Abstract
We rely on our long-term memories to guide future behaviors, making it adaptive to prioritize the retention of goal-relevant, salient information in memory. In this review, we discuss findings from rodent and human research to demonstrate that active processes during post-encoding consolidation support the selective stabilization of recent experience into adaptive, long-term memories. Building upon literatures focused on dynamics at the cellular level, we highlight that consolidation also transforms memories at the systems level to support future goal-relevant behavior, resulting in more generalized memory traces in the brain and behavior. We synthesize previous literatures spanning animal research, human cognitive neuroscience, and cognitive psychology to propose an integrative framework for adaptive consolidation by which goal-relevant memoranda are “tagged” for subsequent consolidation, resulting in selective transformations to the structure of memories that support flexible, goal-relevant behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We rely on our ability to draw on memories from past experiences to adaptively guide interactions with the world. Yet, in everyday life, we encounter incoming sensory information in far greater detail than it is biologically plausible, or useful, to retain in long-term memory, raising the questions: Which memories are retained, and how are they translated into long-term memories? In this review, we draw on recent rodent and human research to suggest that the active processes involved in memory consolidation – particular systems-level consolidation –provide adaptive functions critical to forming flexible long-term memories.
Given the capacity limitations on memory, it is adaptive for memory systems to actively prioritize features from experiences that are goal-relevant and forget more mundane information (Hardt et al., 2013; Richards & Frankland, 2017). Selectively storing such significant information (such as outcomes and immediately preceding events) in memory increases the likelihood of appropriately avoiding threats or attaining positive outcomes when faced with similar scenarios in the future (Shohamy & Adcock, 2010). For example, imagine one day on your commute, rushing down the stairs to catch a departing train, you trip and fall (Fig. 1a). The next time you are running late, the ability to specifically recall the relevant incident on the stairs – rather than the types of cars you had passed blocks earlier – can help prevent another fall. We operationalize this bias towards the retention of goal-relevant features in long-term memory as adaptive memory. In an adaptive memory system, the goal relevance of given information can be signaled by multiple factors, including (1) the significance of the stimulus itself, such as highly arousing emotional events, (2) associations with a valenced outcome, such as neutral items paired with reward or threat, and (3) less intense, more idiosyncratic features that are related to a primary goal. Such a system therefore encompasses domains across neuroscience and psychology research, such as emotional memory, motivated memory, and fear conditioning, which each have long examined prioritization.
Schematic illustration of the selective prioritization and transformation of experience across consolidation. (a) All aspects of an experience that unfolds across time will not necessarily be remembered equally in memory. If a commute ends in falling down the subway steps, it would be adaptive to prioritize the retention of the goal-relevant details – in this case, the intrinsically salient fall on the steps – compared to the peripheral and less informative scenery from the preceding walk. (b) Memory consolidation also transforms memories, extracting and integrating across overlapping features from distinct but related experiences. Integration across distinct but related experiences of falling on the stairs will result in a memory trace that abstractly represents links between the overlapping features from the experiences (e.g., stairs, fall, and a common pair of sandals). Such an integrated trace can more flexibly generalize to novel, related experiences
However, our memories are not simply a static collection of individual snapshots of meaningful experiences, but rather represent integrated memories across experiences (McClelland et al., 1995; Moscovitch et al., 2016). Neural processes underlying consolidation thus need to transform as well as prioritize memory traces. For example, as shown in Fig. 1b, consolidation-dependent transformations will extract and integrate across shared features from distinct but related experiences of falling on stairs, resulting in an abstracted memory trace representing the overlapping central features (e.g., the fall, stairs, and a pair of shoes common to all the scenarios). In this way, integrating across overlapping features can also help uncover underlying patterns (Landmann et al., 2014), such as that running in a specific pair of sandals leads to falls on stairs, and facilitates more flexibly generalization to novel events. Therefore, in addition to prioritization, an adaptive memory system also necessitates mechanisms for transformation.
Memory consolidation, the process of stabilizing new memories for the long-term, involves mechanisms that can both prioritize and transform new experiences. In general, memory consolidation can refer to mechanisms at two different levels: cellular consolidation – changes in the structure of the synapse, and systems-level consolidation – changes in the distribution of memory representations across brain regions (Dudai et al., 2015; Frankland & Bontempi, 2005; Kandel et al., 2014). However, research on the adaptive nature of consolidation has generally overlooked the contributions of systems consolidation in favor of cellular consolidation-based explanations, focusing on local influences of experience-dependent changes in synaptic weights (Cahill & McGaugh, 1998; Lisman & Grace, 2005). Such explanations tend to be limited, accounting only for the prioritization of significant information such as intrinsically threatening items, without considering further transformations to the way memories are represented. At the same time, recent animal and human research has provided considerable emerging evidence demonstrating the neural and behavioral effects that systems consolidation has on the organization of long-term memories, although these studies primarily characterize neutral memories. The siloed nature of these literatures has therefore prevented the field from gaining a holistic understanding of how memory consolidation mechanisms adaptively prioritize and transform memories for the long term.
The goal of this review is to demonstrate how a synthesized account of memory consolidation mechanisms can advance our understanding about how memory consolidation acts as an adaptive process. We review behavioral evidence that consolidation prioritizes and transforms goal-relevant memories for long-term retention, and unpack evidence for the mechanisms that underlie these behavioral results. We particularly focus on how emerging research demonstrates the importance of systems-level consolidation mechanisms for adaptive memory. Finally, we draw on this prior work to propose an integrative framework by which goal-relevant memoranda are selectively “tagged” by cellular mechanisms and prioritized for subsequent systems consolidation, resulting in transformations to the structure of memories. As detailed later, we highlight how information can be “tagged” either through intrinsic value or through association with subsequent outcomes, and how such tags may bias subsequent cross-regional interactions to support the long-term retention of more abstracted representations. This framework therefore brings together these disparate literatures to expand the scope of our understanding about how novel information is adaptively retained through consolidation.
Behavioral evidence for adaptive consolidation: Prioritization
Selectivity is critical to adaptive memories, and historically much research has been focused on characterizing which memories are retained in long-term memory. In this section, we detail the behavioral evidence that consolidation leads to the prioritization of goal-relevant information, resulting in selective delay-dependent memory enhancements, and demonstrate that significance can be defined broadly depending on a given situation.
Emotional information is perhaps the clearest example of the type of goal-relevant experiences targeted by consolidation. Intrinsically emotional information, such as highly arousing images or words, is remembered better compared to neutral information (Dolcos et al., 2005; Ochsner, 2000; Ritchey et al., 2008; Sharot & Phelps, 2004; Sharot & Yonelinas, 2008). This emotional memory benefit seems to depend on processes that occur during consolidation, as memory performance diverges for emotional and neutral information most strongly after a delay (Kleinsmith & Kaplan, 1963; LaBar & Cabeza, 2006; Sharot & Phelps, 2004; Sharot & Yonelinas, 2008; Yonelinas & Ritchey, 2015), suggesting that consolidation selectively renders emotional stimuli more resistant to forgetting compared to neutral stimuli.
Interestingly, biases in memory do not extend to all aspects of an emotional experience; central details seem to be preferentially retained compared to the broader context, such as a neutral background scene (Payne & Kensinger, 2011; Payne et al., 2008; Sharot & Phelps, 2004; Yonelinas & Ritchey, 2015). This selectivity could also be adaptive, enhancing the components particular to the salient cue rather than peripheral, more uninformative information. Indeed, it has been theorized that arousal not only leads to enhanced memory for prioritized information, but also impairs memory for irrelevant information (Mather & Sutherland, 2011), thus reducing the risk of proactive and retroactive interference.
Similar selective memory benefits have also been reported for neutral information encoded in a heightened motivational state. An extrinsic goal state can shift the prioritization of otherwise mundane information, as it is also critical to encode the features that lead to reward attainment or avoidance of threats. In the reward domain, studies have shown that extrinsic reward motivation leads to superior delayed memory performance for neutral stimuli directly associated with high-value stimuli or encountered in a high-reward state (Adcock et al., 2006; Igloi et al., 2015; Murayama & Kitagami, 2014; Murayama & Kuhbandner, 2011; Murty et al., 2017; Nielson & Bryant, 2005; Wittmann et al., 2005). Mirroring these reports, motivation to avoid the threat of punishment, for example an aversive shock, also leads to selective delay-dependent memory enhancements (Bauch et al., 2014; Clewett et al., 2018; Dunsmoor et al., 2012; Murty et al., 2012; Schwarze et al., 2012). The prioritization of neutral information in long-term memory via the association with positive or negative events links the literatures on emotional enhancement of episodic memory with associative learning paradigms such as Pavlovian conditioning (Dunsmoor & Kroes, 2019). These findings also help mitigate the potential confounds that arise when considering intrinsically arousing stimuli, as memory benefits in these cases could be driven by stimulus specific factors, such as limited thematic content for emotional stimulus sets, irrespective of selective consolidation (Talmi, 2013).
Mechanisms for consolidation: Prioritization
Cellular consolidation was the first candidate mechanism put forward to explain how adaptive memories are selectively retained in long-term memory. This account was based on reports that neuromodulators such as dopamine – released in response to emotion, novelty, and reward – modulate synaptic plasticity in the hippocampus, which in turn can bias the persistence of these valuable events in long-term memory (for more detailed review, see Lisman & Grace, 2005; Shohamy & Adcock, 2010). The hippocampus is innervated by dopaminergic projections, particularly to the CA1 subfield, and studies have shown that manipulating dopamine receptor activity, through drug application or genetic knock out, can either enhance or block the induction of long-term potentiation (LTP; Bethus et al., 2010; Huang & Kandel, 1995; Lisman & Grace, 2005; Rossato et al., 2009). For example, one study found that exposure to novelty leads to the induction of LTP in a dopamine-dependent manner, such that a salient change in the environment could trigger the dopaminergic system to selectively strengthen synapses coding for those recent events in the hippocampus (Li et al., 2003). As such, the neuromodulatory enhancement of plasticity at specific synapses could facilitate the selective retention of meaningful information, whether it be intrinsically emotional or neutral information imbued with extrinsic motivational significance.
However, the relatively straightforward explanation for the prioritization of goal-relevant information provided by cellular consolidation is complicated by the fact that in everyday life we often learn contingences between a relevance cue and target stimulus indirectly, such that the significance of encountered stimuli may only become clear after an experience as further information is obtained. For example, if you are on a hike and see a section of flattened grass near the trail that you later learn was from a bear’s movements, you can retroactively re-assess the importance of the grass as a signal for potential danger. Prioritization in an adaptive memory system must also include information based on such indirect “tags” of goal-relevance.
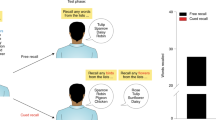
While theories of cellular consolidation cannot fully explain such indirect prioritization effects, recent work has provided evidence that the retroactive prioritization of information does depend on consolidation. In a behavioral study, Dunsmoor and colleagues (2015) found that exposure to Pavlovian category conditioning selectively enhanced memory for previously encoded neutral stimuli from the same category (but not directly associated with the shock). Critically, this memory enhancement was only observed when memory was tested after a delay and was not present immediately, suggesting a role of consolidation (Fig. 2). A recent study found that this retroactive memory effect is related to errors in source memory attribution, such that items learned during the pre-conditioning phase are misattributed to the salient conditioning context (Hennings et al., 2021). Further, using a similar paradigm but with reward, Patil, Murty, et al. (2017) showed that high-value reward information can retroactively enhance conceptually related, but not directly associated, information with a delay (Patil et al., 2017). The extent of this backwards “tag” may be modulated by the proximity to the salient signal, as work shows memory is more accurate for neutral events experienced closer to a reward outcome (Braun et al., 2018). In line with an adaptive memory system, in these studies only the information conceptually related to the goal-relevance signal (in this case, indirectly associated) is prioritized in later memory. In this way, consolidation does not only select memories to strengthen, but promotes the flexible generalization to preserve information related to a future meaningful event.
(a) Immediately after encoding, stimuli directly paired with the shock during conditioning (CS+, dark bars) were remembered better compared to CS- stimuli, but there was no difference in memory for the pre-conditioning phase CS- and CS+ categories (of note, these stimuli were never directly associated with the shock, but came from the same semantic/conceptual category). (b) After a 24-h delay, the pre-conditioning stimuli from the CS+ category were better remembered compared to those from the CS- category, suggesting that conceptually related exemplars were preserved in memory over a period of consolidation. Figure adapted from Dunsmoor et al. (2015)
What mechanisms can explain how consolidation facilitates the retroactive application of such “salience tags”? One model, known as synaptic tag-and-capture, proposed a means by which weak memories can be strengthened through subsequent activation (Frey & Morris, 1997, 1998). According to this model, weakly potentiated synapses representing information that would otherwise be forgotten can be upregulated if encoding is followed by a strong salience cue, which can evoke the downstream cellular cascades that lead to long-term potentiation of the synapses (Frey & Morris, 1998; Redondo & Morris, 2011). The behavioral instantiation of this model, known as “behavioral tagging,” likewise suggests that weakly formed hippocampal-dependent memories can be strengthened for long-term retention via exposure to a strong learning event around the time of the initial learning (Ballarini et al., 2009; Moncada & Viola, 2007). However, both models propose that memory benefits from a sufficiently strong subsequent cue would generalize to all weakly encoded information, which is at odds with both the selectivity expected in an adaptive memory system and the findings from Dunsmoor et al. (2015) and Patil, Murty, et al. (2017). We argue it is necessary to consider mechanisms beyond the cellular level, turning instead to the emerging literature on systems-level consolidation, to account for how consolidation selectively and flexibly facilitates the retention of goal-relevant information in memory.
Mechanisms of systems consolidation
Systems-level consolidation theories focus on the stabilization of memories through interactions between the hippocampus and areas of the neocortex (Alvarez & Squire, 1994; McClelland et al., 1995; Nadel & Moscovitch, 1997; Robin & Moscovitch, 2017; Winocur & Moscovitch, 2011). It is hypothesized that during offline periods following an experience – such as sleep – hippocampal and neocortical traces are reactivated, facilitating a dialogue between these regions that allows the cortex to extract the central tendencies, resulting in an abstracted memory trace that can be integrated into existing memory stores without interference (McClelland et al., 1995; Moscovitch et al., 2016). Such transformation is also adaptive, as integrating across episodes and building more generalized, abstracted memory traces could underlie schemas and heuristics and facilitate the extraction of information about causal relationships from recent experiences and help guide responses to related experiences in the future (Landmann et al., 2014; McClelland et al., 1995; Moscovitch et al., 2016; Robin & Moscovitch, 2017). To return to our subway example in Fig. 1b, with repeated instances of falling on the stairs, our memories can dynamically integrate features shared across these disparate experiences. Generating a more abstracted memory trace representing these central, shared features (e.g., stairs, the fall, and a common pair of sandals), rather than distinct traces, allows us to extrapolate patterns, and, critically, can facilitate generalization to new experiences in a more flexible manner. In other words, if confronted with a similar, but not identical, set of circumstances, such as a staircase in a new context, the abstracted memory can still be reactivated and applied to these novel circumstances to help prevent another fall.
However, models of systems consolidation are often agnostic to the prioritization of goal-relevant content. These theories tend to focus primarily on the mechanisms of the hippocampal-neocortical dialogue and resulting changes to the representational structure of memories, and do not make predictions for mechanisms in the selection of memories, nor the utility of such generalization. Therefore, a framework of consolidation that integrates across cellular and systems level mechanisms is better suited to capture how consolidation adaptively retains information. As we propose in more detail later, in an adaptive memory framework, the cross-regional interactions critical in systems consolidation provide a mechanism by which memories tagged as relevant could be both selectively strengthened and transformed.
Beyond prioritization: Adaptive transformation of memories
Emerging work has provided considerable evidence that memories are transformed with consolidation. Behavioral studies have shown that consolidation benefits the extrapolation of relationships between stimuli that were only indirectly associated (e.g., transitive inference) (Ellenbogen et al., 2007; Lau et al., 2011; Werchan & Gómez, 2013), as well as enhancements in memory for features shared across stimuli (Schapiro et al., 2017, 2018). Further, underlying statistical regularities or hidden rules can be uncovered or extracted with a delay (Batterink et al., 2014; Durrant et al., 2013; Nieuwenhuis et al., 2013; Wagner et al., 2004; for review, see Landmann et al., 2014; Rasch & Born, 2013; Walker & Stickgold, 2004). These data are in line with systems consolidation as an adaptive transformation process, as promoting shared structure and forming links across distinct experiences can emphasize patterns that will be beneficial for optimizing future related behaviors.
Indeed, much research has sought to understand the implications of retaining such abstracted memories on new learning. Prior knowledge, including existing schemas, has been shown to influence the subsequent encoding of novel stimuli (Bein et al., 2020; Craik & Tulving, 1975; DeWitt et al., 2012; Ghosh & Gilboa, 2014; Tompary & Thompson-Schill, 2021; Tse et al., 2007; van Kesteren et al., 2010). Reports also suggest memory for the abstracted structure of a prior learning experience can bias the way novel information is represented (Liu et al., 2019; Whittington et al., 2020), and that applying previously learned knowledge about task structure to a subsequent learning experience benefits the accuracy of later behaviors (Mark et al., 2020). These findings are perhaps in line with the idea that abstracted knowledge may be flexibly represented as a cognitive map, facilitating inferential reasoning, the generation of predictions, and future planning (Barron et al., 2020; Behrens et al., 2018; Biderman et al., 2020; Mark et al., 2020; Stachenfeld et al., 2017; Vikbladh et al., 2019). While more work is needed to fully understand the role of consolidation processes in such decision-making dynamics, this work underscores the adaptive utility of consolidation-dependent transformations to modulate future learning experiences.
Neural evidence for systems-level adaptive consolidation
All of the behavioral evidence noted thus far illustrates that memories are selectively prioritized and transformed through consolidation. In this section, we highlight neural evidence for the adaptive functions of systems consolidation that support a model by which goal-relevant memories are prioritized for subsequent transformation. When this work does not directly consider the selectivity of the effects, we summarize evidence of neural processes that drive the transformation of memories overall and discuss their application to adaptive memories.
Replay and neural evidence for adaptive systems consolidation
The hippocampal-cortical dialogue critical to systems-level consolidation theories is thought to be instantiated through the reactivation of memory traces during offline periods following encoding, such as sleep. The best mechanistic evidence for this process comes from rodent studies of “replay,” whereby patterns of hippocampal activity observed during encoding are reactivated in temporally compressed sequences during sharp wave-ripples, high-frequency oscillatory signals (Buzsáki, 1989; Girardeau & Zugaro, 2011; Joo & Frank, 2018; Skaggs & McNaughton, 1996; Wilson & McNaughton, 1994). Evidence for coordinated replay in the hippocampus and areas of the cortex, including prefrontal cortex and visual cortex (Ji & Wilson, 2007; Lansink et al., 2009; Peyrache et al., 2009; Wierzynski et al., 2009), suggest these reactivations could facilitate the cortical memory trace (Maingret et al., 2016), though more work is needed to directly link replay with the reorganization of memory traces.
In an adaptive memory system, biasing the content of replay could result in the selective stabilization and transformation of goal-relevant, or valuable, memories. In line with this prediction, rewarded information is more likely to be replayed than non-rewarded information (Lansink et al., 2009; Singer & Frank, 2009), as illustrated in Fig. 3. Likewise, it’s been shown that exposure to a rewarded associative learning task leads to increased activity of the replay-supporting sharp wave-ripple oscillations during subsequent sleep (Eschenko et al., 2008). Evidence suggests dopaminergic inputs enhances replay in the hippocampus (McNamara et al., 2014), suggesting neuromodulators may drive the prioritized reactivation of goal-relevant information.
Biased replay of rewarded experiences. (a) An example of two types of experiences, akin to typical replay rodent studies, only one of which ends in obtaining a reward (top). As animals traverse the environments, hippocampal place cells fire corresponding to given locations. (b) During subsequent periods of sleep, the pattern of place cell activity in the hippocampus is reactivated, in sequential order but temporally compressed, during sharp wave ripples (blue), with the content of replay biased toward the rewarded experience. The hierarchical coupling between sharp wave ripples, thalamocortical spindles (red), and slow oscillations (purple) can then coordinate communication between the hippocampus and neocortex during sleep and facilitate the reactivation in the cortex and the cortical memory trace
Replay activity is also modulated by reward during wakeful rest. Wake replay activity may reflect the relative magnitude of rewards available in the task (Ambrose et al., 2016). Interestingly, replay during wake, particularly following a reward outcome, tends to unfold in reverse order, presenting a potential mechanism to associate an outcome with its preceding events, thus solving the “credit assignment problem” (Ambrose et al., 2016; Foster & Wilson, 2006; Joo & Frank, 2018; Singer & Frank, 2009). Reverse replay may also play a role in inferential reasoning, providing a means to establish relationships between indirectly learned items (Barron et al., 2020). In contrast, forward replay during wakeful rest has been connected to future planning and decision-making processes (Diba & Buzsáki, 2007; Ólafsdóttir et al., 2018; Pfeiffer & Foster, 2013; Singer et al., 2013), and can also signal locations that were never visited (Gupta et al., 2010; Liu et al., 2019; Ólafsdóttir et al., 2015), suggesting a potential role in generalization of goal-relevant information into knowledge structures (for more in-depth reviews of wake replay, see Carr et al., 2011; Joo & Frank, 2018; Pfeiffer, 2020). While understanding the connection between replay during wake and sleep in service of consolidation still requires further research, this work highlights the dynamic means by which neural activity is modulated by the goal-relevance of the encoded material.
Sleep-dependent oscillations underlying systems-level adaptive consolidation
The coordinated replay of memories in the hippocampus and areas of the cortex is thought to be supported by the hippocampal-cortical dialogue achieved through the hierarchical temporal coupling between sharp wave ripples (80–100 Hz), which support replay events (Siapas & Wilson, 1998; Wilson & McNaughton, 1994), with more global slow oscillations (< 1 Hz) and thalamocortical sleep spindles (11–16 Hz) (Clemens et al., 2007; Mölle & Born, 2011; Rasch & Born, 2013; Siapas & Wilson, 1998; Sirota et al., 2003; Staresina et al., 2015; Steriade, 2006). Spindles in particular have been related to measures of the cross-regional dialogue, including hippocampal-cortical functional connectivity and the reactivation of memory traces (Andrade et al., 2011; Antony et al., 2018, 2019; Bergmann et al., 2012; Cairney et al., 2018; Cowan et al., 2020; Gais et al., 2007; Schabus et al., 2007; Schönauer et al., 2017). Spindle activity can induce LTP in neocortical synapses (Peyrache & Seibt, 2020; Rosanova, 2005; Steriade & Timofeev, 2003; Timofeev et al., 2002), suggesting that these oscillations help strengthen the cortical memory trace. Supporting the role of spindles in the stabilization of new memories, many studies have found the density of spindles during post-learning sleep is related to measures of subsequent memory performance, including retention across the sleep period, in humans (Clemens et al., 2005, 2006; Cox et al., 2012; Fenn & Hambrick, 2012; Gais et al., 2002; van der Helm et al., 2011; Hennies et al., 2016; Kurdziel et al., 2013; Schabus et al., 2004; Tamminen et al., 2010, 2013).
Sleep-dependent mechanisms seem to support the prioritization and transformation of goal-relevant information central to adaptive memory consolidation. Broadly, a period of sleep, but not an equal wake period, benefits the selective retention of emotional (Hu et al., 2006; Kim & Payne, 2020; Nishida et al., 2009; Payne & Kensinger, 2011; Payne et al., 2008; Sterpenich et al., 2009) and rewarded information (Fischer & Born, 2009; Igloi et al., 2015; Oudiette et al., 2013) in memory. Dopamine signaling may particularly support this selectivity, as pharmacologically enhancing dopamine activity can spread retention benefits to low-reward memories during sleep (Feld et al., 2014). Likewise, in line with sleep benefiting the prioritization of goal-relevant information, studies have shown specific benefits of sleep for the retention of task goals (Diekelmann et al., 2013; Scullin & McDaniel, 2010) and content determined to be relevant for future use ( van Dongen et al., 2012; van Rijn et al., 2017; Wilhelm et al., 2011; cf.: Reverberi et al., 2020; Wamsley et al., 2016). In particular, spindle activity seems to be sensitive to the goal relevance of the prior experience; reports indicate increases in the density of sleep spindles during post-encoding sleep after tasks that involve episodic learning (Gais et al., 2002; Schabus et al., 2004; Schmidt, 2006), and when newly learned information is explicitly instructed to be of future use (Wilhelm et al., 2011). It is possible that these oscillations may dynamically reflect the need to consolidate relevant learned information, and therefore act as a critical mechanism for adaptive consolidation. Spindle density during post-encoding sleep is also related to increased overlap in the representational pattern amongst recently learned information, suggesting a role in the organization of memory representations (Cowan et al., 2020). These effects may reflect the active mechanisms during sleep that promote adaptive functions of systems consolidation.
Sleep is also a period that may promote the adaptive forgetting of superfluous or redundant details accumulated throughout the day that do not necessitate preservation in long-term episodic memory. That is, sleep may be a period to cull memories that were not deemed significant throughout the day, either through direct experience or through temporal association with meaningful experiences (Hardt et al., 2013; Stickgold & Walker, 2013). Simultaneously, sleep may be the optimal time to extract generalized knowledge across waking experiences tagged with salience, to integrate information into existing frameworks (schemas), and to extract regularities to guide future goal-directed behavior to related cues and situations.
Coupling between the hippocampus and cortex as a mechanism for adaptive consolidation
In addition to research on replay and the coupling between oscillations, human neuroimaging research has provided evidence of a putative marker for the hippocampal-cortical dialogue critical to systems consolidation mechanisms. These formative studies report enhanced functional coupling between the hippocampus and areas of the cortex following a learning experience in a manner that predicts later memory performance – particularly with regions previously engaged during learning (Collins & Dickerson, 2018; de Voogd et al., 2016; Murty et al., 2017; Schlichting & Preston, 2014; Tambini et al., 2010; Tompary et al., 2015; Vilberg & Davachi, 2013), providing support for the idea that memories are stabilized through the cross-regional interactions of the hippocampus and cortex. Reports also have found increased hippocampal-cortical functional coupling after a period of sleep or short delay (Gais et al., 2007; Sterpenich et al., 2007; Vilberg & Davachi, 2013; but see Baran et al., 2016; Takashima et al., 2009), and one report found such functional coupling enhancements are related to the density of sleep spindles during the prior night of sleep, specifically for memories initially learned prior to the sleep period (Cowan et al., 2020). Together, this work provides further support for the importance of the hippocampal-cortical dialogue in facilitating systems consolidation.
Most critically, research on functional coupling changes suggest the hippocampal-cortical dialogue facilitates the retention of goal-relevant material. In a study using a reward-motivated encoding paradigm in which word-image pairs were either associated with a high- or low-reward cue depending on the category of the image, Murty et al. (2017) found that subsequent benefits for high-reward memory were related to increased post-encoding functional coupling between anterior hippocampus and a region of sensory cortex sensitive to the category associated with the reward (Fig. 4). Likewise, Gruber et al. (2016) found that memory benefits for reward-related information correlated with post-encoding dynamics, including enhancements in hippocampal functional coupling with reward-related regions, the VTA (ventral tegmental area) and substantia nigra, and greater reactivation of reward-related information. Similar effects have also been reported using a category conditioning task, with greater post-encoding reactivation of stimuli associated with a shock, and, again, enhanced hippocampal-cortical functional connectivity correlated with subsequent benefits for these stimuli (de Voogd et al., 2016). Together, this work suggests long-term adaptive memories may be due to the hippocampal-cortical dialogue that preferentially targets cortical regions specialized for the content of the goal-relevant information. Further, in line with consolidation as an adaptive process, the mechanisms underlying the prioritization and transformation aspects of consolidation are not fully separable and are part of a larger, cross-regional system.
Murty et al. (2017) demonstrated experience-dependent changes in hippocampal-cortical functional coupling following a motivated encoding task in which word-image pairs were either associated with a high or a low reward based on category (word-face, word-scene pairs). Functional coupling between anterior hippocampus and category selective cortex region associated with the high reward (top left) significantly correlated with subsequent benefits for high-reward memory (top right, green line) but not low-reward memory (gray line). Figure adapted from Murty et al. (2017)
Integration of memory traces
Thus far, we have demonstrated evidence for the neural processes underlying the hippocampal-cortical dialogue that seem to support subsequent memory, in line with systems-level cross-regional interactions providing a mechanism to slowly build up the cortical trace (McClelland et al., 1995; Moscovitch et al., 2016). However, beyond strengthening, the cortex is thought to represent regularities across experiences, integrating such abstracted traces into existing knowledge stores without interference, and facilitating generalization (McClelland et al., 1995). As detailed above, such a representation adaptively supports the extrapolation of meaning or relationships across distinct, but related, experiences. Neural evidence has provided support for the transformation of memories in cortex with consolidation, with a pattern emerging particularly implicating the ventromedial prefrontal cortex (vmPFC) in this process. The vmPFC has been associated with the development of integrated representations for related experiences over time (Richards et al., 2014; Tompary & Davachi, 2017), which may be related to sleep spindle density during overnight sleep (Cowan et al., 2020), as well as the formation and maintenance of schemas (Preston & Eichenbaum, 2013; Richards et al., 2014; Schlichting & Preston, 2015; Spalding et al., 2018; Tompary & Davachi, 2017; Tse et al., 2007; van Kesteren et al., 2013), and the retrieval of consolidated memories more broadly (Gais et al., 2007; Sterpenich et al., 2007, 2009; Takashima et al., 2006, 2007, 2009).
Given its role in flexible integration of memories, the vmPFC might also be expected to play a role in adaptive memory consolidation. While there has been limited research looking at the intersection of the prioritization and reorganization of goal-relevant neural traces with consolidation, research from decision-making has also shown that heuristic development and the formation of rule spaces rely on engagement of the vmPFC. This type of vmPFC-based knowledge representation may result from the transformation of learned information over time (Boorman et al., 2013; Wimmer et al., 2018). Critically, future work is needed to characterize the role of the vmPFC in adaptive memory consolidation more explicitly and to delineate how this region intersects with the mechanisms demonstrated across the hippocampus and other regions of the cortex. For example, the orbitofrontal cortex (OFC) has also been implicated in functions including the formation of schemas and representation of mental maps of task space (Boorman et al., 2016; Schuck et al., 2016; Wikenheiser & Schoenbaum, 2016; Wilson et al., 2014; Zhou et al., 2019, 2021), raising the possibility that this region, potentially together with vmPFC, facilitates adaptive behaviors with consolidation. As such, one hypothesis that warrants further testing is that regions of cortex support different types of generalization processes, such that the vmPFC may generalize across multiple features of an experience, while regions of sensory cortex generalize traces within specific modalities. In addition, future work must also consider consolidation-dependent transformations of memory traces within the hippocampus itself, a topic that remains the center of active debate (Moscovitch et al., 2016; Robin & Moscovitch, 2017; Sekeres et al., 2018).
An integrative framework for adaptive consolidation
Synthesizing this prior research, we propose a unified framework by which consolidation prioritizes and transforms goal-relevant memories, allowing us to remember information that will help optimize future outcomes, such as obtaining rewards or avoiding threats (Fig. 5). As in the tag-and-capture model, relevant encoded information is signaled by a “tag,” which can be assigned by two different routes depending on the nature of the stimuli: an encoding-based tag, for information directly paired with a salience cue such as intrinsically emotional stimuli, or a retroactive tag, derived from an experience signaling the relevance of otherwise neutral information initially encoded earlier (Frey & Morris, 1998; Redondo & Morris, 2011). In both cases, the salience tag will facilitate late-LTP cellular cascades within the hippocampus, leading to a selective, persistent potentiation of synapses related to the goal-relevant information (Dudai et al., 2015; Kandel et al., 2014).
Schematic illustrating the integrative framework of adaptive consolidation. In this framework, goal-relevant information (circles) can be signaled by a salience tag either during initial encoding (top left) or through a retroactive tag learned after encoding (bottom left). In both cases, the salience cue will facilitate selective strengthening of the synapses representing the goal-relevant information (red circles), while the neutral information (triangles) will be more likely to be forgotten. During subsequent replay these potentiated synapses are also more likely to be reactivated in the hippocampus, and – through the coupling between sleep oscillations – in areas of the neocortex, facilitating the transformation towards a more generalized or abstracted cortical trace
These tags can therefore serve as a first level of pruning, denoting what synapses should be maintained for subsequent systems-level consolidation processes. During offline periods following the encoding experience, such as sleep, the sequences reactivated in the hippocampus in sharp wave ripples during replay could then reflect the information held in these strengthened, potentiated synapses (Atherton et al., 2015). The hierarchical coupling of sharp wave ripples with thalamocortical spindles and slow oscillations (Clemens et al., 2007; Rasch & Born, 2013; Siapas and Wilson, 1998; Sirota et al., 2003; Staresina et al., 2015) then facilitates the coordinated reactivation of these traces in cortex, particularly in those cortical regions involved in encoding the relevant stimuli. Thus the hippocampal-cortical dialogue, and by proxy, measures of functional coupling, will be biased towards the regions representing the tagged stimuli (e.g., as in Murty et al., 2017). Since these cortical regions are thought to have slower learning rates and more overlapping representations (McClelland et al., 1995), as the information is repeatedly replayed in cortex, similar or related memory traces can be integrated, leading to a more generalized or abstracted cortical trace. These abstracted traces can support more flexible behaviors that rely on integration across memories or the extraction of rules, and can be used to guide future actions when presented with similar, but not necessarily identical, experiences in the future.
Critically, in this framework the relevance tags do not necessarily have to be affective in nature. In instances in which only neutral information is encoded, adaptive consolidation processes may simply be oriented by different definitions of “relevance.” For example, studies have implicated several factors as potential dimensions that can be selectively emphasized by consolidation processes including: weak memories requiring strengthening (Schapiro et al., 2017, 2018), the goals of the task (such as if knowledge is necessary for future tests), the context of the learning schedule, or error-driven feedback information. It is possible that these tags can function in much the same way, either defined in the moment during encoding or defined as new information is provided (e.g., via feedback at the end of a task). Again, through replay and the hippocampal-cortical dialogue, the cortical traces can become defined by their shared structure or overlap. However, future work will be needed to identify the boundary conditions on such processes, to better understand when labels of “significance” rise to the level of signaling goal-relevance.
In addition to integrating evidence, this framework generates several testable predictions for future work. Throughout this review we have demonstrated that adaptive consolidation mechanisms both prioritize and transform goal-relevant memories, however, little research has directly combined these two processes to test the selectivity of integration processes. Our framework suggests that, because replay will be biased towards goal-relevant information, the cortex will build up abstracted representations specific to that information. It will be necessary for future work to examine if information deemed significant, whether intrinsically or through associations with a goal-relevant outcome, is selectively represented in all neural markers of consolidation, including replay and cross-regional measures including functional connectivity, and in an abstract, integrated manner in cortex. In other words, our framework suggests that memories tagged as goal-relevant that are selectively retained are also those that undergo transformation, but little work has directly examined how such relevant versus non-relevant information is represented after consolidation. Interestingly, Clewett and Murty (2019) recently speculated that memory selectivity and integration are facilitated through separable mechanisms, driven by the noradrenergic and dopaminergic neuromodulatory systems, respectively. The authors suggest that the outcome in memory may be driven by the contextual details of the encoding experience that determines which neuromodulatory system is stimulated. The engagement of these different neuromodulatory systems may lead to qualitatively different forms of memory prioritization and integration with previous episodes, such that noradrenergic systems facilitate de-contextualization of salient information from other experiences, while dopamine facilitates integration with surrounding pieces of the concurrent environment and prior episodes. Future work will therefore be important to test how processes of selectivity and integration might differ depending on the stimulated neuromodulatory systems.
Relatedly, an intriguing question arising from this framework is how adaptive memories impact subsequent learning experiences. Prior work has shown that learned task structure can modulate later learning (Liu et al., 2019; Mark et al., 2020), but it is not clear if memory for otherwise mundane information is biased by reactivating existing memories of goal-relevant contexts. If in a prior experience given information is deemed “goal-relevant,” and thus undergoes adaptive consolidation processes, does reactivating this representation when encountering similar information in a context that this information is no longer goal relevant still facilitate selective retention of the novel information? The utility of understanding how we generalize priority to novel, related experiences will help our understanding of the adaptive nature of long-term memory.
The framework proposed here draws on previously proposed theories concerning the adaptive functions of memory. In particular, the memory modulation hypothesis (Cahill & McGaugh, 1998; McGaugh, 2015; Roozendaal & McGaugh, 2011) and Arousal Biased Competition model (Mather & Sutherland, 2011) provide a foundation for discussions of the prioritization of salient information and the neuromodulatory systems that facilitate the retention of such information with consolidation. However, while these models tend to focus on emotionally arousing memoranda, we summarize similar effects across domains, including reward and threat-based conditioning, and extend these findings into the domain of memory integration and generalization. Thus, our model incorporates the findings from these prior models into a more generalizable framework. Similarly, our proposed framework has antecedents in the sleep triage model, which illustrates sleep’s role in the selective retention and integration of new information (Stickgold & Walker, 2013). However, our proposed integrative framework for adaptive consolidation links these theories with more recent advances in systems consolidation research, providing evidence for a more holistic view of the mechanisms by which goal-relevant information is prioritized for subsequent replay and cross-regional systems consolidation processes.
Additionally, our integrative framework for adaptive consolidation complements recent models focused on mechanisms of memory-guided decision making. These models describe the hippocampal functions that utilize existing memories to form predictions, and the importance of reactivating relevant memories in future planning (Biderman et al., 2020; Bulley & Schacter, 2020), and therefore are focused on the consequences of the adaptive processes by which consolidation facilitates the retention of goal-relevant information of interest in the current review. Likewise, the focus of our model of adaptive memory is distinct from, but complementary to, the constructive episodic simulation hypothesis, which posits that existing episodic memories are recombined to adaptively facilitate future thoughts and simulations (Bulley & Schacter, 2020; Schacter & Addis, 2007; Schacter et al., 2007). We do not see these as inconsistent ideas, but rather as models focusing on distinct aspects of episodic memory: from the formation of long-term memory representations to the use of these abstract representations. Situating our framework within the landscape of existing theories of adaptive memory functions therefore begins to provide a larger picture of how aspects of experience are retained, transformed, and ultimately utilized.
The explanatory power of the integrative adaptive memory framework proposed here is that it reconciles the converging evidence from fields that have tended to be siloed. Data from the affective domain demonstrating the flexibility of adaptive memory behaviorally, with selective enhancements for goal-relevant stimuli, have rarely been connected with the emerging literature on the consolidation-related changes in the structure of memories. When integrating recent research across these fields, it becomes clear that consolidation acts as an adaptive process, filtering the continuous, surplus of stimuli encountered in the world to selectively prioritize, strengthen, and transform relevant information into long-term memories.
Data availability
There are no data or experiments associated with this article; all results discussed are from publicly available published work.
References
Adcock, R.A., Thangavel, A., Whitfield-Gabrieli, S., Knutson, B., and Gabrieli, J.D.E. (2006). Reward-Motivated Learning: Mesolimbic Activation Precedes Memory Formation. Neuron 50, 507–517.
Alvarez, P., and Squire, L.R. (1994). Memory consolidation and the medial temporal lobe: a simple network model. Proceedings of the National Academy of Sciences of the United States of America 91, 7041–7045.
Ambrose, R.E., Pfeiffer, B.E., and Foster, D.J. (2016). Reverse Replay of Hippocampal Place Cells Is Uniquely Modulated by Changing Reward. Neuron 91, 1124–1136.
Andrade, K.C., Spoormaker, V.I., Dresler, M., Wehrle, R., Holsboer, F., Samann, P.G., and Czisch, M. (2011). Sleep Spindles and Hippocampal Functional Connectivity in Human NREM Sleep. The Journal of Neuroscience 31, 10331–10339.
Antony, J.W., Piloto, L., Wang, M., Pacheco, P., Norman, K.A., and Paller, K.A. (2018). Sleep Spindle Refractoriness Segregates Periods of Memory Reactivation. Current Biology 28, 1736-1743.e4.
Antony, J.W., Schönauer, M., Staresina, B.P., and Cairney, S.A. (2019). Sleep Spindles and Memory Reprocessing. Trends in Neurosciences 42, 1–3.
Atherton, L.A., Dupret, D., and Mellor, J.R. (2015). Memory trace replay: the shaping of memory consolidation by neuromodulation. Trends in Neurosciences 38, 560–570.
Ballarini, F., Moncada, D., Martinez, M.C., Alen, N., and Viola, H. (2009). Behavioral tagging is a general mechanism of long-term memory formation. Proceedings of the National Academy of Sciences of the United States of America 106, 14599–14604.
Baran, B., Mantua, J., and Spencer, R.M.C. (2016). Age-related Changes in the Sleep-dependent Reorganization of Declarative Memories. Journal of Cognitive Neuroscience 28, 792–802.
Barron, H.C., Reeve, H.M., Koolschijn, R.S., Perestenko, P. V., Shpektor, A., Nili, H., Rothaermel, R., Campo-Urriza, N., O’Reilly, J.X., Bannerman, D.M., et al. (2020). Neuronal Computation Underlying Inferential Reasoning in Humans and Mice. Cell 183, 228-243.e21.
Batterink, L.J., Oudiette, D., Reber, P.J., and Paller, K.A. (2014). Sleep facilitates learning a new linguistic rule. Neuropsychologia 65, 169–179.
Bauch, E.M., Rausch, V.H., and Bunzeck, N. (2014). Pain anticipation recruits the mesolimbic system and differentially modulates subsequent recognition memory. Human Brain Mapping 35, 4594–4606.
Behrens, T.E.J., Muller, T.H., Whittington, J.C.R., Mark, S., Baram, A.B., Stachenfeld, K.L., and Kurth-Nelson, Z. (2018). What Is a Cognitive Map? Organizing Knowledge for Flexible Behavior. Neuron 100, 490–509.
Bein, O., Reggev, N., and Maril, A. (2020). Prior knowledge promotes hippocampal separation but cortical assimilation in the left inferior frontal gyrus. Nature Communications 11, 4590.
Bergmann, T.O., Mölle, M., Diedrichs, J., Born, J., and Siebner, H.R. (2012). Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage 59, 2733–2742.
Bethus, I., Tse, D., and Morris, R.G.M. (2010). Dopamine and memory: Modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. The Journal of Neuroscience 30, 1610–1618.
Biderman, N., Bakkour, A., and Shohamy, D. (2020). What Are Memories For? The Hippocampus Bridges Past Experience with Future Decisions. Trends Cogn. Sci. 24, 542–556.
Boorman, E.D., Rushworth, M.F., and Behrens, T.E. (2013). Ventromedial prefrontal and anterior cingulate cortex adopt choice and default reference frames during sequential multi-alternative choice. The Journal of Neuroscience 33, 2242–2253.
Boorman, E.D., Rajendran, V.G., O’Reilly, J.X., and Behrens, T.E. (2016). Two Anatomically and Computationally Distinct Learning Signals Predict Changes to Stimulus-Outcome Associations in Hippocampus. Neuron 89, 1343–1354.
Braun, E.K., Wimmer, G.E., and Shohamy, D. (2018). Retroactive and graded prioritization of memory by reward. Nature Communications 9, 4886.
Bulley, A., and Schacter, D.L. (2020). Deliberating trade-offs with the future. Nature Human Behaviour 4, 238–247.
Buzsáki, G. (1989). Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience 31, 551–570.
Cahill, L., and McGaugh, J.L. (1998). Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences 21, 294–299.
Cairney, S.A., Guttesen, A. A. V., El Marj, N., and Staresina, B.P. (2018). Memory Consolidation Is Linked to Spindle-Mediated Information Processing during Sleep. Current Biology 28, 948-954.e4.
Carr, M.F., Jadhav, S.P., and Frank, L.M. (2011). Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nature Neuroscience 14, 147–153.
Clemens, Z., Fabó, D., and Halász, P. (2005). Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience 132, 529–535.
Clemens, Z., Fabó, D., and Halász, P. (2006). Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neuroscience Letters 403, 52–56.
Clemens, Z., Molle, M., Eross, L., Barsi, P., Halasz, P., and Born, J. (2007). Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain 130, 2868–2878.
Clewett, D., and Murty, V.P. (2019). Echoes of Emotions Past: How Neuromodulators Determine What We Recollect. Eneuro 6, ENEURO.0108-18.2019.
Clewett, D. V., Huang, R., Velasco, R., Lee, T.H., and Mather, M. (2018). Locus coeruleus activity strengthens prioritized memories under arousal. The Journal of Neuroscience 38, 1558–1574.
Collins, J.A., and Dickerson, B.C. (2018). Functional connectivity in category-selective brain networks after encoding predicts subsequent memory. Hippocampus 1–11.
Cowan, E., Liu, A., Henin, S., Kothare, S., Devinsky, O., and Davachi, L. (2020). Sleep Spindles Promote the Restructuring of Memory Representations in Ventromedial Prefrontal Cortex through Enhanced Hippocampal–Cortical Functional Connectivity. The Journal of Neuroscience 40, 1909–1919.
Cox, R., Hofman, W.F., and Talamini, L.M. (2012). Involvement of spindles in memory consolidation is slow wave sleep-specific. Learning & Memory 19, 264–267.
Craik, F.I.M., and Tulving, E. (1975). Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology. General 104, 268–294.
DeWitt, M.R., Knight, J.B., Hicks, J.L., and Ball, B.H. (2012). The effects of prior knowledge on the encoding of episodic contextual details. Psychonomic Bulletin & Review 19, 251–257.
Diba, K., and Buzsáki, G. (2007). Forward and reverse hippocampal place-cell sequences during ripples. Nature Neuroscience 10, 1241–1242.
Diekelmann, S., Wilhelm, I., Wagner, U., and Born, J. (2013). Sleep Improves Prospective Remembering by Facilitating Spontaneous-Associative Retrieval Processes. PLoS One 8, e77621.
Dolcos, F., LaBar, K.S., and Cabeza, R. (2005). Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences 102, 2626–2631.
van Dongen, E. V., Thielen, J.W., Takashima, A., Barth, M., and Fernández, G. (2012). Sleep supports selective retention of associative memories based on relevance for future utilization. PLoS One 7.
Dudai, Y., Karni, A., and Born, J. (2015). The Consolidation and Transformation of Memory. Neuron 88, 20–32.
Dunsmoor, J.E., and Kroes, M.C. (2019). Episodic memory and Pavlovian conditioning: ships passing in the night. Current Opinion in Behavioral Sciences 26, 32–39.
Dunsmoor, J.E., Martin, A., and LaBar, K.S. (2012). Role of conceptual knowledge in learning and retention of conditioned fear. Biological Psychology 89, 300–305.
Dunsmoor, J.E., Murty, V.P., Davachi, L., and Phelps, E.A. (2015). Emotional learning selectively and retroactively strengthens memories for related events. Nature 520, 345–348.
Durrant, S.J., Cairney, S.A., and Lewis, P.A. (2013). Overnight consolidation aids the transfer of statistical knowledge from the medial temporal lobe to the striatum. Cerebral Cortex 23, 2467–2478.
Ellenbogen, J.M., Hu, P.T., Payne, J.D., Titone, D., and Walker, M.P. (2007). Human relational memory requires time and sleep. Proceedings of the National Academy of Sciences 104, 7723–7728.
Eschenko, O., Ramadan, W., Molle, M., Born, J., and Sara, S.J. (2008). Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learning & Memory 15, 222–228.
Feld, G.B., Besedovsky, L., Kaida, K., Münte, T.F., and Born, J. (2014). Dopamine D2-like Receptor Activation Wipes Out Preferential Consolidation of High over Low Reward Memories during Human Sleep. Journal of Cognitive Neuroscience 26, 2310–2320.
Fenn, K.M., and Hambrick, D.Z. (2012). Individual differences in working memory capacity predict sleep-dependent memory consolidation. Journal of Experimental Psychology. General 141, 404–410.
Fischer, S., and Born, J. (2009). Anticipated Reward Enhances Offline Learning During Sleep. Journal of Experimental Psychology. Learning, Memory, and Cognition 35, 1586–1593.
Foster, D.J., and Wilson, M.A. (2006). Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683.
Frankland, P.W., and Bontempi, B. (2005). The organization of recent and remote memories. Nature Reviews. Neuroscience 6, 119–130.
Frey, U., and Morris, R.G. (1997). Synaptic tagging and long-term potentiation. Nature 385, 533–536.
Frey, U., and Morris, R.G.M. (1998). Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends in Neurosciences 21, 181–188.
Gais, S., Mölle, M., Helms, K., and Born, J. (2002). Learning-dependent increases in sleep spindle density. The Journal of Neuroscience 22, 6830–6834.
Gais, S., Albouy, G., Boly, M., Dang-Vu, T.T., Darsaud, A., Desseilles, M., Rauchs, G., Schabus, M., Sterpenich, V., Vandewalle, G., et al. (2007). Sleep transforms the cerebral trace of declarative memories. Proceedings of the National Academy of Sciences 104, 18778–18783.
Ghosh, V.E., and Gilboa, A. (2014). What is a memory schema? A historical perspective on current neuroscience literature. Neuropsychologia 53, 104–114.
Girardeau, G., and Zugaro, M. (2011). Hippocampal ripples and memory consolidation. Current Opinion in Neurobiology 21, 452–459.
Gruber, M.J., Ritchey, M., Wang, S.F., Doss, M.K., and Ranganath, C. (2016). Post-learning Hippocampal Dynamics Promote Preferential Retention of Rewarding Events. Neuron 89, 1110–1120.
Gupta, A.S., van der Meer, M.A.A., Touretzky, D.S., and Redish, A.D. (2010). Hippocampal Replay Is Not a Simple Function of Experience. Neuron 65, 695–705.
Hardt, O., Nader, K., and Nadel, L. (2013). Decay happens: The role of active forgetting in memory. Trends in Cognitive Sciences 17, 111–120.
van der Helm, E., Gujar, N., Nishida, M., and Walker, M.P. (2011). Sleep-Dependent Facilitation of Episodic Memory Details. PLoS One 6, e27421.
Hennies, N., Lambon Ralph, M.A., Kempkes, M., Cousins, J.N., and Lewis, P.A. (2016). Sleep Spindle Density Predicts the Effect of Prior Knowledge on Memory Consolidation. The Journal of Neuroscience 36, 3799–3810.
Hennings, A.C., Lewis-Peacock, J.A., and Dunsmoor, J.E. (2021). Emotional learning retroactively enhances item memory but distorts source attribution. Learning & Memory 28, 178–186.
Hu, P., Stylos-Allan, M., and Walker, M.P. (2006). Sleep Facilitates Consolidation of Emotional Declarative Memory. Psychological Science 17, 891–898.
Huang, Y.Y., and Kandel, E.R. (1995). D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proceedings of the National Academy of Sciences of the United States of America 92, 2446–2450.
Igloi, K., Gaggioni, G., Sterpenich, V., and Schwartz, S. (2015). A nap to recap or how reward regulates hippocampal-prefrontal memory networks during daytime sleep in humans. Elife 4.
Ji, D., and Wilson, M.A. (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neuroscience 10, 100–107.
Joo, H.R., and Frank, L.M. (2018). The hippocampal sharp wave–ripple in memory retrieval for immediate use and consolidation. Nature Reviews. Neuroscience 19, 744–757.
Kandel, E.R., Dudai, Y., and Mayford, M.R. (2014). The molecular and systems biology of memory. Cell 157, 163–186.
van Kesteren, M.T.R., Fernandez, G., Norris, D.G., and Hermans, E.J. (2010). Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proceedings of the National Academy of Sciences 107, 7550–7555.
van Kesteren, M.T.R., Beul, S.F., Takashima, A., Henson, R.N., Ruiter, D.J., and Fernández, G. (2013). Differential roles for medial prefrontal and medial temporal cortices in schema-dependent encoding: from congruent to incongruent. Neuropsychologia 51, 2352–2359.
Kim, S.Y., and Payne, J.D. (2020). Neural correlates of sleep, stress, and selective memory consolidation. Current Opinion in Behavioral Sciences 33, 57–64.
Kleinsmith, L.J., and Kaplan, S. (1963). Paired-associate learning as a function of arousal and interpolated interval. Journal of Experimental Psychology 65, 190–193.
Kurdziel, L., Duclos, K., and Spencer, R.M.C. (2013). Sleep spindles in midday naps enhance learning in preschool children. Proceedings of the National Academy of Sciences 110, 17267–17272.
LaBar, K.S., and Cabeza, R. (2006). Cognitive neuroscience of emotional memory. Nature Reviews. Neuroscience 7, 54–64.
Landmann, N., Kuhn, M., Piosczyk, H., Feige, B., Baglioni, C., Spiegelhalder, K., Frase, L., Riemann, D., Sterr, A., and Nissen, C. (2014). The reorganisation of memory during sleep. Sleep Medicine Reviews 18, 531–541.
Lansink, C.S., Goltstein, P.M., Lankelma, J. V., McNaughton, B.L., and Pennartz, C.M.A. (2009). Hippocampus Leads Ventral Striatum in Replay of Place-Reward Information. PLoS Biology 7, e1000173.
Lau, H., Alger, S.E., and Fishbein, W. (2011). Relational memory: A daytime nap facilitates the abstraction of general concepts. PLoS One 6, 27139.
Li, S., Cullen, W.K., Anwyl, R., and Rowan, M.J. (2003). Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nature Neuroscience 6, 526–531.
Lisman, J.E., and Grace, A.A. (2005). The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron 46, 703–713.
Liu, Y., Dolan, R.J., Kurth-Nelson, Z., and Behrens, T.E.J. (2019). Human Replay Spontaneously Reorganizes Experience. Cell 178, 640-652.e14.
Maingret, N., Girardeau, G., Todorova, R., Goutierre, M., and Zugaro, M. (2016). Hippocampo-cortical coupling mediates memory consolidation during sleep. Nature Neuroscience 19, 959–964.
Mark, S., Moran, R., Parr, T., Kennerley, S.W., and Behrens, T.E.J. (2020). Transferring structural knowledge across cognitive maps in humans and models. Nature Communications 11, 4783.
Mather, M., and Sutherland, M.R. (2011). Arousal-biased competition in perception and memory. Perspectives on Psychological Science 6, 114–133.
McClelland, J.L., McNaughton, B.L., and O’Reilly, R.C. (1995). Why There Are Complementary Learning Systems in the Hippocampus and Neo-cortex: Insights from the Successes and Failures of Connectionists Models of Learning and Memory. Psychological Review 102, 419–457.
McGaugh, J.L. (2015). Consolidating Memories. Annual Review of Psychology 66, 1–24.
McNamara, C.G., Tejero-Cantero, Á., Trouche, S., Campo-Urriza, N., and Dupret, D. (2014). Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nature Neuroscience 17, 1658–1660.
Mölle, M., and Born, J. (2011). Slow oscillations orchestrating fast oscillations and memory consolidation. In Progress in Brain Research, pp. 93–110.
Moncada, D., and Viola, H. (2007). Induction of long-term memory by exposure to novelty requires protein synthesis: Evidence for a behavioral tagging. The Journal of Neuroscience 27, 7476–7481.
Moscovitch, M., Cabeza, R., Winocur, G., and Nadel, L. (2016). Episodic Memory and Beyond: The Hippocampus and Neocortex in Transformation. Annual Review of Psychology 67, 105–134.
Murayama, K., and Kitagami, S. (2014). Consolidation power of extrinsic rewards: Reward cues enhance long-term memory for irrelevant past events. Journal of Experimental Psychology. General 143, 15–20.
Murayama, K., and Kuhbandner, C. (2011). Money enhances memory consolidation - But only for boring material. Cognition 119, 120–124.
Murty, V.P., LaBar, K.S., and Alison Adcock, R. (2012). Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. The Journal of Neuroscience 32, 8969–8976.
Murty, V.P., Tompary, A., Adcock, R.A., and Davachi, L. (2017). Selectivity in Postencoding Connectivity with High-Level Visual Cortex Is Associated with Reward-Motivated Memory. The Journal of Neuroscience 37, 537–545.
Nadel, L., and Moscovitch, M. (1997). Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology 7, 217–227.
Nielson, K.A., and Bryant, T. (2005). The effects of non-contingent extrinsic and intrinsic rewards on memory consolidation. Neurobiology of Learning and Memory 84, 42–48.
Nieuwenhuis, I.L.C., Folia, V., Forkstam, C., Jensen, O., and Petersson, K.M. (2013). Sleep Promotes the Extraction of Grammatical Rules. PLoS One 8.
Nishida, M., Pearsall, J., Buckner, R.L., and Walker, M.P. (2009). REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cerebral Cortex 19, 1158–1166.
Ochsner, K.N. (2000). Are affective events richly recollected or simply familiar? The experience and process of recognizing feelings past. Journal of Experimental Psychology. General 129, 242–261.
Ólafsdóttir, H.F., Barry, C., Saleem, A.B., Hassabis, D., and Spiers, H.J. (2015). Hippocampal place cells construct reward related sequences through unexplored space. Elife 4, e06063.
Ólafsdóttir, H.F., Bush, D., and Barry, C. (2018). The Role of Hippocampal Replay in Memory and Planning. Current Biology 28, R37–R50.
Oudiette, D., Antony, J.W., Creery, J.D., and Paller, K.A. (2013). The Role of Memory Reactivation during Wakefulness and Sleep in Determining Which Memories Endure. The Journal of Neuroscience 33, 6672–6678.
Patil, A., Murty, V.P., Dunsmoor, J.E., Phelps, E.A., and Davachi, L. (2017). Reward retroactively enhances memory consolidation for related items. Learning & Memory 24, 65–69.
Payne, J.D., and Kensinger, E.A. (2011). Sleep leads to changes in the emotional memory trace: Evidence from FMRI. Journal of Cognitive Neuroscience 23, 1285–1297.
Payne, J.D., Stickgold, R., Swanberg, K., and Kensinger, E.A. (2008). Sleep preferentially enhances memory for emotional components of scenes. Psychological Science 19, 781–788.
Peyrache, A., and Seibt, J. (2020). A mechanism for learning with sleep spindles. Philosophical Transactions of the Royal Society B Biology Science 375, 20190230.
Peyrache, A., Khamassi, M., Benchenane, K., Wiener, S.I., and Battaglia, F.P. (2009). Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nature Neuroscience 12, 919–926.
Pfeiffer, B.E. (2020). The content of hippocampal “replay.” Hippocampus 30, 6–18.
Pfeiffer, B.E., and Foster, D.J. (2013). Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–79.
Preston, A.R., and Eichenbaum, H. (2013). Interplay of Hippocampus and Prefrontal Cortex in Memory. Current Biology 23, R764–R773.
Rasch, B., and Born, J. (2013). About Sleep’s Role in Memory. Physiological Reviews 93, 681–766.
Redondo, R.L., and Morris, R.G.M. (2011). Making memories last: The synaptic tagging and capture hypothesis. Nature Reviews. Neuroscience 12, 17–30.
Reverberi, S., Kohn, N., and Fernández, G. (2020). No evidence for an effect of explicit relevance instruction on consolidation of associative memories. Neuropsychologia 143, 107491.
Richards, B.A., and Frankland, P.W. (2017). The Persistence and Transience of Memory. Neuron 94, 1071–1084.
Richards, B.A., Xia, F., Santoro, A., Husse, J., Woodin, M.A., Josselyn, S.A., and Frankland, P.W. (2014). Patterns across multiple memories are identified over time. Nature Neuroscience 17, 981–986.
van Rijn, E., Lucignoli, C., Izura, C., and Blagrove, M.T. (2017). Sleep-dependent memory consolidation is related to perceived value of learned material. Journal of Sleep Research 26, 302–308.
Ritchey, M., Dolcos, F., and Cabeza, R. (2008). Role of Amygdala Connectivity in the Persistence of Emotional Memories Over Time: An Event-Related fMRI Investigation. Cerebral Cortex 18, 2494–2504.
Robin, J., and Moscovitch, M. (2017). Details, gist and schema: hippocampal–neocortical interactions underlying recent and remote episodic and spatial memory. Current Opinion in Behavioral Sciences 17, 114–123.
Roozendaal, B., and McGaugh, J.L. (2011). Memory Modulation. Behavioral Neuroscience 125, 797–824.
Rosanova, M. (2005). Pattern-Specific Associative Long-Term Potentiation Induced by a Sleep Spindle-Related Spike Train. The Journal of Neuroscience 25, 9398–9405.
Rossato, J.I., Bevilaqua, L.R.M., Izquierdo, I., Medina, J.H., and Cammarota, M. (2009). Dopamine controls persistence of long-term memory storage. Science (80). 325, 1017–1020.
Schabus, M., Gruber, G., Parapatics, S., Sauter, C., Klösch, G., Anderer, P., Klimesch, W., Saletu, B., and Zeitlhofer, J. (2004). Sleep spindles and their significance for declarative memory consolidation. Sleep 27, 1479–1485.
Schabus, M., Dang-Vu, T.T., Albouy, G., Balteau, E., Boly, M., Carrier, J., Darsaud, A., Degueldre, C., Desseilles, M., Gais, S., et al. (2007). Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proceedings of the National Academy of Sciences 104, 13164–13169.
Schacter, D.L., and Addis, D.R. (2007). The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 362, 773–786.
Schacter, D.L., Addis, D.R., and Buckner, R.L. (2007). Remembering the past to imagine the future: The prospective brain. Nature Reviews. Neuroscience 8, 657–661.
Schapiro, A.C., McDevitt, E.A., Chen, L., Norman, K.A., Mednick, S.C., and Rogers, T.T. (2017). Sleep Benefits Memory for Semantic Category Structure While Preserving Exemplar-Specific Information. Scientific Reports 7, 14869.
Schapiro, A.C., McDevitt, E.A., Rogers, T.T., Mednick, S.C., and Norman, K.A. (2018). Human hippocampal replay during rest prioritizes weakly learned information and predicts memory performance. Nature Communications 9, 3920.
Schlichting, M.L., and Preston, A.R. (2014). Memory reactivation during rest supports upcoming learning of related content. Proceedings of the National Academy of Sciences 111, 15845–15850.
Schlichting, M.L., and Preston, A.R. (2015). Memory integration: neural mechanisms and implications for behavior. Current Opinion in Behavioral Sciences 1, 1–8.
Schmidt, C. (2006). Encoding Difficulty Promotes Postlearning Changes in Sleep Spindle Activity during Napping. The Journal of Neuroscience 26, 8976–8982.
Schönauer, M., Alizadeh, S., Jamalabadi, H., Abraham, A., Pawlizki, A., and Gais, S. (2017). Decoding material-specific memory reprocessing during sleep in humans. Nature Communications 8, 15404.
Schuck, N.W., Cai, M.B., Wilson, R.C., and Niv, Y. (2016). Human Orbitofrontal Cortex Represents a Cognitive Map of State Space. Neuron 91, 1402–1412.
Schwarze, U., Bingel, U., and Sommer, T. (2012). Event-related nociceptive arousal enhances memory consolidation for neutral scenes. The Journal of Neuroscience 32, 1481–1487.
Scullin, M.K., and McDaniel, M.A. (2010). Remembering to execute a goal: Sleep on it! Psychological Science 21, 1028–1035.
Sekeres, M.J., Winocur, G., and Moscovitch, M. (2018). The hippocampus and related neocortical structures in memory transformation. Neuroscience Letters 680, 39–53.
Sharot, T., and Phelps, E.A. (2004). How arousal modulates memory: Disentangling the effects of attention and retention. Cognitive, Affective, & Behavioral Neuroscience 4, 294–306.
Sharot, T., and Yonelinas, A.P. (2008). Differential time-dependent effects of emotion on recollective experience and memory for contextual information. Cognition 106, 538–547.
Shohamy, D., and Adcock, R.A. (2010). Dopamine and adaptive memory. Trends in Cognitive Sciences 14, 464–472.
Siapas, A.G., and Wilson, M.A. (1998). Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128.
Singer, A.C., and Frank, L.M. (2009). Rewarded Outcomes Enhance Reactivation of Experience in the Hippocampus. Neuron 64, 910–921.
Singer, A.C., Carr, M.F., Karlsson, M.P., and Frank, L.M. (2013). Hippocampal SWR Activity Predicts Correct Decisions during the Initial Learning of an Alternation Task. Neuron 77, 1163–1173.
Sirota, A., Csicsvari, J., Buhl, D., and Buzsaki, G. (2003). Communication between neocortex and hippocampus during sleep in rodents. Proceedings of the National Academy of Sciences 100, 2065–2069.
Skaggs, W.E., and McNaughton, B.L. (1996). Replay of Neuronal Firing Sequences in Rat Hippocampus During Sleep Following Spatial Experience. Science (80). 271, 1870–1873.
Spalding, K.N., Schlichting, M.L., Zeithamova, D., Preston, A.R., Tranel, D., Duff, M.C., and Warren, D.E. (2018). Ventromedial prefrontal cortex is necessary for normal associative inference and memory integration. The Journal of Neuroscience 38, 2501–2517.
Stachenfeld, K.L., Botvinick, M.M., and Gershman, S.J. (2017). The hippocampus as a predictive map. Nature Neuroscience 20, 1643–1653.
Staresina, B.P., Bergmann, T.O., Bonnefond, M., van der Meij, R., Jensen, O., Deuker, L., Elger, C.E., Axmacher, N., and Fell, J. (2015). Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nature Neuroscience 18, 1679–1686.
Steriade, M. (2006). Grouping of brain rhythms in corticothalamic systems. Neuroscience 137, 1087–1106.
Steriade, M., and Timofeev, I. (2003). Neuronal Plasticity in Thalamocortical Networks during Sleep and Waking Oscillations. Neuron 37, 563–576.
Sterpenich, V., Albouy, G., Boly, M., Vandewalle, G., Darsaud, A., Balteau, E., Dang-Vu, T.T., Desseilles, M., D’Argembeau, A., Gais, S., et al. (2007). Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biology 5, 2709–2722.
Sterpenich, V., Albouy, G., Darsaud, A., Schmidt, C., Vandewalle, G., Dang-Vu, T.T., Desseilles, M., Phillips, C., Degueldre, C., Balteau, E., et al. (2009). Sleep Promotes the Neural Reorganization of Remote Emotional Memory. The Journal of Neuroscience 29, 5143–5152.
Stickgold, R., and Walker, M.P. (2013). Sleep-dependent memory triage: Evolving generalization through selective processing. Nature Neuroscience 16, 139–145.
Takashima, A., Petersson, K.M., Rutters, F., Tendolkar, I., Jensen, O., Zwarts, M.J., McNaughton, B.L., and Fernández, G. (2006). Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proceedings of the National Academy of Sciences of the United States of America 103, 756–761.
Takashima, A., Nieuwenhuis, I.L.C., Rijpkema, M., Petersson, K.M., Jensen, O., and Fernández, G. (2007). Memory trace stabilization leads to large-scale changes in the retrieval network: a functional MRI study on associative memory. Learning & Memory 14, 472–479.
Takashima, A., Nieuwenhuis, I.L.C., Jensen, O., Talamini, L.M., Rijpkema, M., and Fernandez, G. (2009). Shift from Hippocampal to Neocortical Centered Retrieval Network with Consolidation. The Journal of Neuroscience 29, 10087–10093.
Talmi, D. (2013). Enhanced Emotional Memory: Cognitive and Neural Mechanisms. Current Directions in Psychological Science 22, 430–436.
Tambini, A., Ketz, N., and Davachi, L. (2010). Enhanced Brain Correlations during Rest Are Related to Memory for Recent Experiences. Neuron 65, 280–290.
Tamminen, J., Payne, J.D., Stickgold, R., Wamsley, E.J., and Gaskell, M.G. (2010). Sleep Spindle Activity is Associated with the Integration of New Memories and Existing Knowledge. The Journal of Neuroscience 30, 14356–14360.
Tamminen, J., Lambon Ralph, M.A., and Lewis, P.A. (2013). The Role of Sleep Spindles and Slow-Wave Activity in Integrating New Information in Semantic Memory. The Journal of Neuroscience 33, 15376–15381.
Timofeev, I., Grenier, F., Bazhenov, M., Houweling, A.R., Sejnowski, T.J., and Steriade, M. (2002). Short- and medium-term plasticity associated with augmenting responses in cortical slabs and spindles in intact cortex of cats in vivo. The Journal of Physiology 542, 583–598.
Tompary, A., and Davachi, L. (2017). Consolidation Promotes the Emergence of Representational Overlap in the Hippocampus and Medial Prefrontal Cortex. Neuron 96, 228-241.e5.
Tompary, A., and Thompson-Schill, S.L. (2021). Semantic influences on episodic memory distortions. Journal of Experimental Psychology. General
Tompary, A., Duncan, K., and Davachi, L. (2015). Consolidation of Associative and Item Memory Is Related to Post-Encoding Functional Connectivity between the Ventral Tegmental Area and Different Medial Temporal Lobe Subregions during an Unrelated Task. The Journal of Neuroscience 35, 7326–7331.
Tse, D., Langston, R.F., Kakeyama, M., Bethus, I., Spooner, P.A., Wood, E.R., Witter, M.P., and Morris, R.G.M. (2007). Schemas and Memory Consolidation. Science (80). 316, 76–82.
Vikbladh, O.M., Meager, M.R., King, J., Blackmon, K., Devinsky, O., Shohamy, D., Burgess, N., and Daw, N.D. (2019). Hippocampal Contributions to Model-Based Planning and Spatial Memory. Neuron 102, 683-693.e4.
Vilberg, K.L., and Davachi, L. (2013). Perirhinal-Hippocampal Connectivity during Reactivation Is a Marker for Object-Based Memory Consolidation. Neuron 79, 1232–1242.
de Voogd, L.D., Fernández, G., and Hermans, E.J. (2016). Awake reactivation of emotional memory traces through hippocampal-neocortical interactions. Neuroimage 134, 563–572.
Wagner, U., Gais, S., Haider, H., Verleger, R., and Born, J. (2004). Sleep inspires insight. Nature 427, 352–355.
Walker, M.P., and Stickgold, R. (2004). Sleep-Dependent Learning and Memory Consolidation. Neuron 44, 121–133.
Wamsley, E.J., Hamilton, K., Graveline, Y., Manceor, S., and Parr, E. (2016). Test expectation enhances memory consolidation across both sleep and wake. PLoS One 11.
Werchan, D.M., and Gómez, R.L. (2013). Generalizing memories over time: Sleep and reinforcement facilitate transitive inference. Neurobiology of Learning and Memory 100, 70–76.
Whittington, J.C.R., Muller, T.H., Mark, S., Chen, G., Barry, C., Burgess, N., and Behrens, T.E.J. (2020). The Tolman-Eichenbaum Machine: Unifying Space and Relational Memory through Generalization in the Hippocampal Formation. Cell 183, 1249-1263.e23.
Wierzynski, C.M., Lubenov, E. V., Gu, M., and Siapas, A.G. (2009). State-Dependent Spike-Timing Relationships between Hippocampal and Prefrontal Circuits during Sleep. Neuron 61, 587–596.
Wikenheiser, A.M., and Schoenbaum, G. (2016). Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nature Reviews. Neuroscience 17, 513–523.
Wilhelm, I., Diekelmann, S., Molzow, I., Ayoub, A., Molle, M., and Born, J. (2011). Sleep Selectively Enhances Memory Expected to Be of Future Relevance. The Journal of Neuroscience 31, 1563–1569.
Wilson, M., and McNaughton, B. (1994). Reactivation of hippocampal ensemble memories during sleep. Science (80). 265, 676–679.
Wilson, R.C., Takahashi, Y.K., Schoenbaum, G., and Niv, Y. (2014). Orbitofrontal Cortex as a Cognitive Map of Task Space. Neuron 81, 267–279.
Wimmer, G.E., Li, J.K., Gorgolewski, K.J., and Poldrack, R.A. (2018). Reward learning over weeks versus minutes increases the neural representation of value in the human brain. The Journal of Neuroscience 38, 7649–7666.
Winocur, G., and Moscovitch, M. (2011). Memory transformation and systems consolidation. Journal of the International Neuropsychological Society 17, 766–780.
Wittmann, B.C., Schott, B.H., Guderian, S., Frey, J.U., Heinze, H.J., and Düzel, E. (2005). Reward-related fMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron 45, 459–467.
Yonelinas, A.P., and Ritchey, M. (2015). The slow forgetting of emotional episodic memories: An emotional binding account. Trends in Cognitive Sciences 19, 259–267.
Zhou, J., Montesinos-Cartagena, M., Wikenheiser, A.M., Gardner, M.P.H., Niv, Y., and Schoenbaum, G. (2019). Complementary Task Structure Representations in Hippocampus and Orbitofrontal Cortex during an Odor Sequence Task. Current Biology 29, 3402-3409.e3.
Zhou, J., Jia, C., Montesinos-Cartagena, M., Gardner, M.P.H., Zong, W., and Schoenbaum, G. (2021). Evolving schema representations in orbitofrontal ensembles during learning. Nature 590, 606–611.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cowan, E.T., Schapiro, A.C., Dunsmoor, J.E. et al. Memory consolidation as an adaptive process. Psychon Bull Rev 28, 1796–1810 (2021). https://doi.org/10.3758/s13423-021-01978-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-021-01978-x