) to segment each participant’s T1 anatomical image. Amygdala ROIs were extracted for each participant, down-sampled to match the functional resolution of the atlas space (3 × 3 × 3 mm), and registered to the common atlas space using each participant’s whole-brain transformation parameters from preprocessing. Mean time courses for each emotion type were extracted for each participant from the group-level functional ROIs and from his or her individually defined anatomical amygdala ROIs. The ANOVA described above was performed on this data using IBM SPSS Statistics 18.0 (SPSS Inc., Chicago, IL). Additionally, correlation analyses were performed to explore the relationship between amygdala activity and age, CDI-C, CDI-P, and CEMS-S scores. Control correlations were also performed with postinduction mood and change in reported mood due to the mood induction to affirm that this prior task had no effect on the present results. These factors were correlated with the average activity from the anatomically defined amygdala ROIs for each emotion type, as well as the comparison of fearful faces to neutral faces and of sad faces to neutral faces.
Whole-brain analyses
The repeated measures ANOVA described above was performed using the FIDL analysis package to examine effects of time, emotion, sex, and pubertal status at the whole-brain level. On the basis of Monte Carlo simulations implemented with in-house software, a significance threshold of 13 contiguous voxels with z values > 3.0 was used to obtain a whole-brain false-positive rate of .05. The resulting significance maps were separated into ROI clusters using in-house peak finding scripts that identify peaks in z scores at least 15 mm apart from each other. These ROIs were used to examine the time course of the hemodynamic response and for post-hoc contrasts to parse the source of significant effects (Bonferroni corrected for the number of post-hoc tests performed for each ROI). Additionally, participant age was correlated at every voxel with the average activity for each emotion type. On the basis of Monte Carlo simulations, a significance threshold of 17 contiguous voxels with z values > 3.0 was used to obtain a whole-brain false positive rate of .05.
Results
Demographic characteristics
In Table 1 we present the demographic characteristics of the sample. Given the interest in potential effects of sex in emotional processing, we explored sex differences in the distribution of the other demographic factors. There was no significant difference in ethnic distribution as a function of sex [χ 2(2, N = 52) = 3.74, p = .15]. Most of the participants were right handed (44/52 participants), and there was no significant difference as a function of sex [χ 2(1, N = 52) = 1.66, p = .44]. The males in this sample were significantly older than the females [t(51) = 2.41, p = .02]. However, no significant difference in the distribution of pubertal status was found as a function of sex [χ 2(2, N = 52) = 4.50, p = .11], though the males were significantly older than females in Stages I [t(25) = 2.81, p = .01] and II [t(9) = 8.42, p = .02]. Additionally, there was a significant relationship between pubertal status and age [Spearman’s ρ(50) = .75, p < .001]. No significant sex differences were found for scores on the CDI-C [t(48) = 0.19, p = .85] or CEMS-S [t(38) = 0.57, p = .57]. However, the females in this sample showed higher t scores on the CDI-P than the males [t(49) = 3.22, p = .002], though both groups were still in the nondepressed range (< 65).
Amygdala ROI results
Amygdala ROI ANOVA
Table 2 presents the results of the ANOVA for the functionally and anatomically defined amygdala ROIs (Fig. 1 shows these ROIs and the average time courses for the anatomically defined ROIs). These results demonstrate that the amygdala is active during face viewing, given the significant main effect of time point found for both the bilateral amygdala, defined both functionally and anatomically [e.g., anatomical left amygdala ROI: F(3.46, 159.17) = 5.63, p < .001, η p 2 = .11]. However, no other effect reached significance in either the functionally or anatomically defined amygdala ROIs. Although it is difficult to draw inferences with certainty from null results, we feel that our sample size provided sufficient power to isolate even a small effect of emotion type. Specifically, using G*Power (Faul, Erdfelder, Lang, & Buchner, 2007), we derived post-hoc estimates of our achieved power to find significant effects of a within-subjects condition (i.e., emotion type) with a repeated measures ANOVA at the small effect sizes observed in these data. With 52 participants, we had power of .62 to detect a small effect with a partial η 2 of .01 and power over .99 to detect an effect with a partial η 2 of .03 or greater. We conducted three additional analyses to confirm these results: (1) a repeated measures ANOVA including all seven time points including only Time and Emotion Type as factors, given that including Pubertal Status and Sex as between-subjects factors could lower power to detect effects of emotion; (2) a repeated measures ANOVA with only Emotion Type as a factor and using only Time Point 3 (the peak time point of the average time courses); and (3) a repeated measures ANOVA with only Emotion Type as a within-subjects factor using data modeled with an assumed hemodynamic response (SPM canonical function). The results of these three additional ANOVAs were essentially identical to those observed in Table 2 (i.e., significant amygdala responsivity to all face types, but no significant effect of Emotion × Time in both the left or right amygdala) and are presented in Supplementary Table 1 and Supplementary Fig. 4. Additionally, to assure that including half-intensity emotional faces did alter the results, we also ran an ANOVA including only the full-intensity faces and still found no effect of emotion type (ps > .78).
Functionally and anatomically defined amygdala regions of interest (ROIs). (a) Functionally defined amygdala ROIs isolated from the whole-brain main effect of time (b) Anatomically defined amygdala ROIs isolated by FreeSurfer segmentation. Heat map values indicate the number of participants with overlapping ROIs at each voxel. (c) Time courses (average of all face types) for left and right anatomically defined ROIs with 95 % confidence intervals. *Significant difference from zero at a given time point (p < .05)
Amygdala ROI correlations
In Table 3, we present the correlations between average activity for the different emotion types from the anatomically defined amygdala ROIs and age, CDI-C, CDI-P, and CEMS-S scores. In addition, we examined the differences in activity between sad and neutral faces and between fearful and neutral faces. To control false positives due to multiple comparisons, we used a false discovery rate (FDR) correction within measure and hemisphere. Partial correlations were used to control for the effect of sex in the age and CDI-P correlations, given that the boys and girls showed significant mean differences on these variables. No significant correlations were found between age and amygdala activity to any emotion type. CDI-C scores were not normally distributed in this sample [Kolmogorov–Smirnov test of normality: Z(47) = 1.59, p = .013], and as such, nonparametric correlations with CDI-C were calculated (Spearman’s ρ). Significant negative correlations were found between bilateral amygdala activity to neutral faces and CDI-C scores, though only the correlation with left amygdala activity survived FDR correction. Significant positive correlations were found between parent report CDI scores and activity to angry, happy, and fearful faces, although only correlations with angry and happy face activity survived FDR correction. Finally, CEMS-S scores were positively correlated with right amygdala activity to sad faces as well as activity to sad–neutral faces and fearful–neutral faces, though only the correlation with fearful–neutral faces survived FDR correction. Postinduction mood and change in reported mood due to negative mood induction were not correlated with average activity, peak activity, or magnitude estimates with a modeled hemodynamic response function for any emotion type, sad–neutral faces, or fearful–neutral faces (all ps > .1).
Our analyses of puberty categorized individuals into pre-, early-, and late-puberty groups. However, other work has examined correlations across a five level puberty range (Moore et al., 2012). To try to replicate these prior findings more directly, we performed Spearman correlations between pubertal status using the five Tanner stages and amygdala responses in addition to the ANOVAs reported. We found that right amygdala responses to sad faces and the sad–neutral faces contrast negatively correlated with pubertal status [sad, ρ(50) = −.285, p = .041; sad–neutral, ρ(50) = −.330, p = .017], though these effects did not pass correction for multiple comparisons.
Whole-brain results
Whole-brain ANOVA
Figure 2 shows the whole-brain ANOVA results for the main effect of time (projected to the surface of the brain rendering). This encompasses a wide range of regions including occipital cortex regions, the fusiform gyrus, response-related motor regions, anterior and posterior cingulate regions, prefrontal regions, and a number of limbic regions, including the amygdala and hippocampus. The interaction between time and face emotion type was significant in several areas, shown in Table 4. These areas included the left superior temporal gyrus, regions of the cerebellum, the cuneus, and the inferior parietal lobule. To clarify the source of these interactions, we computed post-hoc contrasts by examining whether an Emotion × Time interaction emerged when each of the four emotional face types was compared to neutral faces (repeated measures ANOVA with two emotion types and seven time points). The results of these post-hoc contrasts are shown in Table 4 (significant comparisons are noted, Bonferroni corrected). Supplementary Fig. 5 shows time courses for each emotion type separately for two example regions. Notably, the amygdala was not among the regions showing a Time × Emotion Type interaction.
Table 4 (and Supplementary Fig. 6) shows the regions demonstrating a significant interaction of sex and time. These regions showed several different time course patterns, as is shown in Fig. 3. First, there were several regions that showed greater activation in females as compared to males (in Table 4: F > M act.), including the right lingual gyrus, right fusiform gyrus, right precuneus, and superior and middle temporal gyri. Several other regions showed greater deactivation in females as compared to males (F > M deact.), including the right and left precuneus, the left cuneus, left anterior and posterior cingulate, left inferior frontal gyrus, and left superior temporal gyrus. Post-hoc ANOVAs (seven time points as the repeated measure) testing for a main effect of time for each sex separately indicated that most of these regions were active in females but not males, as is noted in Table 4. The middle frontal gyrus, declive, and putamen showed greater activity in males than females, with no significant main effect of time in these regions for females (M > F act., F: n.s.).
Example time courses in brain regions showing three different types of interactions between sex and time. Lines indicate the average time courses for each sex. (a) Fusiform gyrus shows greater activity in females than in males (F > M, act). (b) Precuneus shows deactivation in females and no significant effect of time in males (F > M deact; M: n.s.). (c) Putamen shows activation in males and no significant effect of time in females (M > F act; F: n.s.)
As is indicated in Table 4, only one region showed a significant Emotion Type × Sex × Time interaction. This right inferior frontal gyrus region is shown in Fig. 4. To understand the source of this interaction, we conducted post-hoc repeated measures ANOVAs separately for each emotion type with time as the repeated measure (seven time points) and Sex as a between-subjects factor. These post-hoc contrasts indicated a significant Sex × Time interaction only for happy [F(4, 88) = 3.49, p = .005] and fearful faces [F(4, 15) = 3.17, p = .014, although this effect did not pass Bonferroni correction]. As is shown in Fig. 4, males tended to show earlier peaks in activity to both happy and fearful faces than did females.
Time course for the inferior frontal gyrus region showing an Emotion × Sex × Time interaction. (a) Surface rendering of the region showing the Emotion × Sex × Time interaction. The color scale (shading, in print) represents z-score values from a whole-brain ANOVA. (b) Lines indicate average time courses for happy- and fearful-face trials (the only emotion types showing a Sex × Time interaction), split by sex
Table 5 and Fig. 5 show the ANOVA results for the interactions with pubertal status. As can be seen in Fig. 5, the regions showing significant interactions with puberty are mainly located in primary and secondary visual cortex regions. As is indicated by the post-hoc tests in Table 5 and the time courses in Supplementary Fig. 7, the regions with a significant Puberty × Time interaction show increased activity in the late and/or early puberty groups as compared to the prepubertal group including the lingual, fusiform, and inferior occipital gyri. The cuneus and middle occipital gyrus show this increased late versus prepubertal group activity as well as differentiation between the late and early groups. Several regions show an Emotion × Puberty × Time interaction, including the fusiform gyrus, lingual gyrus, and transverse and superior temporal gyri. A variety of visual regions also show a Puberty × Sex × Time interaction. Post-hoc tests showed that the interaction in most regions was driven by Puberty × Time interactions in the females where the early puberty females show increased activity. The lingual gyrus and precuneus also show differential activity in the males across puberty groups where the males in late puberty show elevated activity.
Brain regions showing interactions with pubertal status. Colors indicate interaction types. The inset shows the ventral posterior right hemisphere, to highlight the region of greatest overlap. Red: Puberty × Time; Green: Puberty × Sex × Time; Blue: Emotion × Puberty × Time; Yellow: overlap of Puberty × Sex × Time and Puberty × Time; Purple: overlap of Emotion × Puberty × Time and Puberty × Time; Pink: overlap between all three contrasts
Whole-brain correlations
Table 6 shows the results of whole-brain partial correlations with age controlling for sex. All of the identified regions showed a negative correlation with age, such that older children exhibit less activity to the indicated emotion type. There was a negative correlation between left supramarginal gyrus activity to neutral faces and age and between left precentral gyrus activity to sad faces and age. Additionally, the responses to fearful faces in the cerebellum, parahippocampal gyrus, insula, cuneus, middle occipital gyrus, and cingulate gyrus were negatively correlated with age.
Discussion
Research exploring emotion processing in humans has shown that the amygdala is central to the processing of fear (e.g., Adolphs et al., 1995; Bishop et al., 2004; Broks et al., 1998; Calder, 1996; LaBar et al., 1998; LeDoux, 2003; Morris et al., 1998; Phillips et al., 1997), although the amygdala also shows increased activity to other emotional stimuli (for meta-analyses, see Costafreda et al., 2008; Phan et al., 2002). Developmental studies of amygdala activity have suggested that emotional responses show an inverted U-shaped developmental trajectory, in which amygdala activity to emotional stimuli is stronger in adolescents than in either children or adults (Guyer et al., 2008; Monk et al., 2003; Moore et al., 2012). However, the available literature offers mixed results and has been limited in its ability to inform us as to whether children show differential activity to emotionally evocative versus neutral faces, due to small sample sizes and broad age ranges (e.g., Britton et al., 2010; Lobaugh et al., 2006; Thomas et al., 2001a, 2001b; Tottenham et al., 2011).
Amygdala response to emotional and neutral faces
Consistent with the large and established literature on this topic, we found that the bilateral amygdala was active in response to face processing in children. However, we found no evidence that the amygdala showed differential activity to emotionally valenced faces or neutral faces, either in ROI-based analyses or in whole-brain analyses. As such, our findings are consistent with prior results suggesting that differential amygdala responses to specific facial emotions observed in adults arise later in development, during adolescence and into early adulthood. Additionally, undifferentiated amygdala responses to emotional faces are consistent with studies of normative emotion development in childhood demonstrating that the ability to discern and understand the emotions of others increases in sophistication through school age and into adolescence. That is, the ability to react to and fully experience differences in emotions expressed by others is developing during this period (Durand, Gallay, Seigneuric, Robichon, & Baudouin, 2007; Gross & Ballif, 1991), and this may be reflected in the overall activity to faces but the lack of differentiation to different emotion types in these data. These findings may be important for understanding emotion processing in children and across development. Particularly, these data indicate that differential amygdala responses to different emotional faces have not fully developed in this school-age range, suggesting that the peak of the proposed inverted U-shaped curve of emotional development occurs after this 7- to 12-year-old range. Additionally, understanding the normative pattern of amygdala responses to emotional faces will be key to further study of the neural correlates of emotion processing and related psychopathology in this age range. However, a crucial next step will be to explore amygdala responses to emotional faces longitudinally, following the same individuals from childhood, across adolescence, and into adulthood to fully characterize the developmental trajectory of amygdala activity. Characterizing the normative developmental patterns of amygdala activity in healthy children is key to understanding emotion processing in child psychopathology.
We also examined whether the degree of amygdala response was associated with either age or puberty status, but we did not find any significant relationships. This result differs from previous results finding that pubertal development positively correlated with amygdala responses to emotional and neutral faces in 13-year-old children and with responses to neutral faces in 10-year-old children (Moore et al., 2012). The differences in results across studies may be due to differences in the sample properties or in analytic approaches, although even when we used a similar analytical approach, the findings of Moore et al. were not replicated in this sample. In addition, a previous study of children and adolescents (9–17 years old) did find a negative correlation between age and left amygdala response to fearful faces (Killgore et al., 2001), which was not evident in our sample. However, the age range in the Killgore et al. study extended much later into adolescence than in our sample, and the direction of the correlation in that study was different than what would be predicted from some other studies comparing amygdala responses in children, adolescents, and adults. In the present study, we did not detect a strong relationship between pubertal status and amygdala activity. However, most of the study participants were either prepubertal or in early pubertal stages, so further study of these participants later in puberty would be important to further elucidate this issue.
Facial emotion-processing tasks tapping amygdala reactivity have been used as a key method to study depression and other psychiatric conditions. In these studies, adult and adolescent patients with major depressive disorder tend to show increased amygdala responses to emotional faces relative to healthy controls (Beesdo et al., 2009; Peluso et al., 2009; Yang et al., 2010). The degree of amygdala overactivity has been shown to predict a more severe clinical course in MDD patients over eight months (Canli et al., 2005). Thus, we were also interested in examining whether the degree of amygdala response to any face type was associated with subclinical depression symptom severity (CDI) or the ability to regulate sadness (CEMS-S) in a nonclinical sample. We found that child-reported CDI scores negatively correlated with left amygdala responses to neutral faces. In addition, parent-reported CDI scores were positively correlated with left amygdala responses to angry, fearful, and happy faces. Interestingly, differences between the child- and parent-report versions of the CDI in their relation to emotion-related brain activity were also noted in previous work with children from this same sample in a different task (Pagliaccio et al., 2011). Although the parent and child versions of the CDI are related, they may access different aspects of MDD symptomology and potentially different aspects of emotion processing, as the parent report may be more representative of external indicators of mood, whereas the child report maybe more representative of internal experiences. Yet increasing scores on both the parent- and child-report versions of the CDI may be consistent with greater amygdala reactivity to emotional versus neutral faces, typically seen in depression, as CDI-C scores correlated negatively with reactivity to neutral faces, and CDI-P scores correlated positively with emotional face activity. It was also of interest that right amygdala activity to sad faces and to the contrasts of sad – neutral and fearful – neutral faces correlated positively with CEMS-S scores (positive scores indicate increasingly maladaptive emotion management skills). In other words, children with poor sadness management skills showed greater amygdala responses to sad and fearful than to neutral faces. A study exploring amygdala reactivity in 4- to 6-year-old children with and without preschool-onset major depression noted a similar relationship between poor emotion regulation skills and amygdala reactivity to emotional and neutral faces (Gaffrey, Barch, Singer, Shenoy, & Luby, 2013).
Differential responses to emotion types outside of the amygdala
Outside of the amygdala, several regions did show differential responses as a function of emotional face type. Particularly, the inferior semilunar lobule of the cerebellum, cuneus, inferior parietal lobule, claustrum, and superior temporal gyrus differentiated between several emotional face types and neutral faces, though the exact patterns differed across regions. These findings provide clues as to what other regions of the brain, outside of the amygdala, are differentially involved in processing emotional and neutral faces. Other studies have found differential activity to emotional versus neutral faces in a range of regions outside of the amygdala. In a meta-analyses, similar regions as noted here have been related to different emotion types though not necessarily in the pattern observed in this pediatric sample (Fusar-Poli et al., 2009). Overall, these patterns of results outside of the amygdala were not highly indicative of any strong conclusions about emotion processing, and they should be examined further in future work.
Sex differences in regions outside of the amygdala
We also found a number of regions that showed interactions with sex. These results may indicate important sex differences in attention to or engagement with emotional face stimuli, with girls showing greater responses in typical face- and object-processing regions (e.g., the fusiform gyrus and superior and middle temporal gyri) and deactivations in regions associated with the default network (e.g., anterior and posterior cingulate) that were not observed among boys. In contrast, the pattern of results in the boys could suggest that they use a more “cognitively” based strategy for processing faces in this task, as they showed greater activation of regions more typically associated with cognitive processing (e.g., middle frontal gyrus and the putamen). Only a single region, inferior frontal cortex, showed a further interaction of sex with emotion type. This sex difference seemed to primarily reflect differences in the timing of activation rather than the degree of activation, with boys showing early peaks than girls to both fearful and happy faces. A similar region also exhibited sex differences in activity evoked by emotionally valenced images in a previous study (Wrase et al., 2003). Additionally, this inferior frontal region has been associated with emotional perspective taking and observing facial expression making (Hynes et al., 2006; Jabbi & Keysers, 2008).
Effects of puberty outside of the amygdala
We also examined influences of pubertal status on regions involved in facial emotion processing outside of the amygdala. The early and late puberty groups showed increased activity relative to the prepubertal group in the middle occipital gyrus, lingual gyrus, cuneus, and fusiform gyrus. A range of largely overlapping visual cortex regions also exhibited Emotion × Puberty × Time and/or Puberty × Sex × Time interactions. The overlap between these interactions with pubertal status suggests the importance of development on visual responses to emotional faces. Interestingly, Moore et al. (2012) also noted positive correlations between pubertal status and extrastriate visual cortex activity to both emotional and neutral faces. Although we cannot determine the facts specifically from these results, it is possible that this effect may indicate increased attention to the face stimuli in the children later in puberty. However, it is difficult to fully interpret the source of these pubertal effects with the given task design. Using a gender judgment task yields no direct measure of identification of or engagement with emotions. The hypothesis of a differential attentional focus or bias as a function of sex or pubertal status could thus be explored through other tasks with explicit measures of attention or engagement.
Limitations
One of the primary limitations of the present study is that one of the main findings (lack of differential amygdala responsivity to emotional faces) is a null result. Again, although the amygdala showed significant main effects of time across the five face types, there was no significant effect of Emotion × Time, indicating that the amygdala is active to all face types but likely does not show differential activation patterns to different emotions in healthy 7- to 12-year-old children. Though it is difficult to draw inferences from null result, we feel that our sample size yields more than sufficient power to identify even small potential effects. Additionally, the effect sizes observed for the Emotion × Time interactions were very small indicating little to no difference between conditions. These results are also consistent at the whole-brain and ROI level (both anatomically and functionally defined) and when examining the magnitude of amygdala activity with an assumed hemodynamic response function, helping to indicate that amygdala activity in children 7–12 years old does not differentiate between facial emotion types.
Another limitation to note is that all children underwent a negative mood induction prior to the facial emotion-processing task. This was included in the scan session on the basis of the goals of the PDS and though not of interest in the present analyses, we also do not believe that this prior task would introduce any bias into the present analyses. Previous work has shown that mood induction can reactivate negative affective biases specifically in previously depressed individual but not in never depressed individuals (Scher et al., 2005). Because all children in this sample were assessed as psychiatrically healthy through the time of scan, we did not expect this mood induction to influence the present task. This was confirmed in control analyses showing that postinduction mood and change in mood due to negative mood induction were not correlated with amygdala response to any face type. Additionally, should the mood induction have had an effect, we would have expected it to increase the salience of negative facial expressions, which was not apparent in our null effect of emotion type.
Conclusion
Overall, we did not find evidence for differential amygdala responses to different emotional face types in 7- to 12-year-old children, but we did find that increased amygdala activity was related to the severity of subclinical depression symptoms, as rated by parents, and to sadness regulation skills. Therefore, although amygdala responses to emotional and neutral faces may be sensitive to differences related to depression symptom severity and emotion management skills, it is important to consider this overall amygdala response to faces but the null effect of emotion type when investigating psychopathology in school-age children. In contrast to findings in the amygdala, other regions of the brain did show differential activity to different emotion types, with influences of sex in frontal, temporal, and visual regions, as well as of pubertal development, particularly in visual cortex regions. Future work will be needed to determine whether these sex and/or pubertal influences on activity in visual-processing regions reflect differential attention to or processing of emotional face stimuli in children. Further study of amygdala processing in healthy children could be aided by the inclusion of targeted high-resolution functional scanning of the amygdala during this type of emotion-processing task in order to assure the best quality signal—although studies in adults have found differential amygdala reactivity with whole-brain scans—and to potentially explore differences among amygdala subregions. Longitudinal follow-up will also be key to further understanding the development of differential amygdala responses to emotional faces, allowing for the assessment of within-subjects trajectories from childhood to adolescence.
References
Abler, B., Hofer, C., Walter, H., Erk, S., Hoffmann, H., Traue, H. C., & Kessler, H. (2010). Habitual emotion regulation strategies and depressive symptoms in healthy subjects predict fMRI brain activation patterns related to major depression. Psychiatry Research: Neuroimaging, 183, 105–113.
Adolphs, R., Tranel, D., Damasio, H., & Damasio, A. R. (1995). Fear and the human amygdala. Journal of Neuroscience, 15, 5879–5891.
Angold, A., & Costello, E. J. (2000). The Child and Adolescent Psychiatric Assessment (CAPA). Journal of the American Academy of Child and Adolescent Psychiatry, 39, 39–48.
Barch, D. M., Gaffrey, M. S., Botteron, K. N., Belden, A. C., & Luby, J. L. (2012). Functional brain activation to emotionally valenced faces in school-aged children with a history of preschool-onset major depression. Biological Psychiatry, 72, 1035–1042. doi:10.1016/j.biopsych.2012.06.009
Beesdo, K., Lau, J. Y., Guyer, A. E., McClure-Tone, E. B., Monk, C. S., Nelson, E. E., . . . Pine, D. S. (2009). Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry, 66, 275–285. doi:10.1001/archgenpsychiatry.2008.545
Bishop, S. J., Duncan, J., & Lawrence, A. D. (2004). State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience, 24, 10364–10368.
Breiter, H., Etcoff, N., Whalen, P., Kennedy, W., Rauch, S., Buckner, R., . . . Rosen, B. (1996). Response and habituation of the human amygdala during visual processing of facial expression. Neuron, 17, 875–887.
Britton, J. C., Stewart, S. E., Killgore, W. D. S., Rosso, I. M., Price, L. M., Gold, A. L., . . . Rauch, S. L. (2010). Amygdala activation in response to facial expressions in pediatric obsessive-compulsive disorder. Depression and Anxiety, 27, 643–651.
Broks, P. P., Young, A. W. A., Maratos, E. J. E., Coffey, P. J. P., Calder, A. J. A., Isaac, C. L. C., . . . Hadley, D. D. (1998). Face processing impairments after encephalitis: Amygdala damage and recognition of fear. Neuropsychologia, 36, 59–70.
Bryant, R. A., Kemp, A. H., Felmingham, K. L., Liddell, B., Olivieri, G., Peduto, A., . . . Williams, L. M. (2008). Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Human Brain Mapping, 29, 517–523.
Buckner, R. L., Head, D., Parker, J., Fotenos, A. F., Marcus, D., Morris, J. C., & Snyder, A. Z. (2004). A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. NeuroImage, 23, 724–738.
Cahill, L. (2004). Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: An fMRI investigation. Learning and Memory, 11, 261–266.
Cahill, L. (2010). Sex influences on brain and emotional memory: The burden of proof has shifted. Progress in Brain Research, 186, 29–40.
Calder, A. J. (1996). Facial emotion recognition after bilateral amygdala damage: Differentially severe impairment of fear. Cognitive Neuropsychology, 13, 699–745.
Canli, T., Cooney, R. E., Goldin, P., Shah, M., Sivers, H., Thomason, M. E., . . . Gotlib, I. H. (2005). Amygdala reactivity to emotional faces predicts improvement in major depression. NeuroReport, 16, 1267–1270.
Casey, B. J., Duhoux, S., & Malter Cohen, M. (2010). Adolescence: What do transmission, transition, and translation have to do with it? Neuron, 67, 749–760. doi:10.1016/j.neuron.2010.08.033
Costafreda, S. G., Brammer, M. J., David, A. S., & Fu, C. H. Y. (2008). Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews, 58, 57–70. doi:10.1016/j.brainresrev.2007.10.012
De Sonneville, L. M. J., Verschoor, C. A., Njiokiktjien, C., Op het Veld, V., Toorenaar, N., & Vranken, M. (2002). Facial identity and facial emotions: Speed, accuracy, and processing strategies in children and adults. Journal of Clinical and Experimental Neuropsychology, 24, 200–213.
Durand, K., Gallay, M., Seigneuric, A., Robichon, F., & Baudouin, J.-Y. (2007). The development of facial emotion recognition: The role of configural information. Journal of Experimental Child Psychology, 97, 14–27.
Egger, H., Ascher, B., & Angold, A. (2003). The Preschool Age Psychiatric Assessment (Version 1.4). Durham, NC: Duke University Medical Center, Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences.
Fales, C. L., Barch, D. M., Rundle, M. M., Mintun, M. A., Snyder, A. Z., Cohen, J. D., . . . Sheline, Y. I. (2008). Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry, 63, 377–384. doi:10.1016/j.biopsych.2007.06.012
Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. doi:10.3758/BF03193146
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., . . . Dale, A. M. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355.
Fischl, B., Salat, D. H., van der Kouwe, A. J. W., Makris, N., Ségonne, F., Quinn, B. T., & Dale, A. M. (2004). Sequence-independent segmentation of magnetic resonance images. NeuroImage, 23(Suppl. 1), S69–S84.
Forbes, E. E., Phillips, M. L., Silk, J. S., Ryan, N. D., & Dahl, R. E. (2011). Neural systems of threat processing in adolescents: Role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology, 36, 429–452.
Fusar-Poli, P., Placentino, A., Carletti, F., Landi, P., Allen, P., Surguladze, S., . . . Politi, P. (2009). Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience, 34, 418–432.
Gaffrey, M. S., Barch, D. M., Singer, J., Shenoy, R., & Luby, J. L. (2013). Disrupted amygdala reactivity as an early biomarker of depression: An fMRI study of depressed 4–6 year old children. Manuscript under review.
Gaffrey, M. S., Luby, J. L., Belden, A. C., Hirshberg, J. S., Volsch, J., & Barch, D. M. (2011). Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: An fMRI study. Journal of Affective Disorders, 129, 364–370.
Gazer, B., & Zieff, H. (1991). My girl [Motion picture]. Hollywood, CA: Columbia Pictures.
Gotlib, I. H., Sivers, H., Gabrieli, J. D. E., Whitfield-Gabrieli, S., Goldin, P., Minor, K. L., & Canli, T. (2005). Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. NeuroReport, 16, 1731–1734.
Gross, A. L., & Ballif, B. (1991). Children’s understanding of emotion from facial expressions and situations: A review. Developmental Review, 11, 368–398.
Guyer, A. E., Monk, C. S., McClure-Tone, E. B., Nelson, E. E., Roberson-Nay, R., Adler, A. D., . . . Ernst, M. (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20, 1565–1582.
Hamann, S. (2005). Sex differences in the responses of the human amygdala. Neuroscientist, 11, 288–293. doi:10.1177/1073858404271981
Hare, T., Tottenham, N., Galvan, A., Voss, H., Glover, G., & Casey, B. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go–nogo task. Biological Psychiatry, 63, 927–934.
Herba, C. M., Landau, S., Russell, T., Ecker, C., & Phillips, M. L. (2006). The development of emotion-processing in children: Effects of age, emotion, and intensity. Journal of Child Psychology and Psychiatry, 47, 1098–1106.
Huettel, S. A., Singerman, J. D., & McCarthy, G. (2001). The effects of aging upon the hemodynamic response measured by functional MRI. NeuroImage, 13, 161–175.
Hung, Y., Smith, M. L., & Taylor, M. J. (2012). Development of ACC–amygdala activations in processing unattended fear. NeuroImage, 60, 545–552. doi:10.1016/j.neuroimage.2011.12.003
Hynes, C. A., Baird, A. A., & Grafton, S. T. (2006). Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia, 44(3), 374–383.
Iidaka, T., Omori, M., Murata, T., Kosaka, H., Yonekura, Y., Okada, T., & Sadato, N. (2001). Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. Journal of Cognitive Neuroscience, 13, 1035–1047. doi:10.1162/089892901753294338
Jabbi, M., & Keysers, C. (2008) Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion, 8(6), 775–780.
Jacobs, J., Hawco, C., Kobayashi, E., Boor, R., LeVan, P., Stephani, U., . . . Gotman, J. (2008). Variability of the hemodynamic response as a function of age and frequency of epileptic discharge in children with epilepsy. NeuroImage, 40, 601–614.
Killgore, W. D. S., Oki, M., & Yurgelun-Todd, D. A. (2001). Sex-specific developmental changes in amygdala responses to affective faces. NeuroReport, 12, 427–433.
Kovacs, M. (1985). The Children’s Depression, Inventory (CDI). Psychopharmacology Bulletin, 21, 995–998.
LaBar, K. S., Gatenby, J. C., Gore, J. C., LeDoux, J. E., & Phelps, E. A. (1998). Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron, 20, 937–945.
Laeger, I., Dobel, C., Dannlowski, U., Kugel, H., Grotegerd, D., Kissler, J., . . . Zwanzger, P. (2012). Amygdala responsiveness to emotional words is modulated by subclinical anxiety and depression. Behavioural Brain Research, 233, 508–516.
LeDoux, J. (2003). The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology, 23, 727–738.
Lobaugh, N. J., Gibson, E., & Taylor, M. J. (2006). Children recruit distinct neural systems for implicit emotional face processing. NeuroReport, 17, 215–219.
Luby, J. L., Si, X., Belden, A. C., Tandon, M., & Spitznagel, E. (2009). Preschool depression: Homotypic continuity and course over 24 months. Archives of General Psychiatry, 66, 897–905. doi:10.1001/archgenpsychiatry.2009.97
Monk, C. S., Klein, R. G., Telzer, E. H., Schroth, E. A., Mannuzza, S., Moulton, J. L., . . . Ernst, M. (2008). Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. American Journal of Psychiatry, 165, 90–98.
Monk, C. S., McClure, E. B., Nelson, E. E., Zarahn, E., Bilder, R. M., Leibenluft, E., . . . Pine, D. S. (2003). Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage, 20, 420–428.
Moore, W. E., Pfeifer, J. H., Masten, C. L., Mazziotta, J. C., Iacoboni, M., & Dapretto, M. (2012). Facing puberty: Associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience, 7, 35–43.
Morris, J. S., Friston, K. J., Büchel, C., Frith, C. D., Young, A. W., Calder, A. J., & Dolan, R. J. (1998). A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain, 121, 47–57.
Ojemann, J. G., Akbudak, E., Snyder, A. Z., McKinstry, R. C., Raichle, M. E., & Conturo, T. E. (1997). Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. NeuroImage, 6, 156–167.
Pagliaccio, D., Luby, J., Gaffrey, M., Belden, A., Botteron, K., Gotlib, I. H., & Barch, D. M. (2011). Anomalous functional brain activation following negative mood induction in children with pre-school onset major depression. Developmental Cognitive Neuroscience, 2, 256–267. doi:10.1016/j.dcn.2011.11.008
Peluso, M. A., Glahn, D. C., Matsuo, K., Monkul, E. S., Najt, P., Zamarripa, F., . . . Soares, J. C. (2009). Amygdala hyperactivation in untreated depressed individuals. Psychiatry Research, 173, 158–161. doi:10.1016/j.pscychresns.2009.03.006
Pessoa, L., Kastner, S., & Ungerleider, L. G. (2002). Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research, 15, 31–45.
Phan, K., Wager, T., Taylor, S., & Liberzon, I. (2002). Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage, 16, 331–348.
Phillips, M. L., Williams, L. M., Heining, M., Herba, C. M., Russell, T., Andrew, C., . . . Gray, J. A. (2004). Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. NeuroImage, 21, 1484–1496.
Phillips, M. L., Young, A. W., Senior, C., Brammer, M., Andrew, C., Calder, A. J., . . . David, A. S. (1997). A specific neural substrate for perceiving facial expressions of disgust. Nature, 389, 495–498.
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59, 2142–2154. doi:10.1016/j.neuroimage.2011.10.018
Ramel, W., Goldin, P. R., Eyler, L. T., Brown, G. G., Gotlib, I. H., & McQuaid, J. R. (2007). Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biological Psychiatry, 61, 231–239.
Richter, W., & Richter, M. (2003). The shape of the fMRI BOLD response in children and adults changes systematically with age. NeuroImage, 20, 1122–1131.
Scher, C. D., Ingram, R. E., & Segal, Z. V. (2005). Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review, 25, 487–510.
Schneider, S., Peters, J., Bromberg, U., Brassen, S., Menz, M. M., Miedl, S. F., . . . IMAGEN Consortium. (2011). Boys do it the right way: Sex-dependent amygdala lateralization during face processing in adolescents. NeuroImage, 56, 1847–1853. doi:10.1016/j.neuroimage.2011.02.019
Sheline, Y. I., Barch, D. M., Donnelly, J. M., Ollinger, J. M., Snyder, A. Z., & Mintun, M. A. (2001). Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biological Psychiatry, 50, 651–658.
Siegle, G., Carter, C., & Thase, M. (2006). Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry, 163, 735.
Somerville, L. H., & Casey, B. (2010). Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology, 20, 236–241.
Surguladze, S., Brammer, M. J., Keedwell, P., Giampietro, V., Young, A. W., Travis, M. J., . . . Phillips, M. L. (2005). A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry, 57, 201–209.
Surguladze, S. A., Brammer, M. J., Young, A. W., Andrew, C., Travis, M. J., Williams, S. C. R., & Phillips, M. L. (2003). A preferential increase in the extrastriate response to signals of danger. NeuroImage, 19, 1317–1328.
Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the human brain: 3-Dimensional proportional system. An approach to cerebral imaging. Stuttgart, Germany: Thieme.
Tanner, J. (1955). Growth at adolescence. Oxford, UK: Blackwell Scientific.
Thomas, K. M., Drevets, W. C., Dahl, R. E., Ryan, N. D., Birmaher, B., Eccard, C. H., . . . Casey, B. J. (2001). Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry, 58, 1057–1063.
Thomas, K. M., Drevets, W. C., Whalen, P. J., Eccard, C. H., Dahl, R. E., Ryan, N. D., & Casey, B. J. (2001). Amygdala response to facial expressions in children and adults. Biological Psychiatry, 49, 309–316.
Thomas, L. A., De Bellis, M. D., Graham, R., & LaBar, K. S. (2007). Development of emotional facial recognition in late childhood and adolescence. Developmental Science, 10, 547–558. doi:10.1111/j.1467-7687.2007.00614.x
Tottenham, N., Hare, T. A., Millner, A., Gilhooly, T., Zevin, J. D., & Casey, B. J. (2011). Elevated amygdala response to faces following early deprivation. Developmental Science, 14, 190–204.
Tottenham, N., Tanaka, J. W., Leon, A. C., McCarry, T., Nurse, M., Hare, T. A., . . . Nelson, C. (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research: Neuroimaging, 168, 242–249.
Vicari, S., Reilly, J. S., Pasqualetti, P., Vizzotto, A., & Caltagirone, C. (2007). Recognition of facial expressions of emotions in school-age children: The intersection of perceptual and semantic categories. Acta Paediatrica, 89, 836–845.
Viding, E., Sebastian, C. L., Dadds, M. R., Lockwood, P. L., Cecil, C. A. M., De Brito, S. A., & McCrory, E. J. (2012). Amygdala response to preattentive masked fear in children with conduct problems: The role of callous-unemotional traits. American Journal of Psychiatry, 169, 1109–1116.
Wager, T. D., Phan, K. L., Liberzon, I., & Taylor, S. F. (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: A meta-analysis of findings from neuroimaging. NeuroImage, 19, 513–531.
Whalen, P. J., Rauch, S. L., Etcoff, N. L., McInerney, S. C., Lee, M. B., & Jenike, M. A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18, 411–418.
Wrase, J., Klein, S., Gruesser, S. M., Hermann, D., Flor, H., Mann, K., et al. (2003). Gender differences in the processing of standardized emotional visual stimuli in humans: A functional magnetic resonance imaging study. Neuroscience Letters, 348(1), 41–45.
Wright, C. I., Fischer, H., Whalen, P. J., McInerney, S. C., Shin, L. M., & Rauch, S. L. (2001). Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. NeuroReport, 12, 379–383.
Yang, T. T., Simmons, A. N., Matthews, S. C., Tapert, S. F., Frank, G. K., Max, J. E., . . . Paulus, M. P. (2010). Adolescents with major depression demonstrate increased amygdala activation. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 42–51.
Zeman, J., Shipman, K., & Penza-Clyve, S. (2001). Development and initial validation of the Children’s Sadness Management Scale. Journal of Nonverbal Behavior, 25, 187–205.
Zhong, M., Wang, X., Xiao, J., Yi, J., Zhu, X., Liao, J., . . . Yao, S. (2011). Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biological Psychology, 88, 233–242. doi:10.1016/j.biopsycho.2011.08.007
Author note
This work was supported by the National Institute of Mental Health (Grant Nos. MH64769 to J.L.L. and MH090786 to J.L.L., D.M.B., and K.N.B.). A.C.B.’s work on the manuscript was supported by a grant from the National Institute of Mental Health (No. 1K01MH090515-01). M.S.G.’s work on the manuscript was supported by a grant from the National Institute of Mental Health (No. K23MH098176). The NIMH had no further role in the design and conduct of the study (collection, management, analysis, or interpretation of the data) or in the preparation, review, or approval of the manuscript. D.P. had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis. We thank all participants and their families who provided time and effort to making this study possible. The authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Amygdala ANOVA results from functionally and anatomically defined ROIs, only examining emotion and time (DOC 248 kb)
Supplementary Figure 1
Histogram of frames remaining following motion scrubbing across all participants (DOC 86 kb)
Supplementary Figure 2
Relationship between z-scores for main effect of time prior to motion scrubbing and after motion scrubbing. Each point represents an ROI isolated from the ANOVA main effect of time for the unscrubbed data. ROIs above the x-y line show an increase in zscore due to motion scrubbing. (DOC 121 kb)
Supplementary Figure 3
Percent ratio of scrubbed/unscrubbed z-scores of main effect of time due to motion scrubbing (DOC 921 kb)
Supplementary Figure 4
Mean activity in the left and right anatomically defined amygdala for each stimulus emotion type with 95 % confidence intervals. Top row: Mean activity at timepoint 3 (unassumed response shape) Bottom row: Magnitude estimate for SPM cannonical HRF. * significantly different than zero at p < 0.05 (DOC 244 kb)
Supplementary Figure 5
Example timecourses for each emotion type from regions showing a significant interaction between emotion and time (DOC 101 kb)
Supplementary Figure 6
Brain regions showing interaction between gender and time (DOC 566 kb)
Supplementary Figure 7
Example timecourses for each pubertal group from regions showing a significant interaction between puberty and time (DOC 95 kb)
Rights and permissions
About this article
Cite this article
Pagliaccio, D., Luby, J.L., Gaffrey, M.S. et al. Functional brain activation to emotional and nonemotional faces in healthy children: Evidence for developmentally undifferentiated amygdala function during the school-age period. Cogn Affect Behav Neurosci 13, 771–789 (2013). https://doi.org/10.3758/s13415-013-0167-5
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-013-0167-5