Abstract
The direction of gaze towards or away from an observer has immediate effects on attentional processing in the observer. Previous research indicates that faces with direct gaze are processed more efficiently than faces with averted gaze. We recently reported additional processing advantages for faces that suddenly adopt direct gaze (abruptly shift from averted to direct gaze) relative to static direct gaze (always in direct gaze), sudden averted gaze (abruptly shift from direct to averted gaze), and static averted gaze (always in averted gaze). Because changes in gaze orientation in previous study co-occurred with changes in head orientation, it was not clear if the effect is contingent on face or eye processing, or whether it requires both the eyes and the face to provide consistent information. The present study delineates the impact of head orientation, sudden onset motion cues, and gaze cues. Participants completed a target-detection task in which head position remained in a static averted or direct orientation while sudden onset motion and eye gaze cues were manipulated within each trial. The results indicate a sudden direct gaze advantage that resulted from the additive role of motion and gaze cues. Interestingly, the orientation of the face towards or away from the observer did not influence the sudden direct gaze effect, suggesting that eye gaze cues, not face orientation cues, are critical for the sudden direct gaze effect.
Similar content being viewed by others
In social situations, potent cues, such as a person directing her gaze at you, do not appear in isolation, but temporally and spatially co-occur with other cues that shape attention. An intriguing example is sudden onset direct gaze—an abrupt change from an averted gaze direction to direct eye contact. This event comprises multiple stimulus features, in particular direct gaze and sudden onset motion, that may attract and capture attention to a greater degree than other stimuli or each of these features separately—direct gaze itself has been shown to capture attention, even in the absence of sudden onset motion (Senju & Johnson, 2009). Researchers have previously suggested that direct gaze is prioritized because it forms a social cue that provides a medium for verbal and nonverbal communication (Csibra & Gergely, 2009); for example, certain emotional expressions such as anger are more readily recognized with direct gaze versus averted gaze (e.g., Adams, Gordon, Baird, Ambady, & Kleck, 2003; Adams & Kleck, 2003). Gaze processing has been argued to hold a special role in social encounters, as the eyes hold both a signaling and a perceiving function (e.g., Gobel, Kim, & Richardson, 2015; Kendon, 1967; Risko, Richardson, & Kingstone, 2016).
Besides direct eye contact, sudden onset gaze (as has been implemented in previous studies; e.g., Böckler, van der Wel, & Welsh, 2014) also comprises motion cues. As a person shifts attention to look at someone, this overt shift involves movements of the eyes and head. Previous studies have shown that sudden onset motion on its own forms a potent cue for attention capture (e.g., Abrams & Christ, 2003; Al-Aidroos, Guo, & Pratt, 2010). Because in naturalistic interactions, the onset of the direct gaze is often accompanied by motion cues, it is important to determine how these cues independently or interactively capture attention.
In previous work (Böckler et al., 2014), we argued that gaze direction and motion cues form two independent cues for attention capture. In those experiments, participants identified a target letter presented randomly on the forehead of one of four heads, with distractor stimuli being presented on each of the remaining three foreheads (a task modeled after Abrams & Christ, 2003). The initial display showed two heads with direct gaze and two heads with averted gaze. After a delay, one head switched from direct gaze to averted gaze, one head switched from averted gaze to direct gaze, and the other two heads remained static. Simultaneous to the changes in gaze direction, the targets and distractors were revealed. This manipulation allowed us to determine how gaze direction and motion cues independently (additively) or interactively influence attention capture. We found that direct gaze and motion both captured attention, and additively resulted in a sudden direct gaze effect (Böckler et al., 2014).
In a subsequent study (Böckler, van der Wel, & Welsh, 2015), we manipulated visibility of the eyes and head orientation to determine whether attention capture would occur when the eyes were closed (to determine if frontal face orientation would be sufficient for attention capture: Experiment 1), when the eyes were covered by sunglasses and presumed to be “open” (to determine if the potential of being looked at would be sufficient for attention capture even when the eyes are not visible; Experiment 2), or when the eyes were open, but the faces were upside-down (to determine if disrupting holistic face processing would eliminate attention capture by direct gaze; Experiment 3). The results revealed that sudden onset direct gaze stimulus only captured attention when the eyes were covered by sunglasses (Experiment 2) and, hence, could potentially be open and looking at the participant. Sudden direct gaze did not capture attention when the eyes were closed (Experiment 1) or when the eyes were open, but the heads were presented upside-down (Experiment 3). Together, the results of these previous studies suggest that sudden onset direct gaze captures attention more strongly than gaze and motion cues alone (Böckler et al., 2014). Furthermore, visibility of the eyes is neither a necessary nor a sufficient precondition for this effect; attention capture by sudden gaze was found even though eyes were hidden behind sunglasses, but it was disrupted in inverted faces even though eyes were visible (Böckler et al., 2015).
So far, the specific contribution of head and eye orientation to the (sudden onset) direct gaze effect is unknown. In particular, previous studies do not allow us to determine whether or not the eyes are sufficient to elicit attention capture independent from head orientation because shifts in eye gaze were always accompanied by shifts in head orientation. In the present study, we sought to differentiate between the contributions of head (face) and eye orientation to the effects of direct gaze and sudden onset motion on attention capture. Doing so is important because the eyes have been noted to hold significance as a potential social cue for attention capture (Gobel et al., 2015). In all our previous experiments, however, the eyes and the head changed orientation at the same time. Thus, it is not clear if (a) head and eyes need to be in the same orientation and move simultaneously to capture attention, if (b) eye orientation and motion can capture attention independent of head orientation and motion, or if (c) effects of eye orientation and motion are contingent on a specific orientation of the head (e.g., directed towards the participant). These different potential outcomes would have distinct implications. First, if simultaneous head motion is necessary for attention capture by gaze direction and motion (the sudden gaze effect only emerges when the head rotates, too), then this finding would suggest that gaze direction and gaze motion alone are not sufficient to capture attention in social settings that provide more complex information (e.g., depicting the entire face). Hence, previously revealed attention capture by sudden onset direct faces (e.g., Böckler et al., 2014) might, at least partly, be based on salient lower level features, such as the total amount of motion in the display, rather than on the inherently social effect of eye gaze. Second, if, the effects of gaze direction and sudden onset motion occur independent of face orientation (the sudden direct gaze effect occurs when the face is oriented towards or away from the observer), this finding would further establish that the sudden direct gaze effect is in fact predominantly about gaze, supporting the notion that the eyes can act as powerful social cues that capture attention. Finally, finding attention capture by gaze direction and motion only when the head is oriented towards participants would suggest that gaze effects are contingent on contextual information, for instance, on information about social approach that is provided by a face directed towards the onlooker.
To assess the potential independent and interactive effects of eye and head orientation, participants completed the experimental task we have previously used while keeping head/face orientation static (constant) within a trial. For one experimental group, heads were always presented with a frontal orientation (i.e., direct faces condition). For the other experimental group, heads were always presented with a rotated orientation (i.e., an averted face condition). Keeping head orientation constant within each group allowed us to determine if the sudden direct gaze effect can occur with eye movements alone, while also examining the influence of head orientation on this effect in the absence of head motion. Hence, if motion of the head is critical to the effects of direction and motion of the eyes, then no processing advantages should be observed in either group because only the eyes are moving. Second, if eye movement and orientation are the critical component to the effects of gaze direction and motion, then we should find the effects in both groups (i.e., independent of head orientation). Finally, if the sudden direct gaze effect is contingent on both head and eye orientation, then the sudden direct gaze effects should only emerge in the direct face condition.
Method
Participants
Sixty-four participants (39 women, 57 right-handed) with a mean age of 21.4 years (ranging from 18 to 42) took part in the study to earn course participation credit. All participants had normal or corrected-to-normal vision and provided written informed consent and background information. The procedures complied with the ethical standards of the 1964 Declaration of Helsinki. Sample sizes were determined by power analyses with G*Power (Faul, Erdfelder, Lang, & Buchner, 2007). Estimates for effect sizes were taken from previous experiments both for the main effect of gaze direction (partial eta squares = .37–.39; Böckler et al., 2014) and for the interaction between motion and gaze direction (partial eta square = .38; Experiment 2 in Böckler et al., 2015). Desired power was set at 1 − β = .80 and the alpha-level was set at .05. The results of these calculations indicated a sample size of 18 participants per group. We explicitly chose larger sample sizes than minimally required to account for data loss (e.g., technical problems or outlier removal) and potential overestimation of effect sizes in previous experiments. Participants were randomly assigned to one of two experimental scenarios: one in which faces maintained a frontal head orientation (direct faces condition, n = 32) and one in which faces maintained an averted head orientation (averted face condition, n = 32).
Experimental procedure
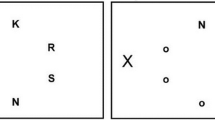
The general procedure was similar to our previous study (Böckler et al., 2014). Participants sat at a desk in front of a 17-in. TFT monitor (screen resolution of 1680 × 1050 pixels) at a distance of 80 cm. Hands were placed on a keyboard. Each trial consisted of two displays (see Figs. 1 and 2). The first display showed four faces around a central fixation, each with the number “8” (placeholder) on their forehead. Images were 200 × 250 pixels (3.8 × 4.7° visual angle) and presented on a black background. All images depicted the same woman but varied in terms of eye gaze direction: eye gaze of two of the faces was directed towards participants, and eye gaze of two of the faces was averted from participants (towards the lower left corner of the screen).
Display across a trial sequence for the group completing the direct face condition. Display first showed four faces with a number 8 placeholder. Between frames, two of the faces changed gaze direction and two maintained static gaze. The placeholders were replaced by target and distractor letters between the frames. Background color has been changed to white for display purposes
The second display appeared after 1,500 milliseconds and contained two changes. First, one of the faces changed from direct eye gaze to averted eye gaze, and one changed from averted eye gaze to direct eye gaze. The gazes of the other two faces remained unchanged. The images and orientations of the eye gazes themselves were irrelevant for the actual task. A second change was that the figure 8 placeholders were replaced by one target letter (H or S) and three distractors (E or U). There was only one target in a display and the remaining three distractor letters were always the same letter. Participants were to maintain fixation on the central cross and identify the target letter as fast and accurately as possible by pressing either the S or the H key on a keyboard with their index fingers of the left and right hand.
There were 384 trials in total. Gaze direction, image position, and target/distractor combination were randomized. Before the experimental trials, participants completed eight practice trials to ensure that they understood the task. We used MATLAB’s PsychToolbox extension (Brainard, 1997; Pelli, 1997) for stimulus presentation and response recording.
The only difference between the two experimental groups was that heads/faces were always directed towards participants in the direct faces condition, and heads/faces were always averted from participants in the averted face condition (independent of gaze direction).
Results
We excluded five participants in the direct face condition and two participants in the averted faces condition due to poor performance, namely reaction times (RT) or error rates larger than two standard deviations above the mean of all participants in the respective experimental condition. We also eliminated RTs associated with incorrect responses (2.8% of the data) and RTs that were outside of ±2 standard deviations of the mean RT for each participant (2.5% of the data).
We conducted a 2 (gaze direction: direct, averted) × 2 (motion: sudden, static) × 2 (head orientation: towards, averted) mixed ANOVA on RTs and error rates, with gaze direction and motion as repeated-measures factors and head orientation as a between-subjects factor. Figure 3 displays RT results. RTs and error rates for all experimental conditions are displayed in Table 1.
Reaction rimes (RTs) as a function of motion and gaze direction for the direct faces (head orientation towards participants) and averted faces groups (head orientation averted from participants). Error bars display 95% within-subjects confidence intervals based on Loftus and Masson (1994)
The results indicated two important findings. First, the results replicated our previous studies by showing significant main effects for both motion and gaze direction and no significant interaction. The main effect for motion revealed that RTs for targets presented on faces that changed eye gaze orientation were shorter than RTs for targets presented on faces with static eyes, F(1, 55) = 73.8, p < .001, η2 = .573. There was also a main effect of gaze direction, F(1, 55) = 6.4, p < .01, η2 = .105, indicating shorter RTs to targets appearing on faces with direct gaze than on faces with averted gaze. As in our original study, the interaction of motion and gaze direction was not significiant. There was a trend for this interaction with gaze effects being numerically, though not statistically, larger when the eyes moved than when the eyes remained static, F(1, 55) = 3.3, p = .075, η2 = .057. It should be noted that, consistent with our previous work, RTs to targets appearing on faces with sudden onset direct gaze were significantly shorter than RTs in all other conditions.
Using Bayesian analysis, we further examined how well the main effects of motion and gaze direction accounted for our data compared to a model that included their interaction as well. To do so, we used JASP and the default uninformed prior of 0.707 (JASP Team, 2016). The model with the strongest explanatory power overall was the model that included the two main effects of gaze direction and motion. This two main effect model was approximately 1.245e +11 times more likely than the null hypothesis. The model that included the two main effects, and the interaction between gaze direction and motion was not as strong, with the model including just the two main effects being 1.51 times more likely than the model that included the two main effects and the interaction. Thus, the pattern of RTs is best accounted for by a model that only includes the main effects for gaze direction and for motion, but not the interaction term.
Second, head orientation did not significantly contribute to the sudden direct gaze effect, as the results failed to show a main effect or interactive effects for head orientation with any of the other factors, p > .10. In line with this observation, our Bayesian analysis also indicated that the between-group factor of head orientation weakened the model. These results are consistent with the hypothesis that the sudden direct gaze effect does not critically rely on motion or orientation of the head and face. That is, the direct gaze effect was present even though the heads were static and was not modulated by the direction of the face.
The analysis of errors indicated that error rates were significantly higher for faces with averted head orientation as compared to faces with direct head orientation, F(1, 55) = 6.1, p < .05, η2 = .100. In contrast to results in RTs, main effects for motion and gaze direction did not reach significance, Fs < 1, though they were numerically present (see Table 1). The interaction of motion and gaze direction was also not significant, F(1, 55) = 1.6, p = .218, η2 = .027. Similar to RTs, head orientation did not significantly interact with any of the other factors, Fs ≤ 1.1.
Discussion
The present study aimed to determine if the orientation of the eyes and/or the head are critical for the sudden direct gaze effect to emerge. Specifically, we sought to disentangle the role of gaze direction and head orientation by presenting heads with either a direct or an averted orientation throughout distinct experiments while manipulating direction and sudden onset motion of the eyes between trials. Doing so was important because in our previous studies, changes in eye gaze direction were always accompanied by changes in head direction (Böckler et al., 2014, 2015).
Consistent with our previous findings and those of others (e.g., Hood, Macrae, Cole-Davies, & Dias, 2003; Senju & Hasegawa, 2005; Vuilleumier, George, Lister, Armony, & Driver, 2005), we observed that direct gaze captured attention and yielded a processing benefit for targets appearing on faces with direct gaze. We also confirmed that sudden onset motion captures attention (Abrams & Christ, 2005) because performance for targets on faces in which the eyes moved was better than for targets on faces with static eyes. In addition, our findings replicated the sudden direct gaze effect, as performance was best for targets on the face in which the eyes with averted gaze shifted to direct gaze (see Böckler et al., 2014). Importantly, the effects of direct gaze, of sudden onset motion and of sudden onset direct gaze, were not influenced by head/face orientation and occurred even though it was only the orientation of the eyes that changed during the trials.
These results extend previous findings by showing that the effects of gaze direction and motion on attention capture do not depend on head/face orientation. This finding is particularly important because changes in eye and head orientation were simultaneous (and thus confounded) in previous studies but are often dissociated in everyday life. Having disentangled the respective information allows us to argue that eye gazes are powerful social cues that capture attention even when embedded in the context of static or averted faces. Interestingly, we showed previously (Böckler et al., 2015, Experiment 3) that presenting faces in an upside-down orientation negated effects of gaze direction on attention capture, indicating that processing of the face in the configural holistic manner is important for this social cueing effect. Based on the current results, it seems that as long as faces are oriented upright (such that holistic processing is likely to occur), direction and sudden onset motion of the eyes are the key features that capture attention. This finding is also informative with respect to a recent study that showed that low-level cues associated with gaze changes, such as luminance changes and motion cues, may interact with gaze processing (e.g., Hayward & Ristic, 2017). Our current findings suggest that the orientation of the face itself does not shape the processing of direct gaze cues.
Future research could address to what extent sudden onset direct gaze captures attention due to low-level features versus due to communicative (i.e., social) intent. Some researchers have emphasized the role of low-level features in attention capture by direct gaze (e.g., Senju & Johnson, 2009), whereas others maintain that direct gaze (partly) captures attention due to its ability to reflect communicative intent (e.g., Csibra & Gergely, 2009). Our previous findings revealed that attention capture can occur even when the low-level feature of direct eye gaze is not visible (see sunglass experiment, Böckler et al., 2015) and that attention capture is absent when the low-level feature of direct gaze is present but cannot be integrated into a meaningful face representation (i.e., when the face is inverted; see Experiment 3, Böckler et al., 2015). This pattern may indicate that attention effects of social cues do not only depend on their low-level features but also on the degree to which they can be processed as communicative signals. Although the current results do not directly disentangle these different possibilities, it is interesting that sudden onset direct gaze captured attention even when the head was oriented away from participants. This finding may, on the one hand, indicate that low-level features of head orientation or (a)symmetry of the location of the pupil in the eyes are not necessary for attention capture associated with direct versus averted gaze—attentional capture by direct gaze occurred when the pupil was symmetrical in the eye (direct faces condition) and when the pupil was asymmetrical in the eye (averted face condition). On the other hand, it is not clear whether direct gaze on an averted face would indicate communicative intent or not. In most communicate acts, head orientation and gaze direction would coincide, such as when someone turns toward you to start a conversation. However, when students attempt to cheat on a test by peeking, they may try to keep head orientation static and direct while moving the eyes only. Thus, whether the coupling of head orientation and gaze direction would result in “natural behavior” may depend on the particular setting in which the two are paired. Future studies could shed light on whether contextual cues modulate the sudden direct gaze effect.
References
Abrams, R. A., & Christ, S. E. (2003). Motion onset captures attention. Psychological Science, 14, 427–432.
Abrams, R. A., & Christ, S. E. (2005). Onset but not offset of irrelevant motion disrupts inhibition of return. Perception & Psychophysics, 67, 1460–1467.
Adams, R. B., Jr., Gordon, H. L., Baird, A. A., Ambady, N., & Kleck, R. E. (2003). Effects of gaze on amygdala sensitivity to anger and fear faces. Science, 300, 1536.
Adams, R. B., Jr., & Kleck, R. E. (2003). Perceived gaze direction and the processing of facial displays of emotion. Psychological Science, 14, 644–647.
Al-Aidroos, N., Guo, R.M., & Pratt, J. (2010). You can’t stop new motion: Attentional capture despite a control set for colour. Visual Cognition, 18, 859–880.
Böckler, A., van der Wel, R. P., & Welsh, T. (2014). Catching eyes: Effects of social and non-social cues on attention capture. Psychological Science, 25(3), 720–727.
Böckler, A., van der Wel, R. P. R. D., & Welsh, T. (2015). Eyes only? Perceiving eye contact is neither sufficient nor necessary for attention capture by gaze cues. Acta Psychologica, 160, 134–140.
Brainard, D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10, 433–436.
Csibra, G., & Gergely, G. (2009). Natural pedagogy. Trends in Cognitive Sciences, 13, 148–153.
Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191.
Gobel, M. S., Kim, H. S., & Richardson, D. C. (2015). The dual function of social gaze. Cognition, 136, 359–364.
Hayward, D. A., & Ristic, J. (2017). Feature and motion-based gaze cuing is linked with reduced social competence. Scientific Reports, 7, 44221.
Hood, B. M., Macrae, C. N., Cole-Davies, V., & Dias, M. (2003). Eye remember you: The effects of gaze direction on face recognition in children and adults. Developmental Science, 6, 67–71.
JASP Team (2016). JASP (Version 0.7.5.5) [Computer software].
Kendon, A. (1967). Some functions of gaze-direction in social interaction. Acta Psychologica, 26, 22–63.
Loftus, G. R., & Masson, M. E. J. (1994). Using confidence intervals in within-subject designs. Psychonomic Bulletin & Review, 1, 476–490.
Pelli, D. G. (1997). The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision, 10, 437–442.
Risko, E. F., Richardson D. C., & Kingstone, A. (2016). Breaking the fourth wall of cognitive science: Real-world social attention and the dual function of gaze. Current Directions in Psychological Science, 25(1), 70–74.
Senju, A., Hasegawa, T. (2005). Direct gaze captures visuospatial attention. Visual Cognition, 12(1), 127–144.
Senju, A., & Johnson, M. H. (2009). The eye contact effect: Mechanism and development. Trends in Cognitive Sciences, 13(3), 127–134.
Vuilleumier, P., George, N., Lister, V., Armony, J., & Driver, J. (2005). Effects of perceived mutual gaze and gender on face processing and recognition memory. Visual Cognition, 12, 85–101.
Acknowledgements
This research was supported by an Early Research Award from the Ontario Ministry of Research and Innovation and Discovery Grants from the Natural Sciences and Engineering Research Council of Canada. We thank Merryn Constable for her contribution to the data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Wel, R.P., Welsh, T. & Böckler, A. Talking heads or talking eyes? Effects of head orientation and sudden onset gaze cues on attention capture. Atten Percept Psychophys 80, 1–6 (2018). https://doi.org/10.3758/s13414-017-1462-y
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-017-1462-y