Abstract
The present study is a replication and extension of previous research examining the effects of others’ gaze direction and gaze shifts on both participants’ (N = 32) manual responses, as an indicator of covert processes, and their visual attention, as an indicator of overt processes, within an experimental response time (RT) paradigm, under both fixed- and free-viewing instructions. Participants viewed arrays of faces displaying direct or averted gaze, which shifted or held their gaze, concurrent with the presentation of a target letter that participants had to identify overlaid on one face, all while their gaze was recorded with an eye-tracking system. Participants’ RTs and eye movements both revealed faster responses when the target face displayed either direct or shifted gaze, and especially when its gaze had shifted from averted to direct, though these effects were modulated by the viewing instructions. Thus, the findings replicate and extend previous research by revealing that direct gaze and dynamic motion onset affect both covert and overt attention.
Similar content being viewed by others
How social and nonsocial cues affect visual processes and social interactions are long-standing yet emerging areas of investigation. The physiology of the human eye makes it an effective communicative cue (Emery, 2000; Frischen, Bayliss, & Tipper, 2007), and gaze direction serves productive and receptive signal functions (Duncan, 1969; Gobel, Kim, & Richardson, 2015). Productively, we intentionally direct others’ attention using our gaze (Böckler, Knoblich, & Sebanz, 2011; Conty, George, & Hietanen, 2016; Hietanen, Myllyneva, Helminen, & Lyyra, 2016; Langton, Watt, & Bruce, 2000; Nummenmaa & Calder, 2009; Otsuka, Mareschal, Calder, & Clifford, 2014; Sebanz, Bekkering, & Knoblich, 2006; Senju & Hasegawa, 2005; Senju & Johnson, 2009; Shirahama, 2012). Receptively, we infer others’ attention from their gaze direction (Carrick, Thompson, Epling, & Puce, 2007; Langton et al., 2000) and reflexively look where others are looking even when doing so is uninformative (Driver et al., 1999; Friesen, Ristic, & Kingstone, 2004; Kuhn & Kingstone, 2009).
Gaze direction also contributes more deeply to social interactions. Beyond following others’ averted gaze, perceived direct gaze, classified as eye contact in actual social interactions, has the phenomenal effect of activating social awareness and capturing attention (Conty et al., 2016; Kendon, 1967). Faces exhibiting direct gaze quicken facial recognition (Hood, Macrae, Cole-Davies, & Dias, 2003), and amplify emotional state awareness (Baltazar et al., 2014). These effects are so exuberant that they have been theorized to represent covert processes, operating preattentively, automatically, and beyond volitional control (Laidlaw, Risko, & Kingstone, 2012; Rothkich, Madipakkam, Rehn, & Sterzer, 2015; Stein, Senju, Peelen, & Sterzer, 2011; Yokoyama, Sakai, Noguchi, & Kita, 2014).
Dynamic motion onset also automatically elicits attention (Abrams & Christ, 2003; Kawahara, Yanase, & Kitazaki, 2012), and is oftentimes coupled with direct gaze in social communication (Hayward & Ristic, 2017), though few studies have investigated how gaze and motion onset cues combine to affect attention and cognition. Böckler, van der Wel, and Welsh (2014), however, explored this with a target detection task. Participants fixated a cross surrounded by four face images, two each displaying direct and averted gaze, and all with a letter overlaying the forehead. After a fixed interval, two of the faces held their gaze direction while the other two shifted to the alternative gaze direction, and, simultaneously, one of the letters was replaced with one of two target letters while the other three were replaced with a distractor letter. Participants identified the target letter faster when it appeared on a face that had shifted rather than held its gaze direction, displayed a held direct gaze rather than a held averted gaze, or transitioned from averted to direct gaze rather than from direct to averted gaze, supporting the predictions that direct gaze and onset motion both attract covert social attention.
Thus, leading theories suggest that we are drawn to others’ eyes and orient in their gaze direction, though how this occurs is not fully understood. It is unknown, for instance, whether others’ gaze direction and gaze shifts are subject to and influence both covert and overt attention, which we explore here by replicating Böckler et al. (2014), with novel manipulation and measure extensions. We manipulate overt attention by varying participants’ instructions. Böckler et al. (2014) provided instructions to fixate a centrally presented cross throughout their experiment, with the expectation that participants could and would do so, and observed effects were therefore inferred to have operated through covert processes. We will contrast participants we instruct to fixate a central cross and those not given this instruction. If effects of others’ gaze are entirely covert, then no differences should emerge between instructions conditions, because covert processes should be similarly accessible across conditions. By contrast, if direct-gaze and gaze-shift effects are influenced by overt processes, then condition differences should emerge. In addition, we incorporate eye-tracking measures to analyze effects on overt attention. We will examine the effectiveness of the instruction to hold fixation, and, more importantly, will test whether participants’ eye movements reflect previously documented performance differences. Thus, our goal was to illuminate the effects of others’ gaze on both covert and overt attention.
Method
Participants
Thirty-two undergraduate students participated (11 males, 21 females; see the supplement, Participants section, for a power analysis). All participants had normal or corrected-to-normal vision, were ≥ 18 years old (MAge = 19.9 years), and earned course credit for participating. One additional participant was tested but excluded as an outlier (mean RT > + 4 SDs from the sample mean). The study was approved by the Georgia Southern University Institutional Review Board.
Apparatus
Participants completed the study on a Tobii TX-300 eye-tracking system with a 512 × 285 mm (1,920 × 1,080 pixels) LCD monitor, at a distance of M = 63.1 cm, with binocular gaze recorded at 60 Hz. Participants responded on a wireless keyboard held on their laps. E-Prime 2.0 (Psychology Software Tools Inc., Pittsburgh, PA, USA) managed the stimulus presentation, eye-tracker functionality, and response measurement. We processed the eye-tracking data with customized Matlab scripts (MathWorks Inc., R2016a), which extracted gaze sequences as a function of tracking quality and clustered gaze sequences into discrete fixations (see the supplement, Eye-Tracking Procedure section).
Stimuli and procedure
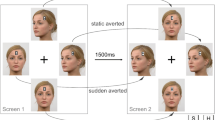
The stimuli were adapted directly from those of Böckler et al. (2014). On each trial, participants viewed an array of four face images, two each displaying direct and averted gaze, all with an “8” overlaid on the forehead (see Fig. 1, left frame), each in 200 × 250 pixel resolution (4.7° × 5.8° visual angle), in a diamond pattern around a central cross. After 1,500 ms, a stimulus shift to another array of faces, two with unchanged gaze, and one each shifted from direct to averted and from averted to direct gaze (see Fig. 1, right frame), co-occurred with a change of three of the “8s” to a distractor letter (either “E” or “U”) and one to a target letter (either “S” or “H”), which participants identified as quickly as possible. Thus, gaze direction was determined by whether the target face displayed direct or averted gaze, and gaze shift by whether the target face was shifted or unchanged across arrays. The response screen appeared until participants had provided a manual response, and it was followed by a 1,000-ms blank screen intertrial interval.
Participants were assigned, in alternation, to a fixed-viewing condition, in which their instructions included (see the Instructions in the supplement), “Please try to hold your eyes on the cross that appears at the center of the screen throughout the trials,” or a free-viewing condition, which omitted this instruction (though the cross was present for all participants). Participants completed 96 trials of each combination of direct versus averted gaze by shifted versus unchanged target face, using each possible unique combination of target and distractor face and letter position, for 384 total trials. Participants took a break, with a self-determined duration, every 50 trials.
Results
Response times
Response times (RTs) were calculated as the differences between the stimulus shift and the key-press response. Following Böckler et al. (2014), incorrect-response trials (M = 2.7%) and those with RTs ± 2 SDs beyond the group mean (M = 2.8%) were excluded (see Table S1 for inclusion rates per condition). We conducted a 2 × 2 × 2 mixed model analysis of variance (ANOVA), with instructions examined between groups, gaze direction and gaze shift as repeated measures, and mean RTs as the dependent variable (DV). This revealed effects of gaze direction, F(1, 30) = 6.56, p = .016, ηp2 = .179, with faster responses for direct (M = 879.2 ms, SEM = 19.1) than for averted (M = 889.7, SEM = 19.8) gazes; and gaze shift, F(1, 30) = 96.63, p < .001, ηp2 = .763, with faster responses on shifted (M = 849.6, SEM = 16.8) than on unchanged (M = 919.3, SEM = 22.2) gazes. Instruction condition was nonsignificant, F(1, 30) = 1.46, p = .237, ηp2 = .046, though instructions and gaze shift did interact, F(1, 30) = 6.43, p = .017, ηp2 = .177. Simple effects analyses indicate a greater gaze-shift effect in the fixed-viewing, F(1, 30) = 76.46, p < .001, ηp2 = .718, than in the free-viewing, F(1, 30) = 26.60, p < .001, ηp2 = .470, condition (see Fig. 2). All other interactions were nonsignificant (p ≥ .125). An analogous ANOVA on response accuracy revealed no effects (all Fs ≤ 2.09, all ps ≥ .159), indicating no speed–accuracy trade-off.
To directly replicate Böckler et al. (2014), we ran a 2 × 2 repeated measures ANOVA for gaze direction and gaze shift in only the fixed-viewing condition. This revealed effects of gaze direction, F(1, 15) = 8.86, p = .009, ηp2 = .371, and gaze shift, F(1, 15) = 54.57, p < .001, ηp2 = .784, and no interaction, F(1, 15) = 0.75, p = .40, ηp2 = .048. Although notably larger than the effect obtained in the preceding analysis with both conditions, this gaze direction effect size was nearly identical to that found by Böckler et al. (2014), and our gaze shift effect size was markedly larger than theirs, essentially providing direct replication.
Eye-tracking data
First, we tested whether participants looked away from the central cross. We ran a 2 × 2 × 2 mixed model ANOVA, as with RTs, with the number of fixations per trial ≥ 1.5° from the central cross as the DV.Footnote 1 Most importantly, this revealed an instructions effect, F(1, 30) = 11.99, p = .002, ηp2 = .286, with fewer fixations away from the cross for the fixed-viewing (M = 3.68, SEM = 0.33) than for the free-viewing (M = 5.17, SEM = 0.27) condition. Gaze shift was also significant, F(1, 30) = 22.29, p < .001, ηp2 = .426, with more fixations for unchanged (M = 4.55, SEM = 0.26) than for shifted (M = 4.30, SEM = 0.25) gazes. Gaze direction and instructions interacted, F(1, 30) = 7.22, p = .012, ηp2 = .194, and simple effects analyses indicated a gaze direction effect in the free-viewing condition, F(1, 30) = 7.56, p = .010, ηp2 = .201 (direct: M = 5.24, SEM = 0.31; averted: M = 5.10, SEM = 0.30), but not in the fixed-viewing condition, F(1, 30) = 1.10, p = .302, ηp2 = .035 (direct: M = 3.66, SEM = 0.31; averted: M = 3.70, SEM = 0.30). Despite these effects, one-sample t tests indicated that both groups looked away from the cross greater than zero times per trial, on average, both ts(15) ≥ 11.07, both ps < .001. Thus, the fixed-viewing instructions likely reduced participants’ eye movements, though they tended to look away from the cross.
Second, we examined eye movements to the faces before the stimulus shift and whether participants looked at the direct-gaze faces faster and longer. We calculated the preshift gaze latencies as the time from the start of each trial until the first look at, and the preshift gaze durations as the cumulative time looking at, either the direct- or the averted-gaze faces. We conducted two 2 × 2 mixed model ANOVAs, with instruction condition examined between groups and gaze direction as a repeated measure, with gaze latencies and gaze durations as DVs. All preshift gaze latency effects were nonsignificant (see the supplement, Preshift, Gaze Latencies section). For preshift gaze durations, however, there were effects of gaze direction, F(1, 30) = 9.91, p = .004, ηp2 = .248, and instructions, F(1, 30) = 24.36, p < .001, ηp2 = .448, as well as their interaction, F(1, 30) = 9.07, p = .005, ηp2 = .232. Fixed-viewing participants looked at the faces less and did not differ between direct (M = 261.0, SEM = 45.3) and averted (M = 260.3, SEM = 43.5) gazes, F(1, 30) = 0.009, p = .924, ηp2 < .001, whereas free-viewing participants looked at the faces longer overall, and at direct gazes longer (M = 553.0, SEM = 34.2) than averted gazes (M = 517.5, SEM = 33.8), F(1, 30) = 18.97, p < .001, ηp2 = .387. Thus, before the stimulus shift, participants’ eye movement speeds did not vary by stimulus gaze or instructions, but free-viewing participants tended to look at the faces, especially those with direct gaze, longer.
Finally, we calculated postshift gaze latencies as the time from the stimulus shift to the first look at the target face (i.e., a visual–motor analogue to the RT measure). We excluded trials with no look at the target face (M = 4.1%), with incorrect responses (M = 2.2%), and with postshift gaze latencies ± 2 SDs beyond the group mean (M = 2.1%; see Table S3 for the inclusion rates per condition).Footnote 2 We conducted a 2 × 2 × 2 mixed-model ANOVA, as in the RT analysis, with mean postshift gaze latencies as the DV. As Fig. 3 illustrates, we found main effects of gaze direction, F(1, 30) = 12.22, p = .016, ηp2 = .289, with shorter latencies when gazes were direct (M = 386.0 ms, SEM = 7.3) rather than averted (M = 398.7, SEM = 7.5); gaze shift, F(1, 30) = 98.28, p < .001, ηp2 = .766, with shorter latencies on shifted (M = 356.6, SEM = 7.0) than on unchanged (M = 428.0, SEM = 9.0) gazes; and instructions, F(1, 30) = 24.06, p < .001, ηp2 = .445, with shorter latencies for free (M = 357.0, SEM = 10.2) than for fixed (M = 427.7, SEM = 10.2) viewing. Gaze shift and instructions interacted, F(1, 30) = 9.50, p = .004, ηp2 = .240, and simple-effect analyses indicated a greater shifted–unchanged difference in fixed viewing, F(1, 30) = 84.44, p < .001, ηp2 = .738, than in free viewing, F(1, 30) = 23.34, p < .001, ηp2 = .438. All other interactions were nonsignificant (p ≥ .178). Thus, these findings replicate and extend the RT analyses and indicate that both direct gaze and gaze shifts, as compared to averted and stable gazes, draw overt attention.
Discussion
This study replicates and extends previous research on how both gaze direction and gaze shifts affect social attention. As in Böckler et al. (2014), participants responded faster when a target appeared on a face that exhibited direct rather than averted gaze and when gaze shifted rather than was held. Using the same sample size, stimuli, procedure, data inclusion criteria, and analyses, these findings mirrored those of Böckler et al. (2014), which contributes to this area of research at a time when replications are highly valued (Nelson, Simmons, & Simonsohn, 2018; Open Science Collaboration, 2015).
We also extended this previous research to examining the role of overt attention, by manipulating participants’ instructions and tracking their gaze. Participants received fixed- or free-viewing instructions. Some have posited that faces exhibiting direct gaze preattentively draw attention, in a way that is reflexive and beyond volitional control (Laidlaw et al., 2012; Rothkich et al., 2015; Senju & Hasegawa, 2005; Stein et al., 2011; Yokoyama et al., 2014). We reasoned that, if others’ gaze direction and gaze shifts affect only covert processes, then the conditions should not differ, because covert processes should be similarly accessible across conditions. If, however, overt processes are involved, then the effects of gaze direction and gaze shifts should be modulated by instruction condition. The participants in both conditions were faster to respond to and look at faces that shifted their gaze, which is consistent with the proposal that covert processes are at play; however, these effects were larger in the fixed- than in the free-viewing condition, suggesting that the free-viewing participants’ freedom to engage overt attention may have reduced the covert motion onset effect (Abrams & Christ, 2003; Hayward & Ristic, 2017; Kawahara et al., 2012). The directionality of this effect is particularly intriguing, as it suggests that constraining participants to fixate the cross amplified the relative advantage for processing the gaze-shift motion information, and in turn that this, more so than gaze direction, is especially likely to capture covert attention. Furthermore, this suggests that gaze shift, onset motion, and direct gaze capture covert and overt attention in different ways, which must be explored further in future research.
The eye-tracking measures revealed faster looks to a target face when it displayed direct rather than averted gaze and shifted rather than unchanged gaze, suggesting that direct gaze and gaze shifts activate overt receptive social attention (Carrick et al., 2007; Conty et al., 2016; Langton et al., 2000). Examining the intersection of the instructions manipulation and eye-tracking measures, the fixed-viewing participants looked away from the cross less than did free-viewing participants, although they too looked at the faces on most trials. This suggests that they were cooperative and invested in performing the task as instructed, but also that there may have been some inability to inhibit overt attention to others’ faces. Before the stimulus shift, the participants in the free-viewing condition looked at direct-gaze faces longer, even though the faces were nonpredictive of where the target letter would appear. This suggests some underlying preference for the direct-gaze stimuli and is consistent with social attention facilitation (Baltazar et al., 2014; Hood et al., 2003; Kendon, 1967), even though the stimuli were mere two-dimensional photographs, which some have argued contribute minimally to self-referential mentalizing processes, and therefore constrain observable effects that may be amplified with genuine social–interactive eye contact (Conty et al., 2016; Hietanen et al., 2016).
In conclusion, our results indicate that free versus fixed viewing modulated the effects of others’ gazes on both participants’ responses and their eye movements. The participants instructed to maintain fixation showed a stronger stimulus gaze shift effect. Our findings, however, reveal that direct and shifting gaze most likely affect not only covert attention processes, as assessed by RTs, but, also, as indicated by eye-tracking measures that revealed highly similar findings, overt attention processes.
Notes
This included all trials, though excluding trials as above produced the same qualitative findings. Also, the analysis of fixation durations provides converging evidence (see the supplement, Fixation Durations section and Table S2).
The rates further indicated that the fixed-viewing participants looked at the faces less, yet did so on most trials.
References
Abrams, R. A., & Christ, S. E. (2003). Motion onset captures attention. Psychological Science, 14, 427–432.https://doi.org/10.1111/1467-9280.01458
Baltazar, M., Hazem, N., Vilarem, E., Beaucousin, V., Picq, J. L., & Conty, L. (2014). Eye contact elicits bodily self-awareness in human adults. Cognition, 133, 120–127. https://doi.org/10.1016/j.Cognition2014.06.009
Böckler, A., Knoblich, G., & Sebanz, N. (2011). Observing shared attention modulates gaze following. Cognition, 120, 292–298.
Böckler, A., van der Wel, R. P. R. D., & Welsh, T. N. (2014). Catching eyes: Effects of social and nonsocial cues on attention capture. Psychological Science, 25, 720–727.
Carrick, O. K., Thompson, J. C., Epling, J. A., & Puce, A. (2007). It’s all in the eyes: Neural responses to the social significance of gaze shifts. NeuroReport, 18, 763–766.
Conty, L., George, N., & Hietanen, J.K. (2016). Watching eyes effects: When others meet the self. Conciousness and Cognition, 45, 184–197.
Driver, J., Davis, G., Ricciardelli, P., Kidd, P., Maxwell, E., & Baron-Cohen, S. (1999). Gaze perception triggers reflexive visuospatial orienting. Visual Cognition, 6, 509–540.
Duncan, S. (1969). Nonverbal communication. Psychological Bulletin, 72, 118–136.
Emery, N. J. (2000). The eyes have it: The neuroethology, function and evolution of social gaze. Neuroscience & Biobehavioral Reviews, 24, 581–604. https://doi.org/10.1016/S0149-7634(00)00025-7
Friesen, C. K., Ristic, J., & Kingstone, A. (2004). Attentional effects of counterpredictive gaze and arrow cues. Journal of Experimental Psychology: Human Perception and Performance, 30, 319–329. https://doi.org/10.1037/0096-1523.30.2.319
Frischen, A., Bayliss, A. P., & Tipper, S. P. (2007). Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin, 133, 694–724. https://doi.org/10.1037/0033-2909.133.4.694
Gobel, M. S., Kim, H. H., & Richardson, D. C. (2015). The dual function of social gaze. Cognition, 136, 359–364.
Hayward, D. A., & Ristic, J. (2017). Feature and motion-based gaze cuing is linked with reduced social competence. Scientific Reports, 7, 44221. https://doi.org/10.1038/srep44221
Hietanen, J. K., Myllyneva, A., Helminen, T. M., & Lyyra, P. (2016). The effects of genuine eye contact on visuospatial and selective attention. Journal of Experimental Psychology: General, 145, 1102–1106. https://doi.org/10.1037/xge0000199
Hood, B. M., Macrae, C. N., Cole-Davies, V., & Dias, M. (2003). Eye remember you: The effects of gaze direction on face recognition in children and adults. Developmental Science, 6, 67–71.
Kawahara, J., Yanase, K., & Kitazaki, M. (2012). Attentional capture by the onset and offset of motion signals outside the spatial focus of attention. Journal of Vision, 12, 10, https://doi.org/10.1167/12.12.10.
Kendon, A. (1967). Some functions of gaze direction in social interaction. Acta Psychologia, 26, 22–63.
Kuhn, G., & Kingstone, A. (2009). Look away! Eyes and arrows engage oculomotor responses automatically. Attention, Perception, & Psychophysics, 71, 314–327. https://doi.org/10.3758/APP.71.2.314
Laidlaw, K. E., Risko, E. F., & Kingstone, A. (2012). A new look at social attention: Orienting to the eyes is not (entirely) under volitional control. Journal of Experimental Psychology: Human Perception and Performance, 38, 1132–1143. https://doi.org/10.1037/a0027075
Langton, S. R. H., Watt, R. J., & Bruce, V. (2000). Do the eyes have it? Cues to the direction of social attention. Trends in Cognitive Sciences, 4, 50–59. https://doi.org/10.1016/S1364-6613(99)01436-9
Nelson, L. D., Simmons, J., & Simonsohn, U. (2018). Psychology’s renaissance Annual Review of Psychology, 69, 511–534.
Nummenmaa, L., & Calder, A. J. (2009). Neural mechanisms of social attention. Trends in Cognitive Sciences, 13, 135–143.https://doi.org/10.1016/j.tics.2008.12.006
Open Science Collaboration. (2015). Estimating the reproducibility of psychological science. Science, 349, 4716:1–8. https://doi.org/10.1126/science.aac4716
Otsuka, Y., Mareschal, I., Calder, A. J., & Clifford, C. W. G. (2014). Dual-route model of the effect of head orientation on perceived gaze direction. Journal of Experimental Psychology: Human Perception and Performance, 40, 1425–1439. https://doi.org/10.1037/a0036151
Rothkich, M., Madipakkam, A. R., Rehn, E., & Sterzer, P. (2015). Making eye contact without awareness. Cognition, 143, 108–114.
Sebanz, N., Bekkering, H., & Knoblich, G. (2006). Joint action: Bodies and minds moving together. Trends in Cognitive Sciences, 10, 70–76. https://doi.org/10.1016/j.tics.2005.12.009
Senju, A., & Hasegawa, T. (2005). Direct gaze captures visuospatial attention. Visual Cognition, 12, 127–144.
Senju, A., & Johnson, M. H. (2009). The eye contact effect: Mechanisms and development. Trends in Cognitive Sciences, 13, 127–134. https://doi.org/10.1016/j.tics.2008.11.009
Shirahama, A. (2012). Stare in the crowd: Frontal face guides overt attention independently of its gaze direction. Perception, 41, 447–459. https://doi.org/10.1068/p7114
Stein, T., Senju, A., Peelen, M. V., & Sterzer, P. (2011). Eye contact facilitates awarenes of faces during interocular suppression. Cognition, 119, 307–311.
Yokoyama, T., Sakai, H., Noguchi, Y., & Kita, S. (2014). Perception of direct gaze does not require focus of attention. Scientific Reports, 4, 3858:1–7. https://doi.org/10.1038/srep03858
Author note
This research was conducted following the relevant ethical guidelines for human research. We thank Anne Böckler for providing the stimulus images, helpful correspondence, and, along with Pessi Lyyra, valuable comments on an earlier version of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 57.2 kb)
Rights and permissions
About this article
Cite this article
Boyer, T.W., Wang, M. Direct gaze, eye movements, and covert and overt social attention processes. Atten Percept Psychophys 80, 1654–1659 (2018). https://doi.org/10.3758/s13414-018-1590-z
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-018-1590-z