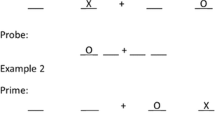Abstract
Responding to a target’s current (probe trial) location is slower when it appears at a former distractor-occupied position (i.e., ignored-repetition [IR] trial), relative to when it arises at a new location (i.e., control trial). This RT(IR) > RT(Control) inequality defines the spatial negative priming (SNP) effect in latency terms. It is uncertain whether the elevated RT(IR) is due to the inhibition of the distractor-occupied location or to the inhibition of this location’s assigned manual response (SNP locus issue). The main aim here was to examine the SNP locus issue. Notably, our SNP design used centrally presented visual events and included having two locations share a common response (many:1 location-to-response mapping) and the use of informative (70 % validity) or uninformative probe-trial response cues. The many:1 mapping trials allowed for the detection of location and response inhibition presence. Results showed that the latter, but not the former, causes inhibitory aftereffects (e.g., SNP) following uninformative response cues. Consistent with this finding, when the informative response cue was valid and was assigned to the many:1 probe response that had just served as the prime distractor response, inhibitory aftereffects were eliminated, when the probe target appeared at the prime distractor position (IR trial) or at a new location (distractor–response repeat trial). Blocked retrieval of stored distractor-processing representations was proposed as the mechanism for inhibitory aftereffect prevention.
Similar content being viewed by others
Introduction
Inhibitory aftereffects refer to those instances where the consequences of prior distractor processing later interfere with ongoing related processing. One exemplar of an inhibitory aftereffect is the spatial negative priming (SNP) phenomenon (e.g., Neill, Terry, & Valdes, 1994; Tipper, Brehaut, & Driver, 1990). With the particular SNP task procedures of interest here, the arrival of a warning signal forecasts the impending appearance of various centrally positioned bar markers on a computer screen that designate the potential locations for upcoming target or distractor event presentations, which can appear singly or together. Typically, each bar marker is assigned a single, spatially compatible, keyboard button response (1:1 location–response mapping). The task is to depress the keyboard button assigned to the location occupied by the target stimulus (e.g., green rectangle) as quickly as possible, while concurrently ignoring any distractor event that might be present (e.g., red rectangle). Trials are then presented in pairs: first the “prime,” and then the “probe,” each lasting about 200 ms. When the probe trial delivery is sufficiently delayed (about 400+ ms), the reaction time (RT) is longer, and buttonpress errors sometimes more numerous, when the probe target appears at the location formerly occupied by the prime distractor event (i.e., ignored-repetition [IR] trial type), relative to when it appears at a previously empty location (i.e., control trial type) (e.g., Buckolz, Avramidis, & Fitzgeorge, 2008; Fitzgeorge & Buckolz, 2008; Neill et al., 1994). The RT(IR) > RT(Control) latency inequality and, sometimes, the IR > control buttonpress error rate imbalance are used to index the presence of the spatial negative priming effect.
Initially, the cause of the visual SNP effect and, hence, the reason for the delayed responding on IR trials were attributed to the inhibition of the distractor-occupied prime-trial location, which somehow interfered with probe target processing when the target later appeared there (i.e., IR trial) (e.g., Christie & Klein, 2001; Connelly & Hasher, 1993; Neill, Valdes, & Terry, 1995). More recently, however, evidence opposing the location-inhibition account of SNP production has been accumulating. This evidence was generated using two distinguishable procedures whose conclusions produced related yet distinctive claims as to the cause of visual SNP.
In one instance, the authors included trials where two acceptable responses were assigned to the same location (i.e., 1:many location-to-response mapping), so that participants could freely choose either response when a probe target appeared at one of these “free choice” locations. On the critical free choice probe trials, participants chose between a control and a prime distractor response (i.e., a distractor–response repeat [DRR] trial), with the probe trial target position being common to both outputs. Individuals showed a significant bias against choosing the prime distractor response, and, when they did select it, its latency significantly exceeded that observed when the control response was chosen (Buckolz, Goldfarb, & Khan, 2004; Fitzgeorge, Buckolz, & Khan, 2011; Lok, 2011). To explain their 1:many subset results, these authors proposed that the prime-trial distractor response was activated and subsequently inhibited, with the latter operation rendering the distractor response resistant to future execution. This execution resistance (ER) would have caused the observed bias against choosing the former prime distractor response and would also have caused the elevated RT on DRR trials when the prime distractor response happened to be chosen, assuming that overriding ER takes time. Because the former distractor response is used on traditional IR trials (1:1 mapping), the time needed to override its ER must at least contribute to IR RT slowing and so, in turn, to the SNP effect. Notably, data arising from 1:many subsets cannot rule out location inhibition also contributing to SNP production (see below).
In the second case, the authors actually claimed that the prime distractor response was the exclusive source of IR trial slowing. Location inhibition, if it occurred, did not contribute at all to SNP production (Guy, Buckolz, & Khan, 2006). These researchers utilized many:1 (rather than 1:many) location-to-response mappings whereby two locations were mapped onto a single response. Within the many:1 mapping subset (Schematic 1), control, DRR, and IR trials could all be generated and were all of the forced choice variety. Hence, the absolute RT values for the three trial types could be compared, and latency differences interpreted in terms of the presence of location (i.e., RT[DRR] vs. RT[IR]) and response (i.e., RT[DRR] vs. RT[Control]) inhibition aftereffects. Guy et al. (2006) found RT(DRR) > RT(IR) > RT(Control). Consistent with other reports (e.g., Buckolz et al., 2004; Fitzgeorge et al., 2011; Lok, 2011), Guy et al. (2006) concluded that the significant RT(DRR) > RT(Control) inequality demonstrated that (prime trial) distractor response inhibition produced reaction delays and, importantly, that the significant RT(DRR) > RT(IR) imbalance indicated that distractor-occupied prime-trial locations are not inhibited. Actually, probe target RT was shorter when it appeared at the prime distractor location (IR trial) than when it arose at a new spatial position (DRR trial). These collective findings pointed to the prime distractor response as the exclusive cause of the SNP phenomenon (henceforth, the exclusivity view). (Note that when 1:many mappings are used, the DRR trials are free choice, while the IR trials are forced choice. Because choice type can affect RT size, RT[DRR-free choice] versus RT[IR-forced choice] cannot be used to determine whether location inhibition is present or not.)
Some trial types arising in location-based negative priming tasks, illustrating ignored-repetition (IR) and distractor–response repeat (DRR) trial types within the many:1 location-to-response mapping subset. Control trials for this subset or for the 1:1 subset occur when the locations related to the required probe response are unoccupied on the prime trial. d = distractor, t = target
Acceptance of the exclusivity view of SNP production has been slow to develop (but see Neill, 2007), perhaps because it opposes the original belief that the SNP phenomenon was caused by the inhibition of prime distractor location and/or because there has been only a single demonstration pointing to the prime distractor response as the exclusive cause of SNP (Guy et al., 2006). Accordingly, the aim of this study was to provide additional support for the exclusivity view. To do this, like Guy et al. (2006), we incorporated many:1 location-to-response mappings within our procedure; however, unlike those authors, we included the use of “uninformative” and “informative (70 % valid)” response cues that appeared between prime- and probe-trial presentations.
When the uninformative response cue (i.e., the “?” symbol) appeared, it naturally signified that all possible probe responses were equiprobable, trials analogous to those used by Guy et al. (2006). The hope was to replicate their findings (RT[DRR] > RT[IR] > RT[Control]). Success would achieve two things. First, it would demonstrate in the same way that processing associated with the former prime distractor response on ignored-repetition trials is the exclusive cause of the visual SNP effect. Second, it would provide the context in which the informative response cue manipulation should reinforce this same conclusion, but, of course, in a different manner. For this to occur, we assume that when an informative response cue validly stipulates the prime distractor response for probe trial use, it results in the removal of this response’s ability to generate inhibitory aftereffects, including SNP (for support of this assumption, see Buckolz, Boulougouris, O’Donnell, & Pratt, 2002; Fitzgeorge & Buckolz, 2008).
With this assumption in mind, the critical trials arose when the informative response cue validly designated the many:1 subset response for use following a prime-trial distractor appearing in one of the two subset locations. In this instance, both IR and DRR trials occurred within the many:1 subset (see Schematic 1). If the valid response cue removes the inhibitory aftereffect otherwise produced by an uncued prime distractor response, and if this distractor response is the exclusive cause of the SNP effect (it is for a DRR trial), we should see the elimination of the IR and DRR trial type response delays. That is, the inhibitory aftereffects for these two trial types should be entirely eliminated, thereby providing additional support for the exclusivity view.
Additionally, if validly cuing the prime distractor response within the many:1 subset does remove its inhibitory aftereffect (i.e., RT [DRR] = RT [Control]), we can engage in needed discussion as to how aftereffect elimination is achieved (Fitzgeorge & Buckolz, 2008).
Method
Refer to Schematics 2 and 3 throughout this section. Four within-subjects factors were manipulated: probe trial configuration (target + distractor, target-only), cue type (informative [valid, invalid] or uninformative), location-to-response mapping (many:1 [M:1] vs. one-to-one [1:1]), and trial type (IR, DRR, control).
An illustration of the prime-probe trial pair sequence. Target (T) = white rectangle, Distractor (D) = black rectangle. Prime trials always contained T + D events (panel 3), while a target + distractor or a target-only could occur on a probe trial (panel 7; here, an ignored-repetition [IR] trial is illustrated)
Ignored-repetition (IR), distractor-response repeat (DRR), and control trial types are illustrated within the many:1 location-to-response mapping subset following valid (panel 1, #3), uninformative (panel 2, ?), and invalid (panel 3, # 2) response cues, for target + distractor (T + D) and target-only (T-only) probe trials. NA = not applicable
Participants
Twenty-two undergraduate students (12 male, 10 female), ranging in age from 19 to 21 years and having normal or corrected-to-normal vision, served as participants.
Apparatus
The input display was presented in a dimly lit room on a 47.3-cm computer screen situated on a tabletop located 73.5 cm above the floor and 196 cm directly in front of the participant. The display contained a fixation cross that appeared in the center of the screen, accompanied on each side by two horizontally arranged bar markers that specified the possible locations for the target and the to-be-ignored distractor stimuli. For ease of exposition, these locations are designated L1–L4, going left to right. The fixation cross and bar markers were white and appeared against a black background. They measured 0.9 cm in width and were separated from each other by a distance of 0.8 cm, generating a horizontal L1–L4 display of about 7.9 cm, or 2.2°. The target (green) and distractor (red) rectangles measured 0.9 cm wide and 1.9 cm high.
Participants sat with their forearms placed on a desktop, with the third digit and index finger of their left hand resting on keyboard buttons “D” and “V,” mapped onto locations L1 and L2, respectively. The right-hand index finger rested upon keyboard button “M” (i.e., the many:1 subset). It was assigned to L3 and L4. Correct responding required pressing the keyboard button assigned to the target-occupied location.
The on-screen stick figure assisted with the discrimination of occupied bar marker positions at the long viewing distance (Fitzgeorge et al., 2011).
Procedure
Trials were presented in pairs, first the prime trial and then the probe. All trial pairs began with a 100-ms warning tone whose offset was immediately followed by the display configuration, which remained on the screen for the entire trial sequence. The prime trial events were delivered 200 ms after the configuration onset and always contained a target and a distractor event that remained on the screen for 157 ms. After a correct target response, one of four possible response cues (1, 2, 3, ?), lasting 400 ms, replaced the central fixation cross. Cues 1, 2, and 3 were informative (70 % validity), designating the likely probe-trial response (going left to right; e.g., panel 5: M buttonpress response cued). The “?” indicated that all responses were equiprobable (uninformative cue). The probe trial appeared 200 ms after cue cessation, containing a target + distractor (50 %) or a target alone. Once a probe target response was performed, the computer screen went blank, indicating the end of the trial pair and, concurrently, beginning a 1,500-ms intertrial interval. This interval ended with the onset of the warning tone and the beginning of the next trial pair sequence.
There were 12 different prime-trial configurations (4 target locations × 3 distractor locations). Each of the three informative number cues (1, 2, and 3) was paired with each prime-trial configuration on 52 trials so that cue validity was 70 %, generating 1,872 prime–probe trial pairs (3 [cues] × 52 [trials] × 12 [prime trials]). Added to this were 576 trial pairs produced by having the uninformative “?” cue follow each prime configuration 48 times (1 × 12 × 48), making for a total of 2,448 trial pairs. There were 16 different probe-trial configurations: 12 target-plus-distractor configurations (4 target locations × 3 distractor locations) plus 4 target-only configurations (a target at each of the four locations). Each prime-trial configuration was paired with each probe-trial configuration so that the number cues were valid 70 % of the time.
Of the 2,448 trial pairs, 1,632 were of the 1:1 mapping type (1,224 [t + d probe], 408 [t-only probe]), while 816 involved the M:1 mapping (576 [target-plus-distractor probe], 240 [target-only probe]). The latter 240 trial pairs provided the data for the analyses of the IR, DRR, and control trial types when they followed valid and uninformative cues. These findings addressed the main questions in this study. When a target and distractor occurred on both the prime and probe trials, a proper control trial was unavailable, and so these trials were not analyzed. The four possible buttonpress probe-trial responses were required with equal frequency following the “?” symbol, and, subsequent to an invalid response cue, the noncued responses were used equally often.
It may be worthwhile to highlight the fact that efforts to examine the prevention of inhibitory aftereffects in location-based tasks, including those in this study, require the use of target-plus-distractor (T + D) trials on 100 % of the primes on the prime trials (Fitzgeorge & Buckolz, 2008; Guy, Buckolz, & Fitzgeorge, 2007) and on at least 50 % of the probe trials (Buckolz, Boulougouris, & Khan, 2002; Fitzgeorge & Buckolz, 2008) administered. In the first instance, this is because aftereffect prevention requires the execution of one of the experimentally assigned responses at the time of prime-trial processing. In the second instance, this is because certain, or highly likely, target-only probe trials themselves motivate inhibitory aftereffect removal. This was unacceptable here because we wanted to examine the impact of valid response cues in this regard and, so, had to avoid this confounding influence. The upshot of the required use of the T + D trials for the reasons just given is that they unavoidably generate trials unnecessary for the examination of the issues at hand. For example, the T + D prime trials produced 816 target-repeat trials that were of no use in the assessment of inhibitory aftereffect prevention. Finally, the required T + D probe trials lacked appropriate control trials. This decision was taken because their production would have required approximately doubling the number of needed trial pairs and because the need for these control trials would arise only if valid response cues eliminated inhibitory aftereffects (i.e., we could test the “restore” SNP result found by Fitzgeorge & Buckolz, 2008). If the latter turns out to be the case, we can then run the larger study that includes control trials that permit the analyses of the T + D probe trials.
Participants naturally had the task demands fully explained to them (emphasizing the role of the response cues and their predictive validities) and were given about 20 practice trials. Once they understood the task, experimental testing began.
Results
Prime-trial distractor location aftereffects for the many:1 location-to-response mapping subset: Target-only probe trials
Informative valid cue versus uninformative cue trials
Two ANOVAs were calculated with trial type (IR, DRR, control) and cue type (valid, uninformative) serving as the main within-subjects factors, one using mean participant RTs, the other buttonpress error rates as dependent variables. The cell means for these analyses are found in Fig. 1 and Table 1 (descriptive statistics for the remaining target-only probe conditions are found in the Appendix).
For the latency data, both cue type, F(1, 21) = 12.47, p < .01, MSE = 2,087, and trial type, F(2, 42) = 4.26, p < .05, MSE = 897, produced significant main effects. Overall, RTs were shorter when the response cue was valid (IR: 389 ms), as opposed to uninformative (417 msec) and when control trial latencies (394 ms) were contrasted with those produced by the IR (404 msec) and DRR (412 ms) trial types. The IR and DRR trial latencies also differed reliably. More important is that these main effects were qualified by their significant interaction, F(2, 42) = 6.05, p < .01, MSE = 810.Footnote 1 The cell means for this interaction are plotted in Fig. 1. Related simple main effects and follow-up Newman–Keuls tests revealed that all pairwise comparisons among the levels of the trial type factor differed reliably when the response cue was uninformative; however, none of these contrasts reached significance when the probe response was validly cued. For the uninformative cue trials, a significant SNP effect was evident (RT[IR] > RT[Control] = 20 ms). More germane, RT(DRR) was significantly larger than RT(IR) (20 ms) and RT(Control) (40 ms).
Buttonpress error rates produced a significant trial type main effect, F(2, 42) = 4.52, p < .02, MSE = 105.84. While the trial type × cue type interaction was nonsignificant, F < 1, Newman–Keuls tests (p < .05) indicated that buttonpress error rates were significantly greater for ignored-repetition (14.8 %) than for control (6.3 %) trials (DRR [12.5 %] vs. control [7.8 %], nonsignificant), but only with the uninformative response cue trials (Guy et al., 2006). Of some note, equivalent error rate values were observed for the IR, DRR, and control trial types when the probe responses were validly cued. This coincides with the latency data showing that validly cued, distractor-related responses do not produce inhibitory aftereffects—in this case, not in error terms.
The foregoing analyses of the many:1 mapping data can be summarized as follows. First, when individuals are forced to execute a response that had been recently assigned to a distractor-occupied (prime-trial) location, its initiation occurs more slowly than that of a control response, and this delay occurs whether the probe target stipulating its use appears at a new location (i.e., DRR trial) or at the location formerly occupied by the probe distractor (i.e., IR trial). Notably, the delay caused by the production of an earlier distractor response is lessened significantly when the probe target arises at the prime-trial distractor position (i.e., RT[IR] < RT[DRR]). Second, when a former distractor response, common to both DRR and IR trial types, is validly forecast by a probe-trial response cue, all inhibitory aftereffects are removed.
Discussion
The Locus Question
First and foremost, two findings here, one a replication, the other new, reinforced the view that the SNP phenomenon as studied herein is caused exclusively by consequences resulting from the inhibition of the prime distractor response. In the first instance, we reproduced the results of Guy et al. (2006). Within our many:1 mapping subset, RT[DRR] > RT[IR] > RT[Control] following uninformative response cue trials (Fig. 1). These results showed that recently suppressed (prime) distractor responses produce their own inhibitory aftereffect delays (i.e., RT[DRR] > RT[Control]). These delays must then contribute to the slowing observed on IR trials (i.e., to SNP production) as well, since the execution of the prime distractor response is required on these occasions. Presumably, some form of ER results when a distractor response is inhibited, which then takes time to override or remove when these responses must be produced. In contrast, there was no evidence that the prime distractor location was inhibited during prime-trial processing (RT[DRR] > RT[IR]), as was originally thought (e.g., Connelly & Hasher, 1993). Placing the probe target at the former prime distractor location did not lengthen RT, relative to when it appeared at a new location (a significant facilitation occurred).
The new evidence here pointing to an exclusive response locus for the SNP effect in location-based tasks took the form of the absence of delays for the DRR and the IR trial types when their common response was validly forecast by the informative response cue (Fig. 1, many:1 subset). The idea here was that if the inhibitory aftereffect produced by the prime distractor response could be removed (i.e., step 1; RT[DRR] = RT[Control]), we should concurrently see the cue-induced elimination of the SNP effect (i.e., step 2; RT[IR] = RT[Control]), if this is the sole cause of the SNP phenomenon. This was the case. Naturally, this RT(IR) = RT(Control) result again indicates that prime distractor locations are not inhibited; otherwise, an SNP effect would still have been observed, even though distractor response aftereffects had been eliminated.
In sum, the SNP effect produced by visual, centrally delivered distractor events is generated solely by the consequences of the inhibition applied to the prime distractor response (i.e., a response locus). The indication here and elsewhere (Guy et al., 2006) that distractor-occupied locations are not, themselves, inhibited is somewhat counterintuitive. So it is reassuring that this same point was made by Klein, Christie, and Morris (2005), and later by Fitzgeorge and Buckolz (2009), for peripherally presented visual distractors, which produce the typical inhibition of return effect. Accordingly, the idea of an exclusive response locus for the visual SNP effect is less controversial.
It bears noting, though, that SNP effects generated using other modalities may not have a response locus. For example, Mayr, Hauke, and Buchner (2009) showed that auditory SNP production depended, at least in part, upon the sounds appearing at the same location on successive trials mismatching (a circumstance that does not seem to hold for visual SNP generation; e.g., Milliken, Tipper, Houghton, & Lupiáñez, 2000). Should future work verify, through more direct testing, that auditory SNP has no response locus component at all, it will be important to explain why prime distractor responses in such tasks are not inhibited or, if they are, why they do not produce inhibitory aftereffects. The latter finding could be especially intriguing, given our present view that inhibited manual responses “will” normally cause inhibitory aftereffects, unless they are prevented by task conditions such as valid informative response cues and/or by infrequent or absent probe-trial distractors (see, e.g., Fig. 1; Fitzgeorge & Buckolz, 2008). If inhibited responses in auditory SNP tasks simply do not generate inhibitory aftereffects (i.e., they are not generated and then prevented, as in the visual SNP case), it will be necessary to add constraints to the claim that inhibited manual responses in location-based tasks will produce inhibitory aftereffects.
There are, of course, a number of reasons for wanting to establish the correct cause of the visual SNP effect. One has to do with the insights gained regarding information processing. For example, it is now clear that we can expect that distractor-occupied locations will not be ignored as instructed but will be extensively (automatically) processed to the point of response activation, with subsequent response, but not location, inhibition producing its own inhibitory aftereffects, such as delaying target processing. Notably, we also have the ability to prevent such inhibitory aftereffects under certain conditions (details later). The other benefit is the accurate interpretation of visual SNP data. The exclusive response-end SNP cause allows us, for example, to interpret the stability of the visual SNP effect with aging (Lok, 2011) and with extensive practice in younger adults (Buckolz, Stoddart, & Edgar, 2012) as showing the persistence of response inhibition ability itself and of its aftereffects.
A cognitive account of inhibitory aftereffect production in location-based tasks
At this point, we set out a more detailed rendering as to how we believe inhibitory aftereffects are produced in location-based tasks such as the one utilized here. Where appropriate, we will use this account to further explain some of the present findings, as well as explain how some of our procedural manipulations function (e.g., informative response cues). Actually, two accounts, arbitrarily labeled “cognitive” and “neural,” are considered in an integrated fashion; specifically, the more extensive cognitive account is presented, with the neural account component identified when it is described. Despite this intermixed approach, explaining how each account differentially explains inhibitory aftereffect production and informative response cue operation is still possible.
When a visible distractor event appears on the prime trial, its identity (Fitzgeorge, 2009; Fitzgeorge & Buckolz, 2008) and location are determined, with the latter leading to the activation and subsequent inhibition of the location’s assigned response. Two things could then occur. The first defines the neural account component (which is incorporated within the cognitive view). The applied inhibition would produce a physiological inhibitory state, whereby the activation level of the inhibited response would be below the resting level of a control response (e.g., Houghton & Tipper, 1994). When the inhibited distractor response is required by the probe target while still in its inhibitory state (i.e., IR or DRR trials), it would take longer to reach the initiation criterion level than would be the case for an uninhibited control response—hence, the delays seen for the IR and DRR trials and their inhibitory aftereffects (SNP and RT[DRR] > RT[Control]). This constitutes the sum total of the neural account.
The second concurrent thing that occurs is that a representation of prime-trial distractor processing is stored and, when retrieved, is capable of generating inhibitory aftereffects (Fitzgeorge, 2009; Fitzgeorge & Buckolz, 2008). This storage feature is rejected by the neural account. However, if this feature is deemed correct, two different sources of SNP production would then exist (i.e., stored distractor representations vs. inhibitory state), and so their respective merits with regard to explaining inhibitory aftereffect dataFootnote 2 would have to be assessed, which we attempt to do here.
The idea that prime (distractor) processing representations are stored is not new (e.g., episodic retrieval theory; Neill, Valdes, Terry, & Gorfein, 1992; see also Tipper, 2001). It continues to be embraced, largely, if not exclusively, on the basis of identity negative priming results, both behavioral (e.g., Fox & deFockert, 1998; Neill, 1997, 2007) and electrophysiological (e.g., Gibbons & Stahl, 2008, 2010) in nature. However, evidence indicative of this representation storage has recently been reported for visual SNP tasks (Fitzgeorge, 2009; Fitzgeorge & Buckolz, 2008). In one of their conditions, for example, Fitzgeorge and Buckolz (2008) randomly intermixed distractor-absent (75 %) and distractor-present (25 %) probe trials. This manipulation eliminated the SNP effect, but only when the probe trial actually lacked a distractor event. When a probe distractor randomly appeared, SNP remained. This so-called “remove SNP (distractor absent)–restore SNP (distractor present)” pattern (Fitzgeorge & Buckolz, 2008) was replicated by Fitzgeorge, who added that it appeared only when the prime- and probe-trial distractor identities fully matched. Partial (either shape matched or color matched) or full identity mismatches did not “restore” SNP. Notably, this reliance of the “restore SNP” component on fully matched distractor identities is indicative of a retrieval role performed by the probe distractor (supporting the object-based retrieval route; Schematic 1B [3]).
Simply put, the remove SNP–restore SNP pattern as defined here reveals that the ingredients needed to produce the SNP effect are actually available even when SNP is not observed (i.e., distractor-absent probe trials). Presumably, this availability is in the form of a stored distractor-processing representation, which is able to produce inhibitory aftereffects when retrieved (i.e., distractor-present probe trial; “SNP-restore”). The latter ability would require that the stored representation of distractor processing include a record that the prime distractor response had been inhibited, thereby causing this response to resist execution (ER) when the distractor representation is retrieved. This ER property causes and is most clearly revealed by the selection bias against former prime distractor responses observed on free-choice probe trials, when it competes with a control response (Fitzgeorge et al., 2011; Lok, 2011); that is, ER steers choice away from the distractor output. ER would also explain the remaining location-based inhibitory aftereffects—namely, (1) delayed IR RT (ER requires override time) and (2) greater buttonpress error rates on IR than on control trials, when obtained (ER opposes the use of the probe target response on IR trials). Just incidentally, there may be a fourth ER-induced inhibitory after effect in the form of protection against probe-trial response selection error, but evidence to this effect is just now being examined with some promise (Buckolz et al., 2012).
The remove SNP–restore SNP pattern has several other important implications, beyond indicating that prime distractor-processing representations are stored. First, the neural account cannot explain the remove SNP–restore SNP pattern.Footnote 3 With the neural view, the SNP prevention observed on unpredictable distractor-absent probe trials requires the dissipation of the physiological inhibitory state. Hence, it cannot then be available to produce SNP when the probe trial randomly contains a distractor.
Second, theoretically, the remove SNP–restore SNP pattern cannot be observed when a physiological inhibitory state is in effect. This is because the inhibitory state would produce the SNP phenomenon whether the probe trial contained a distractor or not. Hence, the presence of a remove SNP–restore SNP pattern signals the dissipation of the physiological inhibitory state. It follows that the SNP-restore component reported by Fitzgeorge and Buckolz (2008) and Fitzgeorge (2009) was caused solely by a retrieved representation of prime-trial distractor processing. Accordingly, the approximate 1,500-ms stimulus onset asynchrony (SOA) used by Fitzgeorge and Buckolz (2008) indicates that the physiological inhibitory state dissipates by this time, unlike the stored distractor representation, which appears to last at least up to 5,000 ms (Buckolz et al., 2008).
Because our SOA was less than 1,500 ms (i.e., about 1,100 ms), we cannot confidently exclude a physiological inhibitory state as a contributor to our inhibitory aftereffect data on this basis. However, it may be helpful in this regard to recall that our prime trials required a manual response. If prime distractor response inhibition is more or less completed by the time the manual target response is executed, the question is how long the physiological inhibitory state persists beyond this response production, a point at which its utility dissipates? The postresponse SOA used here was 600 ms. If the longevity of the physiological inhibitory state is less than this, this state would not have affected our data. Bear in mind, too, that the mere presence of an inhibitory state does not mean that it caused observed inhibitory aftereffects, since the concurrent presence of retrieved prime distractor-processing representations also could have done so.
The third consequence of the remove SNP–restore SNP pattern is that we can say with some certainty that the prevention of inhibitory aftereffects must include blocking the retrieval of the prime distractor representations, a retrieval that would otherwise occur for all IR and DRR trials, for the life of the stored distractor representation. Consequently, the removal of inhibitory aftereffects achieved by our valid informative response cues (Fig. 1) reflects a blocking of distractor–response representation retrieval (Fitzgeorge & Buckolz, 2008). We can be somewhat more specific about this cue-induced retrieval block by considering the possible retrieval routes used to access stored distractor representations.
In addition to the object-based (probe distractor) route identified above (Schematic 4 [3]), Fitzgeorge and Buckolz (2008) posited the existence of a location-based route (Schematic 4 [1]) to account for the presence of SNP effects when probe trials lack a distractor (e.g., Guy, Buckolz, & Pratt, 2004). They proposed that the location-based retrieval route is triggered when the probe target appears at a former distractor-occupied location. Unlike the object-based route, it is blocked when inhibitory aftereffect prevention is motivated (i.e., directed forgetting; Anderson & Levy, 2007). Hence, a blocked location-based route would theoretically cause SNP removal on distractor-free probe trials and would cause SNP to be reinstated when the probe contains a distractor.
An illustration of the possible pathways used to retrieve stored prime distractor representations in a location-based task: [1] location-based, [2] response-based, [3] (distractor) object-based. d = distractor, t = target, A = activation, DRP = distractor response processing, DRR = distractor–response repeat, IR = ignored-repetition
A third possible retrieval route needs to be considered, for two reasons. One is that the present results considered earlier indicated that the location-based route may be nonexistent (RT[DRR] = RT[IR]; Fig. 1, informative response cue). Second, the RT(DRR) > RT(Control) finding, seen with distractor-free probe trials within the M:1 trial subset (Fig. 1; Fitzgeorge & Buckolz, 2008), could not possibly achieve representation retrieval via the two routes already proposed. A third option is that access to prime-trial distractor-processing representations occurs when the probe target activates the former distractor response on DRR and IR probe trials, thus triggering a response-based retrieval route (Schematic 4, [2]). Notably, this same target-induced response activation would also result in the removal of a preexisting inhibitory state, should it be in force. When the probe target serves this dual function (up to some SOA), the cause of inhibitory aftereffect production would be confounded, following uninformative response cues.
Informative response cues
At this point, we can speculate that informative response cues assigned to prior distractor responses activate them in preparation for their expected use, causing the elimination of any preexisting inhibitory state while, at the same time, blocking, rather than triggering, the “response-based” retrieval route. This would explain the absence of inhibitory aftereffects for both the DRR and IR trial types, when the response common to them both was validly cued (i.e., many:1 subset; Fig. 1). Again, this result indicates that (1) visual SNP has an exclusive response locus and (2) there is no location-based retrieval route. The absence of a location-based route may reflect the fact that distractor-occupied locations are not inhibited and, so, have no access to stored distractor-processing representations. Hence, probe targets occupying such locations would not cause distractor representation retrieval, as our data show.
Finally, the faster reactions associated with valid (response) cues are not likely responsible for SNP prevention. Using a cue validity value similar to that employed here, Buckolz, Boulougouris, O’Donnell, and Pratt (2002) produced significant and large RT reductions, yet SNP size remained unaltered, a finding replicated by Fitzgeorge and Buckolz (2008). In both cases, the probe trial contained a distractor. So, the faster response production with validly cued (ignored-repetition) trials did not escape inhibitory aftereffects presumably caused by the distractor’s retrieval of prime-trial distractor representations.
Conclusions
According to the cognitive account, the data here indicate that the inhibitory aftereffects associated with distractor-occupied locations, including the visual SNP phenomenon, are produced solely by consequences arising from the inhibition of the response assigned to the distractor location and that these inhibitory aftereffects can be prevented (Fig. 1; Fitzgeorge & Buckolz, 2008). This prevention is achieved, at least in part, by blocking the retrieval of prime distractor-processing representations. Finally, the idea that inhibitory aftereffects in visual, location-based tasks are generated solely by a physiological inhibitory state arising from distractor response inhibition (i.e., the neural account) is questionable; it is inconsistent with some existing data (e.g., Buckolz et al., 2008; Fitzgeorge, 2009; Fitzgeorge & Buckolz, 2008), and, in any event, the impact of this inhibitory state on inhibitory aftereffect production is confounded by the normal presence of retrieved distractor representations.
Notes
The data pattern exhibited in Figure 1 is not restricted to the use of the preferred hand. We calculated an ANOVA using mean within-subjects RTs, with cue validity (valid, uninformative), trial type (IR, DRR, control), and preferred hand (left hand [n = 8], right hand [n = 14]) serving as the main factors. Most notably, the significant cue validity × trial type interaction, F(2, 52) = 3.82, p = .028, MSE = 856, did not interact with hand preference.
We are indebted to Friederike Schlaghecken for drawing our attention to the need to consider a neural account of inhibitory aftereffect production.
The neural model could account for the remove SNP–restore SNP pattern differently by suggesting that the probe distractor has an activation modulation rather than a retrieval role. Specifically, probe target activation level would be reduced when accompanied by a distractor, relative to when it appeared alone. In the latter case, target-induced response activation level would be sufficient to erase a preexisting inhibitory state on IR or DRR trials, and so no inhibitory aftereffect appears. The weaker target response activation level with distractor-present probe trials is less able to offset the existing inhibitory state, and so an inhibitory aftereffect is seen. This challenge to the retrieval interpretation of the distractor-free versus distractor-present effects following informative cues is readily set aside because such cues eliminate the inhibitory state. So, any interactive effects that probe distractor-free and distractor-present trials might have had with a preexisting inhibitory state would have been eliminated by the cue. In any event, there is no evidence that probe distractors lower target activation levels. For example, SNP sizes are the same for distractor-present and distractor-absent probes with uninformative cue conditions (e.g., Buckolz et al., 2002a; Neill et al., 1994). Furthermore, target activation reductions caused by a probe distractor should be independent of whether it matches or mismatches the prime distractor. This is not the case, however (Fitzgeorge, 2009). Hence, the retrieval account of the remove SNP–restore SNP pattern, together with its implications for inhibitory aftereffect production, remain viable.
References
Anderson, M., & Levy, B. J. (2007). Theoretical issues in inhibition: insights from research and human memory. In D. S. Gorfein & C. M. MacLeod (Eds.), Inhibition in Cognition (pp. 81–102). Washington, D. C: American Psychological Association.
Buckolz, E., Stoddard, A., & Edgar, C. (2012). Inhibitory after-effects in visual location-based tasks persist over practice and guard against response selection error on some probe trials (manuscript in preparation).
Buckolz, E., Avramidis, C., & Fitzgeorge, L. (2008). Prime-trial processing demand and their impact on distractor processing in a spatial negative priming task. Psychological Research, 72, 235–248.
Buckolz, E., Goldfarb, A., & Khan, M. (2004). The use of a distractor-assigned response slows later responding in a location negative priming task. Perception & Psychophysics, 66, 837–845.
Buckolz, E., Boulougouris, A., & Khan, M. (2002a). The influence of probe-trial selection requirements on the location negative priming effect. Canadian Journal of Experimental Psychology, 56, 274–283.
Buckolz, E., Boulougouris, A., O’Donnell, C., & Pratt, J. (2002b). Disengaging the negative priming mechanism in location tasks. European Journal of Cognitive Psychology, 14, 207–225.
Christie, J., & Klein, R. (2001). Negative priming for spatial location? Canadian Journal of Experimental Psychology, 55, 24–38.
Connelly, S. L., & Hasher, L. (1993). Aging and the inhibition of spatial location. Journal of Experimental Psychology. Human Perception and Performance, 19, 1238–1250.
Fitzgeorge, L. (2009). Cognitive Inhibition: Inhibitory After-effects. Ph.D. Thesis (Chapter 4), The University of Western Ontario, London, Canada.
Fitzgeorge, L., & Buckolz, E. (2009). Automatic versus volitional orienting and the production of the inhibition-of-return effect. Canadian Journal of Experimental Psychology, 63, 94–102.
Fitzgeorge, L., & Buckolz, E. (2008). Spatial negative priming modulation: The influence of probe-trial target cueing, distractor presence, and an intervening response. European Journal of Cognitive Psychology, 20(6), 994–1026.
Fitzgeorge, L., Buckolz, E., & Khan, M. (2011). Recently inhibited responses are avoided for both masked and nonmasked primes in a spatial negative priming task. Perception & Psychophysics. doi:10.3758/s13414-011-0125-7
Fox, E., & deFockert, W. (1998). Negative priming depends on prime-probe similarity: Evidence for episodic retrieval. Psychonomic Bulletin & Review, 5, 107–113.
Gibbons, H., & Stahl, J. (2010). Cognitive load reduces visual identity negative priming by disabling the retrieval of task-appropriate prime information: An ERP study. Brain Research, 1330, 101–113.
Gibbons, H., & Stahl, J. (2008). Early activity in the lateralized readiness potential suggest prime-response retrieval as a source of negative priming. Experimental Psychology, 55, 164–172.
Guy, S., Buckolz, E., & Fitzgeorge, L. (2007). Disengagement of the location negative priming effect: The influence of an intervening response. European Journal of Cognitive Psychology, 19, 789–812.
Guy, S., Buckolz, E., & Khan, M. (2006). The locus of repetition latency effects. Canadian Journal of Experimental Psychology, 60, 307–318.
Guy, S., Buckolz, E., & Pratt, J. (2004). The influence of distractor-only prime trials on the location negative priming mechanism. Experimental Psychology, 51, 4–14.
Houghton, G., & Tipper, S. P. (1994). A model of inhibitory mechanisms in selective attention. In D. Dagenbach & T. H. Carr (Eds.), Inhibitory processes in attention, memory, and language (pp. 53–112). San Diego: Academic Press.
Klein, R., Christie, J., & Morris, E. P. (2005). Vector averaging of inhibition of return. Psychonomic Bulletin & Review, 12, 295–300.
Lok, M. (2011). On the preservation of response inhibition and free choice behaviour in the elderly for location-based tasks. (Unpublished MA thesis). University of Western Ontario, London, Ontario, Canada.
Mayr, S., Hauke, R., & Buchner, A. (2009). Auditory location negative priming: A case for feature mismatch. Psychonomic Bulletin & Review, 16, 845–849.
Milliken, B., Tipper, S. P., Houghton, G., & Lupiáñez, J. (2000). Attending, ignoring, and repetition: On the relation between negative priming and inhibition of return. Perception & Psychophysics, 62, 1289–1296.
Neill, W. T. (1997). Episodic retrieval in negative priming: A replication. Journal of Experimental Psychology: Learning, Memory, and Cognition, 23, 1291–1305.
Neill, W. T. (2007). Mechanisms of transfer-inappropriate processing. In D. S. Gorfein & C. M. MacLeod (Eds.), Inhibition in Cognition (pp. 63–78). Washington D.C: American Psychological Association.
Neill, W. T., Valdes, L. A., & Terry, K. M. (1995). Selective attention and the inhibitory control of cognition. In F. N. Dempster & C. J. Brainerd (Eds.), Interference and inhibition in cognition (pp. 207–255). San Diego, CA: Academic Press.
Neill, W. T., Terry, K. M., & Valdes, L. A. (1994). Negative priming without probe selection. Psychonomic Bulletin and Review, 1, 119–121.
Neill, W. T., Valdes, L. A., Terry, K. M., & Gorfein, D. S. (1992). The persistence of negative priming: II. Evidence for episodic trace retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition, 27, 993–1000.
Tipper, S. P. (2001). Does negative priming reflect inhibitory mechanisms? A review and integration of conflicting views. The Quarterly Journal of Experimental Psychology, 54A(2), 321–343.
Tipper, S. P., Brehaut, J. C., & Driver, J. (1990). Selection of moving and static objects for the control of spatially directed action. Journal of Experimental Psychology. Human Perception and Performance, 16, 492–504.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Buckolz, E., Edgar, C., Kajaste, B. et al. Inhibited prime-trial distractor responses solely produce the visual spatial negative priming effect. Atten Percept Psychophys 74, 1632–1643 (2012). https://doi.org/10.3758/s13414-012-0366-0
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-012-0366-0