Abstract
Temporal organization of human behavior is particularly important when several action requirements must be processed around the same time. A crucial challenge in such multitasking situations is to control the temporal response order. However, multitasking studies usually focus on temporal processing dynamics after a specific response order – which is usually triggered by stimulus sequence and instructions – has been determined, whereas a comprehensive study of response-order scheduling mechanisms is still lacking. Across three psychological refractory period (PRP) experiments, we examined the impact of stimulus order, response characteristics, and several other factors on response order. Crucially, we utilized a combination of effector systems (oculomotor and manual) that are known to ensure reasonable response order variability in the first place. The results suggest that – contrary to previous assumptions – bottom-up factors (e.g., stimulus order) are not the primary determinant of temporal action scheduling. Instead, we found a major influence of effector-based characteristics (i.e., oculomotor task prioritization) that could be attenuated by both instructions and changes in the task environment (providing temporally predictable input). Effects of between-task compatibility suggest that a dedicated stimulus-code comparison process precedes and affects response-order scheduling. Based on the present results and previous findings, we propose a multi-phase framework of temporal response-order control that emphasizes the extent to which cognitive control of action scheduling is dynamically adaptive to particular task characteristics.
Similar content being viewed by others
Introduction
Research on human action control can be subdivided into two main areas of inquiry, namely determining what to do, ensuring task-appropriate action, and determining when to do it, which requires that actions must be scheduled along a time line. Since humans frequently handle more than just one task at once they need to schedule the tasks at hand to efficiently execute the required actions. Given that our bodily and cognitive limitations usually do not allow us to easily perform many actions simultaneously, an important question of cognitive research relates to understanding the mechanisms of action order scheduling in multitasking – a field of research that has received only sparse attention to date. In the present paper, we provide a comprehensive picture of mechanisms that determine the outcome of temporal action scheduling by studying both bottom-up and top-down factors and their effects on response order.
Temporal processing dynamics and response scheduling in the psychological refractory period (PRP) paradigm
A classic paradigm used to study the temporal processing dynamics of dual-task control is the psychological refractory period (PRP) paradigm (Telford, 1931; Welford, 1952), which allows for systematic manipulation of the temporal overlap between two tasks and hence a detailed analysis of the underlying processing time course. Usually, it is utilized to study structural limitations in dual-task control, not temporal task-order scheduling. However, the theoretical framework underlying the typical interpretation of PRP data also allows for deriving hypotheses regarding response-order control.
In the PRP paradigm, two stimuli – S1 and S2 – each requiring a separate response – R1 and R2 – are presented sequentially while the time delay between the two stimuli, the stimulus onset asynchrony (SOA), is varied systematically. The PRP effect refers to the typical finding that the reaction time for the second response (RT2) is larger at short than at long SOAs (Herman & Kantowitz, 1970; Pashler, 1984, 1994), while RT1 remains largely unaffected. The most prominent explanation for this pattern is based on the conceptualization of task processing as consisting of three consecutive stages: stimulus processing, response selection, and response execution, and the assumption that this sequence of task-processing stages immediately starts with stimulus onset. The prolongation of RT2 at short SOAs is explained by the presence of a structural (generic) bottleneck that coerces a sequential processing schedule at the central stage of response selection (response selection bottleneck, or RSB model). Hence, processing of this stage cannot proceed simultaneously for both tasks, so that response selection of Task 2 has to wait until response selection of Task 1 is finished (e.g., Pashler, 1984, 1994; McCann & Johnston, 1992). Especially within the RSB framework, response-order scheduling according to stimulus presentation order, that is, a non-reversed response order, represents an important precondition for the interpretation of the data. Here, it is assumed that the task that is entering central processing first is defined by the task in which stimulus processing finishes first. For comparable processing durations of S1 and S2, RS1 should directly follow S1, which, for similar RS durations, results in execution of R1 prior to R2.
An alternative explanation for the PRP effect is based on the idea of parallel processing at the central response selection stage (Navon & Miller, 2002; Tombu & Jolicoeur, 2003), assuming that limited central capacity is shared among the two tasks. Within this framework, capacity is assumed to be allocated asymmetrically (e.g., 90% : 10%), so that one task receives more capacity than the other. Such a capacity-sharing model predicts a very similar data pattern to an RSB model (i.e., execution of R1 prior to R2) when the task in which the stimulus is presented first receives the major proportion of capacity. Under the same conditions as discussed above (i.e., similar durations of S1 and S2), a parallel processing model based on capacity sharing could only then explain response reversals (R2 prior to R1) when assuming that the major capacity proportion would go to Task 2 instead of Task 1. However, capacity-sharing models usually presume that the resource allocation regime is based on stimulus presentation order and do not discuss potential mechanisms for reversed resource allocation (e.g., Navon & Miller, 2002).
Taken together, the idea of a first-come first-served principle, implying that all resources (in the case of RSB models) or most resources (in the case of capacity-sharing models) are shifted to the task associated with S1, predicts that stimulus order should be the major determinant of response order – an assumption that represents a strict bottom-up account of response-order control. In line with this prediction, many previous studies show that R1 is indeed mostly observed to be initiated prior to R2, whereas response reversals occur only rarely and could, for example, be attributed to random fluctuations of stimulus processing stage durations.
Interestingly, in typical PRP studies the likelihood of observing a response order in line with stimulus order is often explicitly increased by instructing participants to prioritize Task 1 processing and to respond in accordance with stimulus order (e.g., Hommel, 1998; Logan & Delheimer, 2001; Logan & Schulkind, 2000; Pashler & Johnston, 1989; Ruthruff, Johnston, & Van Selst, 2001). Thus, PRP experiments that are focused on studying structural limitations (instead of response-order scheduling) usually regard reversals as being “wrong” or “abnormal” (cf., Pashler, 1990) or as occurring “accidentally” (cf., Wu & Liu, 2008). As such, they are excluded from further analysis (e.g., Bratzke, Rolke, & Ulrich, 2009; Hommel, 1998; Tombu & Jolicœur, 2002) or not reported in the first place. Based on the theoretical assumptions and methodological practices reported so far, at least two factors should therefore play an important role in response-order scheduling: stimulus order and explicit instructions to prioritize Task 1.
Similar to the basic assumptions of (flexible) capacity-sharing models outlined above, some researchers explicitly disagreed with the notion that the PRP effect reflects a generic cognitive limitation. Instead, they suggested that both stimulus order and instructions might lead to a strategic prioritization of Task 1 within a serial-processing strategy (e.g., Meyer & Kieras, 1997a; Schumacher et al., 2001), although parallel processing might principally be possible. In favor of the view that serial processing might represent a strategy (instead of a generic structural limitation), Schumacher et al. (2001) showed that the RSB could be greatly reduced after a certain amount of practice (see also Hazeltine, Teague, and Ivry, 2002; Strobach, Liepelt, Pashler, Frensch, & Schubert, 2013). Similarly, Israel and Cohen (2011) trained participants to execute dual tasks without significant costs at SOA = 0 ms, but demonstrated a re-emergence of the PRP effect in trials with SOA = 0 ms when these trials were later presented in the context of other trials with different SOAs ≠ 0 ms. These findings can be interpreted as evidence that dual-task processing can also be strongly influenced by top-down factors related to strategies and context, and that serial stimulus presentation might also trigger strategically (top-down)mediated serial response processing. Note, however, that neither the standard RSB model nor parallel-processing models based on capacity sharing include any specific control mechanisms regarding response order.
In general, all major dual-task frameworks are mainly concerned with explaining the PRP effect and associated findings by discussing cognitive limitations in terms of serial or parallel-processing capabilities (Hommel, 1998; Logan & Gordon, 2001; Navon & Miller, 2002; Tombu & Jolicoeur, 2003; Pashler, 1994) and strategic considerations (Lehle & Hübner, 2009; Meyer and Kieras, 1997a, b, see also Fischer & Plessow, 2015). Importantly, possibly due to the prevalence of certain research paradigms and the methodological constraints, response-order variability was usually considered a nuisance factor and therefore seldom at the central focus of research.
Previous studies on response-order scheduling in the PRP paradigm
Previous dual-task frameworks were mainly developed to account for performance deterioration instead of response order. However, in line with the cited evidence for a more flexible, strategic view on cognitive control in multitasking, some researchers explicitly suggested that response order in the PRP paradigm might to some extent be scheduled actively (e.g., De Jong, 1995; Leonhard et al., 2011; Luria & Meiran, 2003; Szameitat, Lepsien, von Cramon, Sterr, & Schubert, 2006). Instead of being determined in a purely stimulus-driven (bottom-up) manner, it was suggested that additional control processes specific to response-order coordination are required, for instance task sequence activation or inhibition processes (e.g., Hirsch, Nolden, Koch, 2017; Sigman & Dehane, 2008). Importantly, most previous research can be characterized as taking a more macroscopic view on response-order control. Specifically, most of these studies concentrated on RT performance increases as a marker for identifying conditions that require additional response-order control demands (e.g., when response order has to be switched compared to fixed stimulus-order conditions). However, little research has experimentally addressed determinants of the actual response order. For example, De Jong (1995) as well as Kübler, Reimer, Strobach, and Schubert (2017) studied the impact of instructions on response-order control by comparing one condition in which participants were instructed to respond in accordance with the stimulus order with another condition in which participants freely chose response order. In both studies, fewer response reversals occurred in the instructed-order condition, indicating top-down control mechanisms of response order. However, the instructed-order condition in De Jong (1995) always involved the presentation of error feedback, which makes it difficult to assess the contribution of instructions alone. Additionally, De Jong (1995) studied stimulus-order predictability by comparing conditions with predictable (constant) and unpredictable (variable) stimulus order. He found a tendency towards repeating the processing order from the previous trial in the unpredictable stimulus-order condition, indicating contextual modulation of response-order control. Probably, this effect can be explained by the intention to avoid performance costs associated with trial-by-trial switches of response order (Luria & Meiran, 2003; Hirsch, Nolden, & Koch, 2017).
Finally, Leonhard, Ruiz Fernández, Ulrich, and Miller (2011) suggested that response-order scheduling may depend on the anticipated duration of the response selection (RS) stage, which they manipulated by employing different task difficulties (see also Ruiz Fernández, Leonhard, Rolke, & Ulrich, 2011). Specifically, these studies reported a tendency to execute the response in a task with a short RS duration first even when the stimulus of this particular task was presented second. The authors argued that this strategy helped to reduce slack time (i.e., the time in which one task has to wait for clearance of the bottleneck) in order to minimize total reaction time (i.e., the sum of RTs in both tasks). While this explanation appears to be plausible, alternative explanations are possible. For example, it could be that, instead of the relatively complex process of anticipating and minimizing slack time, a general a priori prioritization of the easy task was responsible for the observed response-scheduling pattern. This would represent a strategy without the additional assumption of an intention to optimize processing efficiency. Despite this limitation, the study represents clear evidence against a simple first-come first-served (pure bottom-up) principle based on stimulus-order presentation to account for response-order control in dual tasks. Crucially, these findings indicate that properties of stages after stimulus processing can be relevant, too. This raises the novel question of whether even characteristics of effector systems (i.e., factors related to the final response execution stage) could also affect response-order scheduling.
Taken together, the few previous studies on response-order scheduling provide first evidence against the assumption that a first-come first-served mechanism that prioritizes the task associated with S1 can fully account for response order in multitasking. Instead, they indicate that instructions, contextual factors (e.g., surrounding trials within a block), or characteristics of processing stages after stimulus processing may play an important role. While on a macroscopic level the presence of a dedicated task-order processing stage has been proposed to account for some of the effects (e.g., Sigman & Dehaene, 2008), previous studies did not fully unravel the potentially complex dynamic interplay of bottom-up and top-down factors affecting the temporal organization of behavior on a microscopic level (i.e., by specifying underlying mechanisms within such a presumable processing stage).
The impact of effector systems and spatial compatibility on response prioritization
Based on the assumption derived above that response characteristics could play a role in response order, we present an overview of the role of response modalities (in terms of effector systems) in dual-task control. Given that the assumption of sequential stage processing within each task is prevalent in current dual-task models, one would not expect a strong influence of features of the response execution stage on central processes (which occur earlier in the processing chain). Probably because of this, the majority of PRP studies utilized only a limited range of potential combinations of effector systems, namely manual-manual or manual-vocal (see reviews by Huestegge & Hazeltine, 2011; Pashler, 1994). However, in line with accumulating evidence for “embodied cognition” in the last decades (see Wilson, 2002), several observations indicate that response modalities might play a more important role for central processing than previously assumed.
First, it has been demonstrated that certain combinations of stimulus (= input) and response (= output) modalities for the two tasks in the PRP paradigm cause much larger dual-task interference that other combinations (input-output modality compatibility effect, e.g., Hazeltine, Ruthruff, & Remington, 2006). Second, recent research (Huestegge & Koch, 2013) suggested that the specific response modality combination of two simultaneous actions (compatible responses triggered by a common stimulus) determine the distribution of performance costs across effector systems. In their study, they utilized the amount of dual-response costs (i.e., the additional time needed to initiate a response when a secondary response is required) as an inverse marker for prioritization. They showed that oculomotor responses are typically prioritized over both vocal and manual responses (oculomotor dominance effect), whereas vocal responses are prioritized over manual responses. Interestingly, this pattern could not simply be explained in terms of the particular RT level associated with effector systems (i.e., features that should be closely related to the duration of the response execution stage within the sequential processing stage logic). Instead, the data appeared to reflect differential attentional weighting of certain output modules. Pieczykolan and Huestegge (2014) confirmed the overall effector prioritization pattern in a slightly modified experimental paradigm. They showed that for two responses that were spatially incompatible with each other, the relative difficulty of response selection (manipulated via S-R (in)compatibility) affected prioritization strength, suggesting a rather flexible weighting mechanism. Taken together, these observations suggest that (a) the specific response modalities and their associated prioritization gradient and (b) spatial compatibility impact on attentional weighting mechanisms in multiple-response control. Therefore, effector system-based prioritization and spatial compatibility might also be relevant determinants of response-order scheduling. These hypotheses still await empirical testing.
The present study
The present study was aimed at elucidating a more comprehensive picture of the mechanisms underlying response order in multitasking. By directly measuring response order as a function of different potential determinants and their interplay we set out to complement previous studies demonstrating that response-order scheduling requires specific control processes. Specifically, De Jong (1995), Luria and Meiran (2003), and Sigman and Dehaene (2008) explicitly suggested that order control should occur within a dedicated process at the beginning of each trial before processing the individual component tasks.
Based on the literature review above, an ideal experimental setting to study temporal scheduling dynamics is the PRP paradigm. Due to its inherent potential for a systematic manipulation of relevant bottom-up factors such as stimulus order and SOA, it is possible to maintain relatively high experimental control over the timing of cognitive processes.
For studying response-order control it is important to establish an experimental situation that is likely to yield substantial variability of response order (i.e., a sufficient amount of response reversals) in the first place to avoid floor effects. A review of previous literature suggested that especially the combination of oculomotor and manual responses in the PRP paradigm yields many response reversals (Pashler et al., 1993). Although this previous study did not explicitly examine response reversals as a central dependent variable, the data showed that even when the stimulus associated with the oculomotor response was presented second, the associated oculomotor response was still initiated earlier than the manual response in a substantial proportion of trials, especially at short SOAs. Furthermore, this particular combination of effector systems provides the opportunity of studying the impact of different response characteristics on response-order control (i.e., asymmetrical prioritization and different response execution stage durations, as outlined in the previous section). Based on these considerations, the combination of oculomotor and manual responses in the PRP paradigm appears ideal for studying response-order control in multitasking.
The present study comprises three PRP experiments. Experiment 1 involved blocks of trials with mixed stimulus order without specific response-order instructions. Experiment 2 addressed the impact of explicit response-order instructions under otherwise identical conditions as in Experiment 1. Finally, Experiment 3 involved a change in task environment by implementing a fixed (instead of mixed) and therefore predictable stimulus order. Similar to De Jong (1995) and Leonhard et al. (2011), we measured the rate of response reversals as an indicator of response-order scheduling, and additionally analyzed errors to assess the impact of response-order scheduling on multitasking performance accuracy.
Since we aimed to assess a relatively broad range of the determinants and mechanisms of response-order control (especially when compared to the specific research questions in each of the previous studies on this issue), we focused on the following six factors and corresponding hypotheses (either within or across experiments):
Characteristics of response modalities
In all three PRP experiments, we combined two effector systems (oculomotor and manual responses) that substantially differ in their response characteristics (e.g., regarding response execution stage duration and a priori prioritization) in order to test whether these response characteristics affect response-order scheduling. If response order is not significantly affected by response characteristics, we would expect that stimulus order (or any of the remaining variables mentioned below) should be the main factor determining response order (indicated by a low number of response reversals). In contrast, a bias towards one effector system in terms of a large number of response reversals would indicate an important influence of response characteristics on response-order scheduling.
Stimulus order
Based on a stimulus order-based first-come first-served principle (e.g., Logan & Gordon, 2001; Pashler, 1994), one would expect stimulus order to represent a major factor determining response order. If the role of stimulus order has been overestimated in previous research, our design allows us to assess the degree to which stimulus order may interact with other factors affecting response-order scheduling, especially with an effector combination involving different response characteristics. The corresponding predictions follow from the reasoning outlined in the previous paragraphs: If stimulus order had no impact on response scheduling, we would expect that, for example, oculomotor responses were nearly always executed first irrespective of stimulus order. In contrast, if stimulus order was the dominant factor determining response order, we would expect low reversal rates regardless of stimulus order.
Stimulus onset asynchrony (SOA)
One essential feature of the PRP paradigm is the SOA manipulation, which is the temporal distance between the onset of S1 and S2. SOA could have the following effect on response order: Long SOAs may increase stimulus-order salience (i.e., the temporal distinctiveness of the stimuli) and as such amplify the overall impact of bottom-up factors (e.g., stimulus order).
Between-task compatibility
Previous research on dual-task control in general and within the PRP paradigm in particular has demonstrated that the compatibility relation between R1 and R2 affects RT1 performance (e.g., backward crosstalk effect; see Ellenbogen & Meiran, 2008; Hommel, 1998; Janczyk, 2016; Miller, 2006; Miller & Alderton, 2006; Watter & Logan, 2006), which in turn propagates further onto RT2. Interestingly, no study (to our knowledge) has addressed the impact of between-task compatibility on response-order scheduling yet. If between-task compatibility played a role in response-order scheduling we would expect different rates of response reversals for spatially compatible and incompatible trials. Furthermore, such a compatibility effect would indicate that between-task compatibility is processed prior to the determination of response order and that response order can also be adjusted when task processing is already under way (and is not fully determined before the onset of the trial; see De Jong, 1995; Leonhard et al., 2011; Luria & Meiran, 2003).
Instructions
A between-experiment comparison of two different instruction conditions (unspecific in Experiment 1, explicit in Experiment 2) under otherwise controlled conditions (unlike in De Jong, 1995) will show the extent to which participants are able to self-regulate response-order control in a top-down manner (without being reminded during the block by feedback). Thus, if participants are able to voluntarily apply an instructed response order in accordance with stimulus order, we expect fewer response reversals compared to a condition without specific order instructions.
Predictability of stimulus order
Finally, we studied the influence of stimulus order variability as a context factor on response-order scheduling. Therefore, we compared performance of identical trials (i.e., under identical SOA and stimulus-order conditions) in mixed stimulus order blocks (Experiment 1) with performance in fixed stimulus order blocks (Experiment 3). A difference in response reversals would suggest that response scheduling in a current trial strongly depends on the context established by the surrounding trials (see similar observations in De Jong, 1995, albeit only with respect to RTs instead of response order, and Kübler et al., 2017). Consequently, response reversals should be less likely under highly predictable circumstances implemented by fixed stimulus-order conditions.
Experiment 1
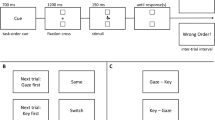
Experiment 1 investigated the impact of response characteristics (i.e., different response execution stage duration and prioritization, see Huestegge & Koch, 2013; Pieczykolan & Huestegge, 2014) and its interplay with stimulus order on response-order scheduling in a PRP setup with varying SOAs. In each trial, two distinct actions were required: a saccade towards a left or right target on the screen and a manual left or right key press. The two actions were comparable in that both were triggered auditorily and required a certain amount of spatial transformation (i.e., hearing a sound on one ear must be transformed into a spatially compatible action that is not simply directed towards the sound source). We used auditory stimuli for both tasks to avoid potential confounds associated with different processing speeds across different input modalities. Specifically, we compared conditions in which the stimulus for the oculomotor response precedes the stimulus for the manual response (SOSM) with conditions in which this order was reversed (SMSO) to measure corresponding effects on response reversal frequency. Additionally, we compared between-task compatible and incompatible conditions. Unlike many PRP studies, we refrained from instructing participants to follow a certain response order (as in Pashler et al., 1993) with the aim of observing scheduling occurrences that are unrestricted by explicit instructions. Finally, we utilized the same SOAs (120, 240, and 360 ms) for both stimulus-order conditions. This symmetrical implementation was intended to minimize any implicit bias towards a certain response order.
Method
Participants
Twenty-four participants (16 female) with a mean age of 23.2 years (range 20–30) took part in Experiment 1. They had normal or corrected-to-normal vision and hearing and received course credits or monetary reimbursement for participation.
Apparatus
Participants were seated 67 cm in front of a 21-in. cathode ray monitor (temporal resolution: 100 Hz; spatial resolution: 1,024 × 768 pixels) with a keyboard in front of them. The space bar of the keyboard was used during calibration routines. Saccades were registered using a head-mounted Eyelink II infrared reflection system (SR Research, Osgoode, Ontario, Canada) by measuring the position of the right eye’s pupil with a temporal resolution of 500 Hz and a spatial resolution of less than 0.0022°. A chin rest was used to minimize head movements.
Stimuli
The visual display consisted of a black background on the screen with a green fixation cross in the center and two green rectangular squares that served as saccade targets at 8.5° visual angle to the left and right of the fixation cross. This display was permanently visible during each experimental block. The size of the fixation cross and the targets was 0.3° (= 10 px) each. Two keys on the keyboard (left Ctrl and right arrow) served as response keys. Two unilateral auditory stimuli (left or right), a 1,000-Hz pure tone (indicating response location in one task) and a pink noise burst (indicating response location in the other task) with a duration of 50 ms each, were presented via supra-aural headphones. Both stimuli were easily distinguishable and of equal perceived loudness.
Procedure
Each block started with an on-screen instruction followed by the calibration of the eye-tracking system. In each trial, the two stimuli appeared sequentially with a variable SOA (120, 240, and 360 ms) in random order. Participants were instructed to execute the two responses as fast and accurately as possible. No information or instruction was given regarding stimulus order and response order. The experiment also included a 0 ms-SOA condition (simultaneous stimulus presentation) in order to compare these data with those from other experiments in our laboratory, but this condition (because it imposes different processing demands compared to sequential stimulus presentation) was irrelevant for the purpose of the present study and thus not further analyzed.
In the oculomotor task, participants were instructed to move their gaze to the target on the screen that was spatially compatible with the stimulus and afterwards return to the central fixation cross. In the manual task, participants were instructed to press the key that spatially corresponded to the stimulus. The assignment of stimulus type (pure tone and noise burst) to response modality (oculomotor and manual) was specified in the instructions and counterbalanced across participants. The interval between the first stimuli of two consecutive trials was 3,500 ms. Each participant completed four dual-task blocks consisting of 56 trials each and two single task blocks containing 32 trials of each component task, respectively. The single-task blocks, in which only one stimulus was presented, were not relevant for the purpose of the present research questions. Block order was counterbalanced across participants.
Design
Stimulus order (SOSM & SMSO), SOA (120, 240, and 360 ms) and task compatibility (spatially compatible and incompatible) were manipulated within participants. Each SOA was presented 32 times. All four possible combinations of stimuli (i.e., both tone and noise on left or right side, tone left + noise right, and tone right + noise left) occurred equally often, resulting in the same amount of spatially compatible and incompatible trials. The relative frequency of response-order reversals, response errors, and RTs as a means to analyze inter-response intervals were measured as the main dependent variables.
Results and discussion
Valid data were selected by removing trials with technical irregularities (e.g., missing data because of loss of eye-tracking signal). Additionally, one participant with a very high error rate (> 40%) was excluded. This procedure led to 94.1% valid data. Response error trials were defined as trials in which a response error occurred in any of the two tasks. The overall error rate amounted to 15%. These trials were discarded from analyses of response order. Prior to the analysis of this dichotomous variable, we wanted to ensure that our experimental setting did not promote an excessive occurrence of grouped responses, which would represent an additional category of response scheduling (simultaneous scheduling, in addition to manual first and oculomotor first) and as such potentially compromise our conclusions. Common definitions of response grouping are usually based on a certain range of temporal inter-response intervals (IRIs), i.e., typically up to 150 ms (Adam, Hommel, & Umiltà, 2003; Ulrich & Miller, 2008). Here, IRIs were defined as the temporal difference between the onset between the manual response tRM and the oculomotor response tRO (IRI = tRM – tRO). Based on the fact that goal-directed saccades can be executed relatively fast (i.e., with a latency of about 125 ms; see, e.g., Findlay & Walker, 1999), we reasoned that a reasonable criterion for grouped responses should not exceed this value in our study. A visual inspection of Fig. 1 clearly rules out that excessive response grouping (in terms of a strong peak of the IRI distribution within the ±125 ms range) occurred in Experiment 1.
Distribution of inter-response intervals (pooled in 50-ms bins) as a function of SOA and spatial between-task compatibility (compatible and incompatible) in Experiments 1 and 2. The gray area indicates the IRI range between −125 and +125 ms (potential grouping range). SOSM indicates that the stimulus for the oculomotor response was presented first (vice versa for SMSO). Negative IRIs indicate that the manual response was initiated first (RMRO; vice versa for positive IRIs: RORM)
Response-order reversals
Response-order reversals were defined as response sequences that did not correspond to the presented stimulus order. Figure 2 depicts the mean relative frequencies of response-order reversals. The overall mean reversal rate of all experimental trials amounted to 42.4% and significantly differed from 0, t(22) = 20.3, p < .001, suggesting that in nearly half of the trials participants did not respond in accordance with the stimulus order. Instead, oculomotor responses were executed first in 89.5% of all trials. Apparently, response modality characteristics had a greater impact on response order than mere stimulus order, at least within the (rather typical) SOA range selected in the present experiment. Since these effects cannot be attributed to differences in stimulus processing-stage duration, they are clear evidence against a simple first-come first-served mechanism without any further assumptions. However, if characteristics of the response modalities were the only factor determining response order (and stimulus order played no meaningful role whatsoever), we would have expected an amount of oculomotor-first trials in the SMSO condition that corresponds to the amount of oculomotor-first trials in the SOSM condition. However, this was not the case (81.1% vs. 96.4%), t(22) = 3.38, p = .003, suggesting that stimulus order still had a significant (although small) impact on response-order scheduling.
Reversal rate as a function of stimulus order (SOSM: stimulus for oculomotor response first, SMSO = stimulus for manual response first), spatial between-task compatibility (compatible and incompatible), and SOA in Experiment 1. Error bars represent standard errors
Taken together, these results suggest that both response-related and stimulus-related factors contribute to response-order scheduling. Let us assume that the prioritization of oculomotor responses (in terms of a tendency to execute oculomotor responses first) represents a top-down regulated attentional weighting process (see Huestegge & Koch, 2013) that affects response-order scheduling. Then, our results would suggest that in the present experimental setting top-down factors had a greater overall impact than bottom-up factors such as stimulus order. Therefore, the present novel results indicate that oculomotor prioritization, which was previously observed in the pattern of dual-response costs across effector systems based on RTs (Huestegge & Koch, 2013; Pieczykolan & Huestegge, 2014), also generalize to response order in the PRP paradigm.
Due to the markedly different reversal rates in the two stimulus-order conditions, we further analyzed the effects of SOA and task compatibility on reversal rates by calculating separate 2 × 3 ANOVAs for each stimulus-order condition. This separation of analyses is important since the occurrence of a reversal refers to opposed empirical phenomena across the two stimulus-order conditions (e.g., oculomotor-first represents a reversal in the SMSO condition, but not in the SOSM condition).
The reversal rate in the SOSM condition was very low (3.6%), but still significantly different from 0, t(22) = 2.9, p = .008. It was not significantly affected by SOA, compatibility, or the interaction of SOA and compatibility, all ps ≥ .14.
In contrast, the reversal rate in the SMSO condition amounted to 81.1%. Here, we observed a significant main effect of SOA, F(2,44) = 13.25, p < .001, η2p = .38, indicating that the overall preference for executing the oculomotor response first was attenuated when stimuli were temporally further apart (88.6%, 80.5%, 74.2% reversals at SOAs of 120, 240, and 360 ms, respectively). Since a pure bottom-up account could already be ruled out, the SOA effect may be explained in several ways. For example, it is possible that “oculomotor-first” represents a default response-order strategy, which is plausible given the substantial proportion of reversals. Probably, this default mode is particularly applied as an economic scheduling regime in situations with relatively high uncertainty (e.g., regarding stimulus order, which changes from trial to trial). In this context, a larger SOA may increase the salience of stimulus order (thus lowering uncertainty) and eventually lead to a decreased necessity for applying the default mode. Such a mechanism would result in a priority shift away from oculomotor prioritization and yield fewer response reversals. However, it is also possible that large SOAs may reduce the likelihood that subjects wait with their final response-order decision until both stimuli are processed (and compared, e.g., by their compatibility, see following paragraph), so that after some time they deliberately switch to a task-processing mode that corresponds to the stimulus order. Thus, one would expect that for more extreme SOAs (in which task-processing overlap is less likely) stimulus order should be the main factor determining response order (see Experiment 3 for corresponding data).
Furthermore, we observed a main effect of between-task compatibility in the SMSO condition, F(1,22) = 5.05, p = .035, η2p = .19, with 87.1% reversals in compatible trials and 75.1% reversals in incompatible trials. The interaction between SOA and task compatibility was not significant, F(2,44) = 2.1, p = .076, η2p = .11. Interestingly, the compatibility effect suggests that final response-scheduling adjustments (at least partially) occur temporally after the processing and comparison of both stimuli, which is not in line with a framework in which stimulus processing in Task 1 is immediately followed by response selection. This interpretation is also consistent with the observation that numerous reversals still occurred at long SOAs in the SMSO condition, suggesting that participants withheld the manual response and therefore established a time window in which a stimulus comparison process could take place (see General discussion for further details).
Finally, it is possible that the compatibility effect in reversal rates (calculated on the basis correct trials only) represents an artifact due to an unequal distribution of errors across compatibility conditions (see the following section on error rates). Therefore, we further analyzed effects of SOA and compatibility on reversal rates in all (valid) trials in SMSO conditions, that is, including error trials. The 3 × 2 ANOVA yielded a significant effect of SOA (88.2%, 83.4%, and 78.3% for 120, 240, and 360 ms), F(2,44) = 9.64, p = .001, η2p = .31, and, crucially, a significant effect of compatibility (86.9% vs. 79.8% for compatible vs. incompatible trials), F(1,22) = 28.69, p < .001, η2p = .57. The interaction was not significant, F < 1. Taken together, this analysis yielded the same statistical pattern as before, which allows us to exclude the possibility that a tradeoff between accuracy and response order occurred.
Error rates
Due to the substantial reversal rates and the fact that only 12 participants responded in accordance with the stimulus order in SMSO conditions more than just occasionally, a meaningful statistical comparison of error rates between reversal and non-reversal trials was not possible (but see the section on Comparison across experiments for a more powerful combined analysis). Nevertheless, an analysis of error rates can still be informative regarding the overall difficulty of the task conditions. In analogy to the dichotomous reversal measure, an error trial was defined as containing an incorrect response in at least one of the two tasks. We computed separate ANOVAs for both stimulus-order conditions (analogous to the reversal rate analysis) with the independent factors compatibility and SOA. Table 1 depicts the mean error rates.
In the SOSM condition errors decreased with longer SOAs (16.7, 10.7, and 8.7% for 120, 240, and 360 ms), F(2,44) = 15.1, p < .001, η2p = .41, confirming the expectation that a larger SOA is accompanied by a lower potential for interference. Error rates differed significantly between compatible (3.5%) and incompatible (20.6%) trials, F(1,22) = 28.69, p < .001, η2p = .57, indicating higher difficulty for incompatible trials. The interaction was significant, too, F(2,44) = 10.03, p < .001, η2p = .31, indicating that for incompatible trials errors rate decreased much faster with increasing SOAs than for compatible trials.
In the SMSO condition, in which responses were reversed in over 80% of the trials, we observed a main effect of compatibility, too, F(1,22) = 66.89, p < .001, η2p = .75, but there was neither a significant SOA effect, F(2,44) = 1.13, p = .33, nor a two-way interaction, F(2,44) = 2.08, p = .14. Overall, these results demonstrate that spatial between-task incompatibility represented a substantial source of interference. Its adverse impact on performance was to some extent reduced by increasing SOAs (but only in the SOSM condition). This further corroborates the assumption that S1 was held in working memory to allow for a comparison with S2, which may have eventually caused confusion of the mapping of spatial codes to the appropriate response modalities.
A comparison across both stimulus-order conditions showed that more errors occurred in the SMSO condition (18.3%) than in the SOSM condition (12.1%), F(1,22) = 9.17, p = .006, η2p = .29. This may indicate that when both bottom-up and top-down factors suggest the same response order (as in the SOSM condition), fewer overall cognitive demands are present than in a condition in which bottom-up factors (related to stimulus order) are in conflict with (and thus attenuate) the default top-down response-order strategy based on effector system prioritization.
Experiment 2
In Experiment 1, oculomotor responses were temporally prioritized over manual responses (i.e., executed first) with only a comparatively small impact of stimulus-order information. One potentially important difference between Experiment 1 and typical PRP studies is that in PRP studies participants are usually explicitly encouraged to respond in accordance with stimulus order, whereas we did not provide any specific instructions regarding response order. Thus, the lack of evidence for a strong role of stimulus order in Experiment 1 might be due to the absence of a corresponding instruction. In Experiment 2, we repeated the previous experiment but added the instruction to respond in accordance with stimulus order. This manipulation should be informative about the extent to which response-scheduling strategies can be actively adjusted in a top-down manner while the remaining conditions are fully comparable (unlike in De Jong, 1995).
Method
Participants
Twenty-four new participants (18 female) with a mean age of 24.9 years (range 19–30) were recruited and received course credits or monetary reimbursement for participation.
Apparatus and stimuli
The experimental hardware setup was exactly the same as in Experiment 1. However, an Eyelink 1000 (instead of an Eyelink II) eye tracker with a temporal resolution of 1,000 Hz was used.
Procedure and design
While the oculomotor and manual tasks were identical to Experiment 1, participants were now explicitly instructed to execute the responses in accordance with stimulus order. As in Experiment 1, they were not informed about the order of stimuli and did not receive any error feedback after each trial (see De Jong, 1995, for a different approach). Stimulus order (SOSM & SMSO), between-task compatibility (compatible and incompatible), and SOA (120, 240, and 360 ms) were manipulated within participants.
Results and discussion
We applied the same data-cleansing procedures as in Experiment 1. One participant was excluded from the analysis because of an unusually high amount of erroneous saccades in incompatible trials (> 60% incorrect). Valid data amounted to 95.2%. The overall error rate was 11.9% (see detailed analysis below). As in Experiment 1, the IRI distribution (see Fig. 1) showed no signs of excessive response grouping.
Response order reversals
Figure 3 depicts the mean reversal rates as a function of stimulus order, compatibility, and SOA. The overall reversal rate amounted to 21.3%, which differed significantly from 0, t(22)= 7.17, p < .001. In 76.5% of the trials, the oculomotor response was executed as the first response, which again is evidence for a strong oculomotor prioritization effect. However, the frequency of oculomotor-first responses significantly differed between the two stimulus-order conditions, t(22) = 9.6, p < .001, with 95% oculomotor-first responses in the SOSM order condition and 37.7% oculomotor-first responses in the SMSO order condition. Thus, similar to Experiment 1, we observed strong oculomotor prioritization, but also a significant influence of stimulus order, which was nominally greater than in the previous experiment (see section on Comparison across experiments for statistical analyses). To further analyze the effects of task compatibility and SOA on reversal rates, we calculated separate 2 × 3 ANOVAs for each stimulus order condition (SOSM and SMSO).
Reversal rate as a function of stimulus order (SOSM and SMSO), spatial between-task compatibility (compatible and incompatible), and SOA in Experiment 2. Error bars represent standard errors
Reversals in SOSM condition. The overall reversal rate was very low (5%). There was a main effect of SOA, F(2,44) = 4.9, p = .012, η2p = .18, indicating a decrease of reversals with increasing SOA (7.9%, 4.2%, and 2.9%). Interestingly, unlike in Experiment 1, reversals here were significantly more frequent for compatible than for incompatible trials (6.4% and 3.5%), F(1,22) = 4.87, p = .038, η2p = .18. The interaction was not significant, F < 1.
Reversals in SMSO condition. The mean reversal rate amounted to 37.7%, showing that despite explicit instructions, participants failed to respond accordingly in more than one-third of the trials. Reversals decreased with increasing SOA (49.7%, 33.8%, and 29.6%), F(2,44) = 18.87, p < .001, η2p = .46, and were more frequent in compatible trials than in incompatible trials (47.1% and 28.3%), F(1,23) = 40.18, p < .001, η2p = .65. There was no two-way interaction, F < 1.
Taken together, participants were to some degree able to voluntarily execute responses in accordance with the stimulus order. Thus, they appear to be capable of attenuating their inherent prioritization of the oculomotor task, which we referred to as a default response mode in Experiment 1. The SOA effect in both stimulus-order conditions likely reflects that a larger temporal distance between stimuli generally helps to disambiguate response order, that is, it facilitates responding in accordance with the stimulus order. Nevertheless, oculomotor prioritization still plays a major role in determining response order, since a notable number of response reversals were still observed (e.g., 60% for compatible trials in the 120 ms SOA condition in the SMSO order). This observation shows that mere instructions are not sufficient to fully overcome the effector system-based prioritization. However, it is important to note that in Experiments 1 and 2 stimulus order changed unpredictably from trial to trial, representing a situation with a degree of uncertainty that might have supported the overall influence of the default (oculomotor-first) response mode.
Interestingly, explicit response-order instructions, which likely directed attention more towards stimulus-order information, were more effective in incompatible than in compatible trials. Probably, the higher resource demand during spatial conflict resolution in incompatible trials further increased the amount of attentional resources directed at processing of stimuli, including their associated features like temporal-order information. In contrast, the less demanding compatible trials may rather support keeping up the default prioritization mode (based on effector systems) irrespective of stimulus characteristics (for details see General discussion section).
The effect of SOA in the SOSM condition, which was not present in Experiment 1, can be explained by differences in stimulus order salience in a similar way as in the SMSO condition. Fewer reversals for incompatible trials in the SOSM condition may again indicate enhanced attention to stimulus features (including stimulus order information) during the presence of spatially incompatible stimuli.
As in Experiment 1, we analyzed whether the compatibility effect in reversal rates represents an artifact due to an unequal distribution of errors across compatibility conditions. When all trials (including error trials) were considered in the analysis of reversal rates, the compatibility effect in the SMSO condition (which was based on the analysis of correct trials only) disappeared (unlike in Experiment 1). Specifically, this analysis yielded 46.9% reversals for compatible and 44.3% reversals for incompatible trials, F < 1. This suggests that in Experiment 2 a trade-off might have occurred between two concurrent demands, namely responding with the correct (left/right) response given the individual stimuli and responding in the correct order (as specified by the response-order instruction, which was only present in Experiment 2).
Error rates
A overall comparison of error rates between the two stimulus-order conditions showed that significantly more errors occurred in the SMSO condition (15.7%) than in the SOSM condition (7.4%), F(1,22) = 33.1, p < .001, η2p = .60.
Furthermore, we analyzed errors in the same way as in Experiment 1 by calculating separate 3(SOA) × 2(compatibility) ANOVAs for the two stimulus-order conditions (see Table 2). In the SOSM condition, errors decreased with increasing SOA (9.5%, 6.2%, and 6.6%), F(2,44) = 15.1, p < .001, η2p = .41, and compatible trials were less error-prone than incompatible trials (3.0% vs. 11.8%), F(1,22) = 28.55, p < .001, η2p = .57. The interaction was also significant, F(2,44) = 6.62, p = .003, η2p = .23, showing that the decrease of errors with increasing SOA was more pronounced in incompatible trials than in compatible trials.
In the SMSO condition, the main effect of compatibility was also significant (2.6% for compatible trials vs. 28.8% for incompatible trials), F(1,22) = 95.16, p < .001, η2p = .81, but there was no significant effect of SOA, F(2,44) = 1.34, p = .272, and no significant two-way interaction, F(2,44) = 2.46, p = .097. The substantial compatibility effect in the SMSO condition is similar to the corresponding effect observed in Experiment 1. Most likely, this indicates confusion of the mapping of spatial codes to effector modalities.
RTs and error rates of reversed versus non-reversed responses
Compared to Experiment 1, reversals and non-reversals were more equally distributed in Experiment 2. This allows us to address the potential functional relevance of response reversals for overall performance within a trial with the specific question of whether response prioritization (as reflected in response reversals) is associated with processing benefits. Although the present study was not specifically designed to examine causal effects of response order on RTs and error rates, a post hoc analysis might still be informative regarding processing efficiency. Specifically, two different scenarios are possible: Responses were reversed strategically in order to optimize processing efficiency, for instance in order to minimize total response time (TRT = RT1 + RT2) as suggested by Leonhard et al. (2011, see also Miller, Ulrich, & Rolke, 2009). Alternatively, performance may have suffered in reversed response trials, suggesting that oculomotor prioritization is a rather generic phenomenon that may be functionally relevant and thus beneficial in everyday life situations, but not in the context of the specific laboratory setting in the present study.
In order to assess if there were significant performance differences between reversed and non-reversed responses, we conducted ANOVAs on RTs and error rates at SOA = 120 ms in the SMSO order condition. This condition was especially suited for the comparison because 49.7% of trials were reversals. Since between-task compatibility manipulations in PRP studies are known to affect the two responses differently (see backward crosstalk effects, in, e.g., Miller, 2006), we subjected compatible and incompatible trials to separate analyses. Response order (reversed vs. non-reversed) and task (oculomotor vs. manual) served as independent factors. Note that the analysis included only a subsample of 15 participants who contributed sufficient valid data to each of the conditions. Results are depicted in Fig. 4.
Response times (RTs) and errors rates of oculomotor and manual responses in SMSO order at SOA = 120 ms as a function of response order (reversed and non-reversed) and spatial between-task compatibility (compatible & incompatible) in Experiment 2. Horizontal lines represent single-task baseline RTs. Error rates virtually amounted to 0 in single-task conditions and were therefore not included in the figure. Error bars represent standard errors
In compatible trials, the main effect of task on RTs was significant with overall greater manual RTs (1207 ms) compared to oculomotor RTs (941 ms), F(1,14) = 117.4, p < .001, η2p = .89, but there was no significant main effect of response order, F(1,14) =1.58, p = .23. However, there was a significant two-way interaction, indicating that in the reversed response order (in which oculomotor responses were executed first), manual responses were slower while oculomotor responses were faster, F(1,14) = 93.21, p < .001, η2p = .87, indicating prioritization regarding both speed and order. For incompatible trials, the pattern was similar with a significant main effect of task (1,567 ms for manual and 1,215 ms for oculomotor responses), F(1,14) = 98.82, p < .001, η2p = .88, and no significant main effect of response order, F = 3.09, p = .10. The two-way interaction was again significant, F(1,14) = 115.31, p < .001, η2p = .89. Crucially, the lack of a significant overall effect of response order on mean (equivalent to total) RTs does not support an optimization account according to which reversals are associated with an increased overall processing efficiency.
An analogous analysis of error rates in compatible trials revealed no significant main effects of task or response order, both Fs < 1, and only a marginally significant interaction, F(1,14) = 4.42, p = .054, η2p = .24, indicating that for both response orders the first executed response tended to be associated with fewer errors. For incompatible trials, however, there was a main effect of task, F(1,14) = 47.88, p < .001, η2p = .77, indicating overall higher error rates for oculomotor responses (23.1%) than for manual responses (5.9%), and a significant main effect of response order, F(1,14) = 24.84, p < .001, η2p = .64, indicating higher error rates in the reversed response order (27.3%) than in the non-reversed response order (1.7%). The two-way interaction, F(1,14) = 19.46, p = .001, η2p = .58, showed that the high error rates for reversed responses were mainly driven by errors in the oculomotor task.
Taken together, the present post hoc analysis showed no convincing evidence for a performance benefit associated with response reversals. Instead, reversing responses were accompanied by lower accuracy in both tasks, and, interestingly, especially in the oculomotor response. Thus, we can exclude the possibility that the a priori prioritization of the oculomotor system represents a successful functional strategy to increase overall performance. The present observations rather suggest that oculomotor prioritization might represent a rather generic (instead of a strategic) bias, which is also consistent with the apparent difficulty of participants to comply with response order instructions.
Experiment 3
Based on the results from Experiment 1 and 2 alone, it still remains possible that the general tendency to prioritize oculomotor responses only emerged because of the unpredictable stimulus order, which is associated with a mixed stimulus-order design. Thus, in Experiment 3 we analyzed performance in a situation with a fixed (SMSO) stimulus order and without explicit response-order instructions.
To selectively assess the impact of a fixed stimulus order on response order, we used a similar design to that in Experiment 1 (i.e., without any explicit response-order instructions). To further emphasize the overall salience of the fixed stimulus order, we additionally added more extreme SOA conditions (range: 120–1,200 ms) but retained the SOA conditions from Experiment 1 (120, 240, and 360 ms) for comparison purposes. Note that in the very large SOA conditions the manual response could principally be executed prior to the onset of the stimulus for the oculomotor response (i.e., non-overlapping task processing). If response reversals observed in the previous experiments mainly resulted from a strategic adaptation to changing stimulus order demands, we would expect much fewer reversal rates in Experiment 3, where task settings implicitly discourage an oculomotor-first strategy (as opposed to the explicit instructions in Experiment 2).
Method
Participants
Twenty-four new participants (23 female) with a mean age of 22.2 years (range = 18–28) were recruited and received course credits or monetary reimbursement for participation.
Apparatus, stimuli, and procedure
The experimental setup and instructions were identical to those in Experiment 1. Participants were neither explicitly informed about stimulus order nor instructed regarding response order.
Design
SOA (120, 240, 360, 480, 720, 960, and 1,200 ms) and between-task compatibility (compatible and incompatible) were manipulated within participants.
Results and discussion
Of the recorded data, 97.7% remained valid after the data-cleansing procedures, as described in Experiment 1. The overall error rate amounted to 5% only. For response-order analyses in correct trials, we applied a 2 × 7 ANOVA with the independent variables compatibility and SOA.
Response-order reversals
The mean reversal rate amounted to 17.2%, which differed significantly from 0, t(23) = 3.86, p < .001. Reversal rates decreased with increasing SOAs, F(6,138) = 28.03, p < .001, η2p = .55, (39.4%, 25.6%, 19.3%, 12.7%, 9.5%, 7.4%, and 6.2% for SOAs from 120 ms to 1,200 ms). Again, reversals were more frequent in compatible (19.4%) than in incompatible trials (14.9%), F(1,23) = 18.09, p < .001, η2p = .44. The interaction between compatibility and SOA was significant, too, F(6,138) = 3.86, p = .001, η2p = .14, showing that the reversal rate dropped faster for incompatible than for compatible trials (specifically at short SOAs until SOA = 480 ms, see Fig. 5).
Reversal rate as a function of between-task compatibility (compatible and incompatible) and SOA in Experiment 3 with fixed stimulus order (SMSO) and without explicit response-order instructions. Error bars represent standard errors
Pairwise Bonferroni-corrected post hoc comparisons statistically corroborated that the reversal rate did not vary significantly for SOAs greater than 360 ms (i.e., all comparisons for data points at SOA = 480 ms against data points at larger SOA levels were non-significant, ps > .25).
The observation that overall reversal rate dropped to around 10% above an SOA of 480 ms provides strong evidence that participants were able to overcome oculomotor prioritization for long SOAs, that is, under conditions in which waiting for the second stimulus prior to manual response execution would be highly dysfunctional for overall processing efficiency. Nevertheless, despite the fixed stimulus order in Experiment 3, reversals still occurred in approximately 40% of the trials at SOA = 120 ms. This observation shows that response modality characteristics and associated prioritization mechanisms exert a strong influence even when the overall task context strongly suggests a fixed processing order.
Unlike in Experiments 1 and 2, we observed an SOA limit beyond which between-task compatibility was no longer relevant for response-order scheduling. Apparently, beyond a certain SOA, participants did not apply a strategy to wait for (and compare) both stimuli in order to decide an appropriate response order. Instead, tasks were processed serially in accordance with the stimulus order.
As in the previous experiments, we checked whether the compatibility effect in reversal rates represents an artifact due to an unequal distribution of errors across compatibility conditions. An analysis of reversal rates based on all trials (i.e., including error trials) yielded the same statistical pattern as before. There was a main effect of SOA (36.1%, 24.0%, and18.1%), F(2,46) = 26.6, p < .001, η2p = .52, and, importantly, a main effect of compatibility (compatible: 32% vs. incompatible: 20.1%), F(1,23) = 18.8, p < .001, η2p = .45. This observation suggests that there was no substantial trade-off between response-order control and accuracy (as in Experiment 1).
Error rates
Error rates (see Table 3) in Experiment 3 were significantly affected by SOA, F(6,138) = 7.45, p < .001, η2p = .25 and by compatibility (compatible: 3% vs. incompatible: 8.6%), F(1,23) = 27.4, p < .001, η2p = .54. The two-way interaction was also significant, F(6,138) = 12.51, p < .001, η2p = .35. Overall, this pattern reflects greater processing demands for processing incompatible spatial codes (likely due to code conflict), while this interference became weaker with greater temporal distance.
RTs and error rates of reversed versus non-reversed responses
As in Experiment 2, we ran a post hoc analysis of RTs and errors in the 120 ms-SOA condition with the question of whether response prioritization (reflected in response reversals) is associated with processing benefits (in terms of faster RTs and/or fewer errors). A subsample of 16 participants contributed sufficient valid data in each relevant condition (see Fig. 6).
Response times (RTs) and errors rates of oculomotor and manual responses in the SMSO order condition at SOA = 120 ms as a function of response order (reversed and non-reversed) and spatial between-task compatibility (compatible and incompatible) in Experiment 3. Horizontal lines represent single-task baseline RTs. Error rates virtually amounted to 0 in single-task conditions and were therefore not included in the figure. Error bars represent standard errors
In compatible trials, there was a significant main effect of task with overall longer manual RTs (640 ms) compared to oculomotor RTs (506 ms), F(1,15) = 96.49, p < .001, η2p = .87, and a significant main effect of response order, F(1,15) = 14.56, p = .002, η2p = .49, indicating longer RTs on average (hence also longer total RTs) for reversed responses (616 ms) than for non-reversed responses (529 ms). The two-way interaction was also significant, F(1,15) = 58.29, p < .001, η2p = .80, indicating that in the reversed (vs. non-reversed) response-order manual responses were postponed while oculomotor responses were slightly prioritized (similar to Experiment 2). A similar pattern was observed for incompatible responses. While the significant main effect of task indicates longer RTs for manual responses (1,082 ms) than for oculomotor responses (959 ms), F(1,15) = 25.74, p < .001, η2p = .63, the significant main effect of response order indicates longer RTs for reversed responses (1,127 ms) than for non-reversed responses (914 ms), F(1,15) = 8.33, p = .011, η2p = .36. The two-way interaction was again significant, F(1,15) = 50.93, p < .001, η2p = .77, suggesting that reversing responses impaired manual RTs more than oculomotor RTs.
An analysis of error rates (0.1% on average) in compatible trials revealed no significant effects. For incompatible trials, there was a significant main effect of task, F(1,15) = 5.64, p = .031, η2p = .27, indicating overall higher error rates for oculomotor responses (15.9%) than for manual responses (5.0%), but no significant main effect of response order, F(1,14) = 3.15, p = .096, η2p = .17. However, there was a nominal trend towards higher error rates for reversed responses (13.8%) compared to non-reversed responses (7.0%). The two-way interaction was not significant, F(1,15) = 2.36, p = .145.
Taken together, these analyses again demonstrate that reversed responding was associated with worse performance compared to responding according to stimulus order, which supports the assumption that oculomotor prioritization does not reflect an optimization strategy but rather a generic a priori bias. However, this additional analysis cannot be interpreted causally in the sense that reversing responses led to impaired performance. This specific question should be tested in future studies.
Comparison across experiments
Experiment 1 provided evidence for strong oculomotor prioritization in a PRP setup with mixed stimulus order and without explicit response-order instructions, which was only to a small degree affected by stimulus order. The identical setup under explicit response-order instructions in Experiment 2 led to a decrease of response reversals. Finally, the fixed (and thus implicitly more predictable) stimulus order in Experiment 3, in which the stimulus for the dominant oculomotor response was always presented second, also led to lower reversal rates than in Experiment 1. Taken together, Experiment 1 can be interpreted as a baseline against which the effectiveness of these two manipulations to attenuate oculomotor dominance can be statistically compared. Based on this reasoning, we ran an ANOVA on reversal rates and error rates in SMSO order conditions including the factors experiment compatibility and SOA, which included those SOA conditions that were present in each experiment (120 ms, 240 ms, and 360 ms, thus representing a subset of the SOA range used in Experiment 3). For the sake of completeness, we also compared the data in SOSM order conditions between Experiment 1 and 2 (this condition was not present in Experiment 3).
Reversal rates across experiments
SMSO order
To investigate the extent to which instructions (Experiment 2) and trial context (Experiment 3) impacted on performance in otherwise identical trials, we compared reversal rates in SMSO order for SOAs of 120, 240, and 360 ms (see Table 4). The mixed ANOVA with the within-subject factors compatibility and SOA and the between-subject factor experiment yielded significant main effects of between-task compatibility, F(1,67) = 38.15, p < .001, η2p = .36, with higher reversal rates for compatible trials (55.6%) than for incompatible trials (42.3%), and of SOA, F(2,134) = 57.23, p < .001, η2p = .46, indicating decreasing reversal rates with increasing SOAs (59.3%, 46.6%, and 41%). Interestingly, the analysis also revealed a significant main effect of experiment (81.1%, 37.7%, and 28.1% in Experiments 1, 2, and 3), F(2,67) = 27.37, p < .001, η2p = .45, while there was no significant interaction between any of the factors, all ps > .17. Post hoc (Bonferroni-corrected) comparisons showed that reversal rates in Experiments 2 and 3 differed from those in Experiment 1 (both ps < .001). However, reversal rates were not significantly different from each other in Experiments 2 and 3, p = .631, although at least nominally reversal rates were lower in Experiment 3 throughout all experimental conditions (see Table 4). This might probably suggest a lack of sufficient statistical power due to the between-subjects comparison. Taken together, the data show that the explicit (Experiment 2) and the implicit (Experiment 3) approach were effective for decreasing reversal rates. Specifically, the effectiveness of explicit instructions suggests that participants are able to control response order in a top-down manner. Interestingly, the implicit effect of a fixed stimulus order was at least as strong as the instruction effect.
SOSM order
Overall response reversal rates did not differ significantly in SOSM conditions between Experiment 1 (3.6%) and Experiment 2 (5.0%), F < 1. Since in this condition the stimulus order corresponds to the effector system-based prioritized response order, these reversal rates likely represent the lower bound of unsystematic response-order fluctuations. However, there was a marginal effect of SOA, F(2,88) = 2.96, p = .066, η2p = .06, indicating decreasing reversal rates with increasing SOA (5.7%, 3.7%, 3.5%) and a significant interaction between SOA and experiment, F(2,88) = 3.53, p = .041, η2p = .06, reflecting that SOA mainly affected reversal rates in Experiment 2. Most probably, the explicit instructions encouraged participants to pay more attention to the stimulus order, and short SOAs might have rendered confusions regarding stimulus order processing slightly more likely.
Error rates across experiments
In order to test if response accuracy was affected by the manipulations across experiments, we calculated an ANOVA with the independent factors SOA, compatibility, and experiment. There were significant main effects of SOA, F(2,134) = 4.74, p = .013, η2p = .07, reflecting a decrease of errors with longer SOAs (15.3%, 14.1%, and 12.3%), and of between-task compatibility (25.1% for incompatible and 2.6% for compatible trials), F(1,67) = 195.23, p < .001, η2p = .74. Importantly, there was a main effect of experiment, F(2,67) = 11.14, p < .001, η2p = .25, demonstrating that a decrease of reversal rates across experiments was accompanied by an accuracy increase (18.3%, 15.7%, and 7.7% errors in Experiments 1, 2, and 3). The interaction of compatibility and experiment was significant, too, F(2,67) = 14.79, p < .001, η2p = .31, signifying that the compatibility effect became smaller with a decreasing reversal likelihood (compatibility effects of 30.8, 26.2, and 10.5 percentage points for Experiments 1, 2, and 3, respectively). Finally, there was a significant interaction of compatibility and SOA, F(2,134) = 9.57, p < .001, η2p = .13, suggesting that spatial code conflict decreased with SOA. There was neither an interaction between SOA and experiment, F < 1, nor a three-way interaction, F(2,134) = 1.14, p = .34.
Post hoc pairwise (Bonferroni-corrected) comparisons of the between-experiment manipulation showed no difference between error rates of the two experiments with mixed stimulus order (Exp. 1: 18.3%, Exp. 2: 15.3%), p = .85. However, error rates were lowest in Experiment 3 involving a fixed stimulus order compared to both Experiment 1, p < .001, and Experiment 2, p = .003. This suggests that a predictable task context (i.e., a fixed stimulus order) is eventually more effective (in terms of performance accuracy) in attenuating effector system-based prioritization than explicit instructions in a less predictable environment.
We observed evidence for two distinct mechanisms based on between-task compatibility. On the one hand, incompatible trials were more error prone, probably due to a greater confusability potential during the mapping of spatial codes to response modalities. On the other hand, the presence of incompatible codes yielded a stronger focus of attention to bottom-up information within the task set (specifically, stimulus-order information), eventually attenuating the occurrence of response-order reversals.
Note that we did not run an experiment in which we combined both factors that, in isolation, evidently lowered the frequency of response reversals, namely explicit instructions and fixed stimulus order. However, based on our results it seems relatively safe to assume that the occurrence of response reversals would be further attenuated, a result that would not provide substantial additional theoretical insight.
General discussion
The aim of the present study was to investigate the underlying processes of the temporal organization of response-order control in multitasking in order to achieve a more detailed understanding of temporal action scheduling. To this end, we examined several potential sources of influence on the rate of response reversals, which served as an empirical marker for response-order scheduling. We devised a dual-task situation involving two effector systems that are known to substantially differ in their characteristics regarding response execution stage duration and overall prioritization (see Huestegge & Koch, 2013; Pieczykolan & Huestegge, 2014), and that are known to produce a substantial variability of response order in the first place (see Pashler et al., 1993). This specific experimental setup allowed us to study the impact of characteristics of the late (motor-related) processing stage on response-order scheduling, which should occur earlier in the processing chain. In three PRP experiments, we systematically examined the influence of stimulus order, response modalities, response-order instructions (unspecific and explicit), between-task compatibility, and stimulus-order context (fixed and mixed stimulus order) in a PRP paradigm involving a variable SOA. Note that the two tasks differed only with respect to their effector modality, since both required comparable left or right decisions.
Top-down and bottom-up determinants of response-order control
A major finding was that response scheduling was not largely determined by stimulus order. The plausibility of such a bottom-up processing account follows from traditional sequential information stage processing logic within dual tasks, according to which the onset of the selection stage for the first response should be solely determined by the end of processing of the first stimulus (e.g., Pashler & Johnston, 1989; Navon & Miller, 2002). However, while some influence of stimulus order was clearly present in our current data, its overall impact was substantially smaller than one would probably expect from previous dual-task studies. The lack of a pronounced influence of bottom-up processing across the range of SOAs that are typical for PRP experiments is especially notable since previous dual-task studies that involved only a limited number of effector systems usually treated response reversals as an abnormality and excluded them from further analyses (e.g., Bratzke, Rolke, & Ulrich, 2009; Hommel, 1998; Logan & Delheimer, 2001; Pashler & Johnston, 1989; Tombu & Jolicœur, 2002). Nevertheless, it should be noted that the impact of stimulus order was to some extent amplified by the bottom-up factor SOA, in that stimulus order became more effective the longer the SOA was.
In contrast to these bottom-up effects, our present data suggest a very strong overall influence of response characteristics (i.e., characteristics of late processing stages) on response-order scheduling. This is especially interesting since within a traditional processing-stage logic the scheduling process should occur prior to the selection of the first response (if response selection is immediately followed by the corresponding response execution). Specifically, the results suggest a strong temporal prioritization of oculomotor responses over manual responses, even when the stimulus for the manual task was presented first. This implies that stimulus processing of Task 1 was not immediately followed by selecting an appropriate response in Task 1. The present finding that processing characteristics of stages after stimulus processing affect response order is in line with previous reports by Leonhard et al. (2011; see also Ruiz Fernández et al., 2011), who demonstrated the occurrence of more response reversals when the anticipated duration of response selection (i.e., response selection difficulty) in Task 2 was shorter (i.e., the task was easier) than in Task 1. Together with these findings, the present results seriously challenge any account of response-order control that assumes prioritized resource allocation to the task triggered by S1.
In addition, the present data present novel evidence that even late (response execution-related) characteristics affect response-order scheduling (e.g., based on anticipation of response features). The present effects of oculomotor prioritization regarding response order extend to similar, previous findings where oculomotor prioritization was reflected in dual-response cost asymmetries regarding RTs (Huestegge & Koch, 2013; Pieczykolan & Huestegge, 2014, 2017, 2018). Specifically, those previous studies showed that oculomotor responses exhibit fewer dual-response costs than manual responses when the two are combined, a finding that could not solely be explained by inherent differences in response execution durations between effector systems (Huestegge & Koch, 2013; Pieczykolan & Huestegge, 2014). This suggests that effector-specific characteristics instead of mere execution stage duration are responsible for oculomotor prioritization. Interestingly, our analysis of how reversed versus non-reversed responses affected RTs and error rates in Experiments 2 and 3 suggested that oculomotor prioritization does not reflect a functional strategy with the aim of achieving performance benefits (for example by minimizing slack time during serial processing, see Leonhard et al., 2011). Instead, oculomotor prioritization rather appeared to be a generic, deeply-rooted processing default that is comparatively difficult to overcome. While being dysfunctional in the present experimental (laboratory) setting, it may nevertheless represent a useful mechanism (looking before acting) in more real-life situations. Since studies on eye-hand coordination have shown repeatedly that eye movements precede hand movements in pointing, reaching, or object manipulation tasks (e.g., Carnahan & Marteniuk, 1991, Land & Hayhoe, 2001), it is possible that this behavior also transfers to situations with non-overlapping targets for eye and hand.
At first sight, one potential explanation for the high overall reversal rate and the decrease of reversals with longer SOAs might refer to a potential confusion of stimuli or stimulus order already at a perceptual level. This is especially plausible since stimuli in both tasks were similar in terms of their (auditory) stimulus modality and their task-relevant (spatial left/right) stimulus dimension (set-level compatibility, see Kornblum, Hasbroucq, & Osman, 1990). A similar point was already raised by De Jong (1995), who showed that participants tended to encounter more difficulties in judging the correct stimulus order when the SOA was short (see also Hendrich, Strobach, Buss, Müller, & Schubert, 2012). However, two counter-arguments clearly speak against this assumption. If stimulus order uncertainty was mainly responsible for the high reversal rates, we should have observed a comparable amount of reversals for both stimulus-order conditions (at the corresponding SOAs) in Experiments 1 and 2, which was clearly not the case. Second, the low error rates in Experiment 3 prove that participants were principally able to accurately distinguish between the two stimuli. This rules out the potential objection that perceptual limitations regarding stimulus processing can account for the reported effects on response-order scheduling (i.e., the occurrence of reversals). Nevertheless, it is still possible that a certain (small) amount of reversals have occurred due to processing errors at the perceptional stage.
Explicit and implicit measures to adjust scheduling and performance in multitasking
Experiment 2 revealed that explicit instructions can initiate a priority shift towards responding in accordance with the stimulus order. This finding shows that instructions alone can affect temporal response order (as suggested by De Jong (1995), who reported an effect of the combination of instructions and feedback). However, despite this evidence for a top-down attenuation of effector system-based prioritization, oculomotor-first scheduling was still prevalent at the shortest SOA. Finally, Experiment 3 demonstrated that even in a condition with constant stimulus order, thus with a more predictable context that comparably facilitated response-order scheduling (as evidenced by the lowest reversal rates), oculomotor prioritization still occurred. However, this experiment again showed that participants were able to overcome oculomotor prioritization to some degree at short SOAs and quite effectively at longer SOAs. Taken together, Experiments 2 and 3 provide evidence that response order is partially under active, deliberate control of participants (see, De Jong, 1995, for similar results). However, in terms of overall response accuracy, a predictable task environment (Experiment 3) was more effective for adjusting performance (in terms of lower reversal rates) than explicit instructions (Experiment 2), a finding that could probably be relevant for applied settings to improve multitasking performance.
Mechanisms of response-order control in dual tasks: Towards a multi-phase framework of temporal action scheduling
That response-order scheduling was strongly affected by between-task compatibility in all three experiments is particularly informative regarding underlying dynamics of action scheduling. Usually, the resolution of spatial incompatibility in dual-task control is known to draw on cognitive resources, thus yielding performance decrements in incompatible trials. However, here spatial incompatibility led to fewer response reversals, a finding that – at least in Experiment 2 – represents better performance (i.e., performance that is more in line with the instructions). Thus, the present findings cannot be simply attributed to known compatibility phenomena. In the following paragraph, we develop a framework that is better in line with our present observations while integrating previous studies on response-order control (see Fig. 7).
Schematic multi-phase framework of temporal action scheduling: p(reversal) denotes the probability of a response reversal that is dynamically adjusted (either positively: “+”, or negatively: “−”) during the progress of processing phases. The default p(reversal)DEFAULT is determined by initial attentional weight parameters αRi associated with specific actions (or effector systems). After being continuously adjusted (p(reversal)ADJ), a final p(reversal)FINAL is reached that eventually determines response order
Initially, we assume that each of the to-be-coordinated actions is associated with an attentional weight (e.g., based on effector system-based prioritization), which determines a default response reversal probability for the specific effector combination at stake. From the beginning of the experiment until the final response order, adjustment in each trial’s new incoming information is taken into account, so that the response reversal probability can be continuously adjusted (either increased or attenuated) throughout several distinguishable phases. Experiment 1 strongly suggested a default mode of response-order control based on effector-system characteristics in the form of an oculomotor prioritization. Since this default mode was relatively strong and only to some extent modulated by factors such as SOA or compatibility, it seems reasonable to assume that it exerts its influence throughout the course of the experiment and is already set prior to the beginning of each experiment. This reasoning is in line with previous suggestions that response order as part of an overall task set is activated early (e.g., at the beginning of a trial, see De Jong, 1995; Leonhard et al., 2011; Luria & Meiran, 2003). Besides these generic attentional weights associated with actions, other factors can also contribute to adjustments of the reversal probability at a very early stage, for example, anticipated response selection difficulty (e.g., Leonhard et al., 2011). While oculomotor responses might generally be prioritized over manual responses, the introduction of spatially incompatible (vs. compatible as implemented in the present study) S-R mappings might alter the strength of prioritization (as shown for dual-response costs; Pieczykolan & Huestegge, 2014) and thus might also affect the reversal probability. Another factor that should be effective in adjusting the reversal probability at the beginning of an experiment are explicit response-order instructions, as utilized in Experiment 2.
In a following phase, response order (i.e., reversal probability) can be adjusted immediately prior to the beginning of each trial by factors like the response order in the previous trial or stimulus-order predictability (unpredictable in Experiments 1 and 2 vs. predictable in Experiment 3; see also De Jong, 1995). Furthermore, our data suggest that probability adjustments can still occur after the beginning of a trial. Importantly, a mechanism that accounts for the substantial portion of reversals in the present experiments needs to include an explanation of why the response that corresponds to the first stimulus is withheld and what kind of processes occur between the end of stimulus processing and response selection. Also, it needs to explain why stimulus order exerted a stronger effect in incompatible than in compatible trials.
A plausible mechanism involves the assumption that after the processing of both stimuli a dedicated stimulus comparison process takes place, in which the two spatial codes (i.e., those coded by the stimuli of the two component tasks) are compared. The assumption of such a comparison is crucial to account for the effects of compatibility on response-order scheduling. In the case of compatible spatial codes, we assume that the outcome of the comparison process yields one common spatial code that can subsequently be utilized to specify both responses. This assumption has the following implications: While relevant spatial information for both responses is completely preserved, any redundant information is discarded (parsimonious information reduction). Specifically, we assume that during the generation of a common spatial code information that is associated with each of the two individual codes, including information about temporal stimulus order, is lost. Consequently, the probability of responding in default mode should be more likely.
Conversely, in the case of incompatible spatial codes both (conflicting) codes remain active after the comparison process because they are necessary to correctly map the two spatial codes to the two effector systems (i.e., to solve the dual-task binding problem; see discussion in the following section). Thus, incompatible stimuli should yield a processing strategy with a stronger attentional focus on stimulus characteristics, including stimulus order. Given that both spatial codes (based on the two stimuli) are retained, we further assume that specific information associated with the stimuli will not be discarded (here: stimulus-order information). In this way, response order in incompatible trials is more strongly affected by stimulus order than when redundant codes are processed in compatible trials. Thus, reversals occur less likely in incompatible trials, for instance by attenuating the default oculomotor-first regime (see Fig. 7). The assumption of rather separate processing of the component tasks in incompatible dual-task conditions is in line with previous assumptions that incompatible tasks generally involve more distinct response-selection processes when compared to compatible tasks (Fagot & Pashler, 1992; Pieczykolan and Huestegge, 2018).
Note that the explicit instruction to focus on stimulus order (in Experiment 2) was effective in compatible as well as in incompatible conditions, while the compatibility effect was comparable in Experiments 1 and 2. This suggests that instructions no longer interfere once stimulus processing has started. Thus, instructions neither alter the specific probability of entering either of the two processing paths (information reduction vs. retention) referred to above nor do they reduce the information reduction process (reflected in the size of the compatibility effect). Instead, they rather appear to attenuate the overall strength of the default (oculomotor prioritization) mode at the beginning of the experiment.
Finally, to explain the lower reversal rates for long SOAs, we additionally assume that there is only a limited temporal interval (ending with a final deadline) following the processing of the first stimulus in which the reversal probability can be adjusted. However, after the deadline any further waiting for the second stimulus for initiating the stimulus comparison process would be too costly. In this case, response selection would be initiated and completed in Task 1 without any dedicated code comparison process. This is especially likely at long SOAs (which are more likely associated with non-overlapping task processing; see Experiment 3), because it would be highly dysfunctional to withhold R1 until the occurrence of S2. This case can also be regarded as equivalent to a very strong attenuation of the default reversal probability settings.
Taken together, our framework of dynamic multi-phase adjustments of response reversal probability integrates the major findings of the present study as well as previously reported effects. As such, it represents a more realistic approach to response-order scheduling than a simple first-come first-served account, which can be clearly rejected. Nevertheless, the particular predictions of this framework should be rigorously tested in future studies of response-order control.
Implications for current theoretical frameworks of dual-task control
Serial processing frameworks
Since the concurrent processing of two actions necessarily requires temporal scheduling, it seems surprising that this issue has not yet received more attention in current theories on dual-task control. While the presence of a dedicated response-order processing stage has previously been suggested, any specific mechanisms associated with such a stage have largely remained elusive. Apparently, the typical procedures associated with the PRP paradigm, namely sequential stimulus presentation, serial processing instructions, and the utilization of response modalities that are rather similar regarding their overall characteristics (i.e., two manual responses), yielded only few response reversals. As a result, there was not much to be explained, and the focus of research rather shifted towards processes that occur given a certain response order. Therefore, it is possible that the absence of a dedicated research focus on response-order control to date (focusing on actual response order as a dependent variable) is the result of the prevalence of certain research paradigms (associated with low response-order variability), but not the result of a lack of theoretical relevance per se. As a consequence, the view that stimulus order should mainly determine response order is reflected in many of the current dual-task frameworks.
As outlined in the introduction, the standard RSB model does not include any explicit control mechanisms regarding task order. Instead, it inherently assumes that the task in which stimulus processing is finished first will be the first to immediately enter the response selection stage. As outlined above, our present results clearly do not correspond with this assumption. Instead, the present results suggest that participants usually processed both stimuli prior to the response selection in Task 1. Thus, at least in the case of reversals, response selection for Task 1 was deferred until S2 processing was finished. The idea of strategic deferment in dual-task control has already been proposed by Meyer and Kieras (1997a, 1997b), who assumed that processing strategies play a greater role in dual-task control than previously assumed. However, within their Executive-Process Interactive Control (EPIC) framework, strategic deferment rather refers to the assumption that response selection in the second task (i.e., based on S2) is strategically deferred until response selection in the first task is finished. Thus, their model does not explicitly consider the possibility of processing deferment (e.g., in terms of effector-based task prioritization mechanisms) as a potential account of response reversals, but instead focuses on strategic processes after response order has already been determined.
Parallel processing frameworks
Another class of theories that principally allow for parallel central processing is built on the assumption of central capacity sharing (e.g., Navon & Miller, 2002; Tombu & Jolicœur, 2003). These theories assume that resources can be allocated to two simultaneous tasks at the same time, with each task receiving a certain proportion of the limited central capacity during response selection. These accounts do not explicitly discuss the issue of response-order control. Instead, they typically assume that response order is determined by stimulus order based on the premise that Task 1 is processed with the majority of available resources (and is therefore prioritized), so that Task 2 processing receives fewer resources until Task 1 processing has been finished. For example, Navon and Miller (2002, p. 232) explicitly stated that for reasons of “simplicity […] [they] exclude the possibility that selection of R2 finishes before selection of R1 because this possibility is remote with the particular tasks ordinarily used in studies with the overlapping tasks paradigm”. Interestingly, at first it appears that response reversals could be principally explained by such models when assuming that resources (specifically those relevant for response selection) are primarily allocated to Task 2 once S2 has been presented. For example, let us assume that in SMSO conditions the majority of the resources could be allocated to the oculomotor task (although triggered second). Then, at short SOAs the appearance of SO – when response selection of the task associated with SM has already started – would induce a shift of resources towards the response selection process associated with the oculomotor task (for example, via a priori weighting parameters of associated effector systems, as suggested above; see Fig. 7). This would result in a relatively short oculomotor response selection stage (compared to that for RM), thereby increasing the possibility of an “oculomotor-first” response order. However, while such a mechanism might principally be responsible for response reversals at short SOAs (e.g., 120 ms), it cannot easily explain reversed responses at longer SOAs (e.g., 360 ms), which we still observed quite frequently.
Additionally, in Experiment 3 we observed that reversed oculomotor RTs (for compatible responses) were approximately 50 ms faster than non-reversed RTs. If this RT gain is assumed to represent the shortening of response selection caused by a resource boost for oculomotor selection, then reversals in long SOA conditions could only occur under two additional assumptions: First, response selection in the manual Task 1 would still have to continue during the onset of SO. Second, manual response selection would then have to be completely interrupted at the onset of SO (for the duration of oculomotor response selection), and only then resumed again. However, two observations render these assumptions highly unlikely: First, reversed oculomotor RTs in incompatible trials in Experiment 3 were slower than non-reversed responses, which does not corroborate the assumption of a selective response selection shortening via corresponding capacity allocation. Second, reversed oculomotor RTs in Experiment 2 were about 400 ms faster than non-reversed RTs, which is almost twice as long as the oculomotor single-task RT level. Taken together, it appears much more likely that the outcome of a stimulus comparison process – deferring the onset of response selection of Task 1 – determined further processing (whether parallel or fully serial) rather than a capacity-sharing mechanism based on task-specific priorities.
Another successful computational approach to dual-task control is the Executive Control Theory of Visual Attention (ECTVA) by Logan and Gordon (2001), which is characterized by the interplay of control parameters related to bottom-up and top-down processing. Basically, this theory also assumes that processing priorities result from stimulus presentation order. Nevertheless, it principally allows for the occurrence of response reversals as a result of parallel processing. Specifically, ECTVA conceptualizes serial stimulus processing by allocating priority to S1 and S2 in succession. This serial mechanism is assumed to solve the dual-task binding problem associated with “knowing which response goes with which stimulus” (p. 402). However, while our present results regarding incompatible trials (and the associated mechanism of information retention) as well as the strong influence of stimulus order to some extent agree with this assumption, the high reversal rates for compatible trials are rather difficult to explain within this framework. At least in these trials, participants are unlikely to have solved this problem by resorting to serial processing as envisioned in ECTVA. Thus, we strongly believe that a dedicated, more complex mechanism (as shown above) is necessary to account for such response-order phenomena. However, these limitations of the current version of ECTVA (which was not designed to account for response order) do not rule out the possibility that a more extended version can be considered feasible in the future (e.g., one that incorporates features of the framework developed in the previous section).
Crosstalk
Finally, it should be noted that the present setup involving between-task compatibility resembles a situation often studied in dual-task research. Specifically, it has been observed that crosstalk occurs whenever incompatible response content has to be processed across tasks, for example, when one task requires a (spatial) “left” response and the other task requires a (spatial) “right” response (Navon & Miller, 1987). Such crosstalk yields RT increases for both tasks and is typically explained by assuming insufficient shielding during parallel processing. Crucially, many studies on crosstalk were conducted with the aim to falsify the RSB account by demonstrating parallel processing of response-related features during response selection. Therefore, these studies only involved tasks that did not overlap in terms of stimulus dimensions, since any prolongation effects on Task 1 that would occur before or during Task 1 response selection (e.g., due to compatibility relations between S2 and S1/RS1) could still be explained with an RSB account, assuming that Task 1 prolongation simply creates a longer slack period that propagates to Task 2 processing. Since the aim of the present study was not to distinguish between parallel or serial processing models, the present compatibility manipulation represents a first attempt to study compatibility effects in the context of response-order control, which has not been investigated in previous research. Our two tasks shared dimensional features both on the level of response dimensions as well as on the level of stimulus dimensions. This principally allows for additional sources of the compatibility effect besides the previously discussed effects based on stimulus processing. Note, however, that even if a certain amount of the compatibility effect originated from response-based crosstalk (i.e., information that was available during parallel response selection), it would still require a comparison process (see above) to explain the different reversal rates. Nevertheless, in future studies it would certainly be interesting to disentangle the contribution of early (i.e., stimulus-based) versus late (i.e., response-based) task characteristics to compatibility effects on response-order scheduling.Footnote 1
Limitations of generalizability
Finally, two potential limitations regarding the generalizability of the present data should be discussed. First, one might argue that the amount of oculomotor prioritization in the present setup might have been amplified by differences in the ease of mapping responses to stimuli between the two tasks. Specifically, it is possible that auditory input is more difficult to be translated into a manual response than into an oculomotor response (e.g., see input output modality compatibility (IOMC) effect; Hazeltine, Ruthruff, & Remington, 2006; Stephan, Koch, Hendler, & Huestegge, 2013). However, recent research has shown that the specific sensory system plays a much smaller role for IOMC effects than the type of the task-relevant stimulus code (e.g., verbal vs. spatial), suggesting that our spatially defined (auditory) input for the manual response should be easily transferable into a respective response (Göthe, Oberauer, & Kliegl, 2016). Furthermore, the two tasks in the present study were largely comparable in that both require a spatial transformation (i.e., of auditory signals on the left or right ear into distinct key-press movements or eye movements to lateralized saccade targets), ensuring the involvement of central processes during S-R mapping requirements (see Nieuwenstein & Wyble, 2014). Second, one might argue that the present study utilizes a quite unique task combination, especially when compared to those studies that provide the database for current dual-task frameworks and that seldom yield notable amounts of response reversals (see Navon & Miller, 2002). Thus, the proposed mechanisms reported in the present study might not be generalizable to other, more conventional settings. However, while dual-task studies combining oculomotor and manual responses are indeed rare, a mandatory precondition to comprehensively study determinants of response-order control is to establish a situation involving a reasonable amount of response-order variability in the first place (to avoid floor effects). Therefore, the lack of a substantial research focus on response-order control, which – in our view – represents a very relevant control process in multitasking situations, may be largely the result of using rather conventional task combinations in previous dual-task research; which, however, appear to be insufficient for the present research purpose. In this context, the present study precisely demonstrates the impact of specific task characteristics (including response modalities) on processing dynamics and shows that the human cognitive architecture works in an adaptive, rather than a generic, manner.
Notes
In a further experiment (not reported here, see Pieczykolan & Huestegge, in preparation) that was identical to Experiment 3 (fixed stimulus order) with the only exception that saccade direction in Task 2 was based on the color of a central stimulus (thereby avoiding cross-task dimensional overlap between stimuli), reversals almost never occurred (1.4% overall, with 4.1% at SOA =120 ms, 2.8% at SOA = 240 ms, and <= 1% for longer SOAs). It appears that a stronger separation of task representations (via using different stimulus dimensions: auditory-spatial vs. color-based visual) may further reduce response reversals. Thus, cross-task dimensional overlap might be a further determinant of response-order control that should be studied more closely in future research.
References
Adam, J. J., Hommel, B., & Umiltà, C. (2003). Preparing for perception and action (I): The role of grouping in the response-cuing paradigm. Cognitive Psychology, 46, 302–358.
Bratzke, D., Rolke, B., & Ulrich, R. (2009). The source of execution-related dual-task interference: Motor bottleneck or response monitoring? Journal of Experimental Psychology: Human Perception and Performance, 35, 1413–1426.
Carnahan, H., & Marteniuk, R. G. (1991). The temporal organization of hand, eye, and head movements during reaching and pointing. Journal of Motor Behavior, 23, 109-119.
De Jong, R. (1995). The role of preparation in overlapping-task performance. The Quarterly Journal of Experimental Psychology, 48, 2–25.
Ellenbogen, R., & Meiran, N. (2008). Working memory involvement in dual-task performance: Evidence from the backward compatibility effect. Memory & Cognition, 36, 968–978.
Fagot, C., & Pashler, H. (1992). Making two responses to a single object: Implications for the central attentional bottleneck. Journal of Experimental Psychology: Human Perception and Performance, 18, 1058–1079.
Findlay, J. M., & Walker, R. (1999). A model of saccade generation based on parallel processing and competitive inhibition. Behavioral and Brain Sciences, 22, 661–721.
Fischer, R., Plessow, F. (2015) Efficient multitasking: parallel versus serial processing of multiple tasks. Frontiers in Psychology, 6
Göthe, K., Oberauer, K., & Kliegl, R. (2016). Eliminating dual-task costs by minimizing crosstalk between tasks: The role of modality and feature pairings. Cognition, 150, 92-108.
Hazeltine, E., Ruthruff, E., & Remington, R. W. (2006). The role of input and output modality pairings in dual-task performance: Evidence for content-dependent central interference. Cognitive Psychology, 52, 291–345.
Hazeltine, E., Teague, D., & Ivry, R. B. (2002). Simultaneous dual-task performance reveals parallel response selection after practice. Journal of Experimental Psychology: Human Perception and Performance, 28, 517–545.
Hendrich, E., Strobach, T., Buss, M., Müller, H., & Schubert, T. (2012). Temporal-order judgment of visual and auditory stimuli: modulations in situations with and without stimulus discrimination. Frontiers in Integrative Neuroscience, 6, 1–9.
Herman, L. M., & Kantowitz, B. H. (1970). The psychological refractory period effect: Only half the double-stimulation story? Psychological Bulletin, 73, 74–88.
Hirsch, P., Nolden, S., & Koch, I. (2017). Higher-order cognitive control in dual tasks: Evidence from task-pair switching. Journal of Experimental Psychology: Human Perception and Performance, 43, 569–580.
Hommel, B. (1998). Automatic stimulus-response translation in dual-task performance. Journal of Experimental Psychology: Human Perception and Performance, 24, 1368–1384.
Huestegge, L., & Hazeltine, E. (2011). Crossmodal action: Modality matters. Psychological Research, 75, 445–451.
Huestegge, L., & Koch, I. (2013). Constraints in task-set control: Modality dominance patterns among effector systems. Journal of Experimental Psychology: General, 142, 633–637.
Israel, M., & Cohen, A. (2011). Involuntary strategy-dependent dual task performance. Psychological Research, 75, 513–524.
Janczyk, M. (2016). Sequential modulation of backward crosstalk and task-shielding in dual-tasking. Journal of Experimental Psychology: Human Perception and Performance.
Kornblum, S., Hasbroucq, T., & Osman, A. (1990). Dimensional overlap: Cognitive basis for stimulus-response compatibility—a model and taxonomy. Psychological Review, 97, 253–270.
Kübler, S., Reimer, C. B., Strobach, T., & Schubert, T. (2017). The impact of free-order and sequential-order instructions on task-order regulation in dual tasks. Psychological Research, 82, 40-53.
Land, M,F., & Hayhoe, M. (2001) In what ways do eye movements contribute to everyday activities? Vision Reseach, 41, 3559–3565
Lehle, C., Hübner, R. (2009) Strategic capacity sharing between two tasks: evidence from tasks with the same and with different task sets. Psychological Research Psychologische Forschung 73 (5):707–726
Leonhard, T., Ruiz Fernández, S., Ulrich, R., & Miller, J. (2011). Dual-task processing when task 1 is hard and task 2 is easy: Reversed central processing order? Journal of Experimental Psychology. Human Perception and Performance, 37, 115–136.
Logan, G. D., & Delheimer, J. A. (2001). Parallel memory retrieval in dual-task situations: II. Episodic memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 27, 668–685.
Logan, G. D., & Gordon, R. D. (2001). Executive control of visual attention in dual-task situations. Psychological Review, 108, 393–434.
Logan, G. D., & Schulkind, M. D. (2000). Parallel memory retrieval in dual-task situations: I. Semantic memory. Journal of Experimental Psychology: Human Perception and Performance, 26, 1072–1090.
Luria, R., & Meiran, N. (2003). Online order control in the psychological refractory period paradigm. Journal of Experimental Psychology: Human Perception and Performance, 29, 556–574.
McCann, R. S., & Johnston, J. C. (1992). Locus of the single-channel bottleneck in dual-task interference. Journal of Experimental Psychology: Human Perception and Performance, 18, 471–484.
Meyer, D. E., & Kieras, D. E. (1997a). A computational theory of executive cognitive processes and multiple-task performance: Part 1. Basic mechanisms. Psychological Review, 104, 3–65.
Meyer, D. E., & Kieras, D. E. (1997b). A computational theory of executive cognitive processes and multiple-task performance: Part 2. Accounts of psychological refractory-period phenomena. Psychological Review, 104, 749–791.
Miller, J. (2006). Backward crosstalk effects in psychological refractory period paradigms: Effects of second-task response types on first-task response latencies. Psychological Research, 70, 484–493.
Miller, J., & Alderton, M. (2006). Backward response-level crosstalk in the psychological refractory period paradigm. Journal of Experimental Psychology: Human Perception and Performance, 32, 149–165.
Miller, J., Ulrich, R., & Rolke, B. (2009). On the optimality of serial and parallel processing in the psychological refractory period paradigm: Effects of the distribution of stimulus onset asynchronies. Cognitive Psychology, 58, 273–310.
Navon, D., Miller, J. (1987) Role of outcome conflict in dual-task interference.. Journal of Experimental Psychology: Human Perception and Performance 13 (3):435-448
Navon, D., & Miller, J. (2002). Queuing or sharing? A critical evaluation of the single-bottleneck notion. Cognitive Psychology, 44, 193–251.
Nieuwenstein, M., & Wyble, B. (2014). Beyond a mask and against the bottleneck: Retroactive dual-task interference during working memory consolidation of a masked visual target. Journal of Experimental Psychology: General, 143, 1409–1427.
Pashler, H. (1984). Processing stages in overlapping tasks: Evidence for a central bottleneck. Journal of Experimental Psychology: Human Perception and Performance, 10, 358–377.
Pashler, H. (1990). Do response modality effects support multiprocessor models of divided attention? Journal of Experimental Psychology: Human Perception and Performance, 16, 826–842.
Pashler, H. (1994). Dual-task interference in simple tasks: Data and theory. Psychological Bulletin, 116, 220–244.
Pashler, H., Carrier, M., & Hoffman, J. (1993). Saccadic eye movements and dual-task interference. The Quarterly Journal of Experimental Psychology, 46A, 51–82.
Pashler, H., & Johnston, J. C. (1989). Chronometric evidence for central postponement in temporally overlapping tasks. The Quarterly Journal of Experimental Psychology, 41A, 19–45.
Pieczykolan, A., & Huestegge, L. (2014). Oculomotor dominance in multitasking: Mechanisms of conflict resolution in cross-modal action. Journal of Vision, 14(13), 1–17.
Pieczykolan, A., Huestegge, L. (2017) Cross-modal Action Complexity: Action- and Rule-related Memory Retrieval in Dual-response Control. Frontiers in Psychology, 8
Pieczykolan, A., & Huestegge, L. (2018). Sources of interference in cross-modal action: Response selection, crosstalk, and general dual-execution costs. Psychological Research, 82, 109–120.
Pieczykolan, A., & Huestegge, L. (in preparation). Oculomotor Control and Dual-Task Interference –Evidence from the PRP paradigm.
Ruthruff, E., Johnston, J. C., & Van Selst, M. (2001). Why practice reduces dual-task interference. Journal of Experimental Psychology: Human Perception and Performance, 27, 3–21.
Schumacher, E. H., Seymour, T. L., Glass, J. M., Fencsik, D. E., Lauber, E. J., Kieras, D. E., & Meyer, D. E. (2001). Virtually perfect time sharing in dual-task performance: Uncorking the central cognitive bottleneck. Psychological Science, 12, 101–108.
Sigman, M., & Dehaene, S. (2008). Brain mechanisms of serial and parallel processing during dual-task performance. The Journal of Neuroscience, 28, 7585–7598.
Stephan, D. N., Koch, I., Hendler, J., & Huestegge, L. (2013). Task switching, modality compatibility, and the supra-modal function of eye movements. Experimental Psychology, 60, 90–99.
Strobach, T., Liepelt, R., Pashler, H., Frensch, P. A., & Schubert, T. (2013). Effects of extensive dual-task practice on processing stages in simultaneous choice tasks. Attention, Perception, & Psychophysics, 75, 900–920.
Szameitat, A. J., Lepsien, J., von Cramon, D. Y., Sterr, A., & Schubert, T. (2006). Task-order coordination in dual-task performance and the lateral prefrontal cortex: An event-related fMRI study. Psychological Research, 70, 541–552.
Telford, C. W. (1931). The refractory phase of voluntary and associative responses. Journal of Experimental Psychology, 14, 1–36.
Tombu, M., & Jolicœur, P. (2002). All-or-none bottleneck versus capacity sharing accounts of the psychological refractory period phenomenon. Psychological Research, 66, 274–286.
Tombu, M., & Jolicœur, P. (2003). A central capacity sharing model of dual-task performance. Journal of Experimental Psychology: Human Perception and Performance, 29, 3–18.
Ulrich, R., & Miller, J. (2008). Response grouping in the psychological refractory period (PRP) paradigm: Models and contamination effects. Cognitive Psychology, 57, 75–121.
Watter, S., Logan, G. D. (2006) Parallel response selection in dual-task situations. Perception & Psychophysics 68 (2):254-277
Welford, A. T. (1952). The psychological refractory period and the timing of high-speed performance—a review and a theory. British Journal of Psychology. General Section, 43, 2–19.
Wilson, M. (2002). Six views of embodied cognition. Psychonomic Bulletin & Review, 9, 625–636.
Wu, C., & Liu, Y. (2008). Queuing network modeling of the psychological refractory period (PRP). Psychological Review, 115, 913–954.
Acknowledgements
The authors thank Andrea Zahn, Marvin Liesner, Nora Gosch, and Aki Schumacher for the collection of the data and data preprocessing, Mark Asbach for technical support, and those who participated in the study. The present research was funded by the Deutsche Forschungsgemeinschaft (HU 1847/3-1, HU 1847/4-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pieczykolan, A., Huestegge, L. Action scheduling in multitasking: A multi-phase framework of response-order control. Atten Percept Psychophys 81, 1464–1487 (2019). https://doi.org/10.3758/s13414-018-01660-w
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-018-01660-w