Published online Nov 14, 2013. doi: 10.3748/wjg.v19.i42.7267
Revised: September 24, 2013
Accepted: October 19, 2013
Published online: November 14, 2013
The pancreas is a major player in nutrient digestion. In chronic pancreatitis both exocrine and endocrine insufficiency may develop leading to malnutrition over time. Maldigestion is often a late complication of chronic pancreatic and depends on the severity of the underlying disease. The severity of malnutrition is correlated with two major factors: (1) malabsorption and depletion of nutrients (e.g., alcoholism and pain) causes impaired nutritional status; and (2) increased metabolic activity due to the severity of the disease. Nutritional deficiencies negatively affect outcome if they are not treated. Nutritional assessment and the clinical severity of the disease are important for planning any nutritional intervention. Good nutritional practice includes screening to identify patients at risk, followed by a thoroughly nutritional assessment and nutrition plan for risk patients. Treatment should be multidisciplinary and the mainstay of treatment is abstinence from alcohol, pain treatment, dietary modifications and pancreatic enzyme supplementation. To achieve energy-end protein requirements, oral supplementation might be beneficial. Enteral nutrition may be used when patients do not have sufficient calorie intake as in pylero-duodenal-stenosis, inflammation or prior to surgery and can be necessary if weight loss continues. Parenteral nutrition is very seldom used in patients with chronic pancreatitis and should only be used in case of GI-tract obstruction or as a supplement to enteral nutrition.
Core tip: The pancreas is a major player in nutrient digestion and malnutrition is frequently found but is often neglected. The severity of malnutrition is correlated with malabsorption and depletion of nutrients (e.g., alcoholism and pain) that causes impaired nutritional status and increased metabolic activity due to the severity of the disease. Good nutritional practice includes screening to identify patients at nutritional risk, followed by a thoroughly nutritional assessment and nutrition plan for risk patients. Treatment should be multidisciplinary and the mainstay of treatment is abstinence from alcohol, pain treatment, dietary modifications and pancreatic enzyme supplementation.
- Citation: Rasmussen HH, Irtun &, Olesen SS, Drewes AM, Holst M. Nutrition in chronic pancreatitis. World J Gastroenterol 2013; 19(42): 7267-7275
- URL: https://www.wjgnet.com/1007-9327/full/v19/i42/7267.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i42.7267
Chronic pancreatitis (CP) is an inflammatory disorder that causes irreversible anatomical changes and damage, including infiltration of inflammatory cells, fibrosis and calcification of the pancreas with destruction of the glandular structure and thereby affects normal digestion and absorption of nutrients.
Maldigestion is often a late complication of CP and depends on the severity of the underlying disease. The medium latency between onset of first symptoms and signs of maldigestion is about 8-9 years in alcoholic CP and more than 15 years in idiopathic non-alcoholic pancreatitis. Nutrient deficiencies are common in CP, driven by many risk factors including malabsorption, diabetes and, in alcoholic CP, alcoholism. However, deficiencies are frequently overlooked, leading to malnutrition[1,2].
The aim of this article is to discuss the definition of malnutrition and good nutritional practice in patients with CP.
Generally about 20%-50% of all patients in hospital are found at risk of undernutrition, dependent on definition, clinical setting and screening tool amended. A large part of these patients are at nutritional risk when admitted to hospital and in the majority of these, undernutrition develops negatively during hospital stay[3,4].
This can be prevented if special attention is paid to nutritional care of patients. Routine identification is paramount as a first stage in a patient’s care in order to identify at-risk patients, with a view to providing nutrition support if necessary[5,6].
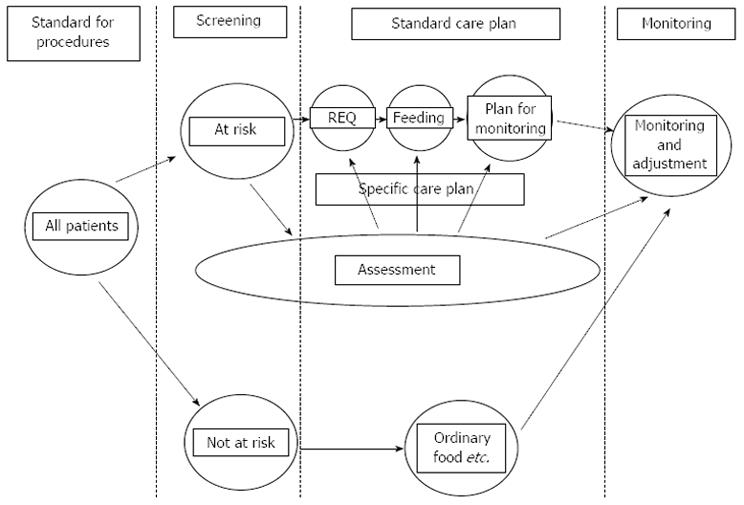
Good nutritional practice for the patient starts by nutrition screening. The European Society for Clinical Nutrition and Metabolism (ESPEN) Guidelines for Nutrition Screening recommend a continuity of issues to be considered in all patients admitted to hospital (Figure 1)[6]. (1) Initially on admission, a simple nutritional screening is to be done, to identify patients at actual nutritional risk; (2) Subsequently for patients at nutritional risk, a thorough nutritional assessment is to be completed; (3) This stage leads to an individual evaluation of nutritional requirements, and a plan for nutrition therapy and care; and (4) Monitoring and defining of targeted outcome should be structured in order to reconsider therapy and care-planning. Finally, communication around results of screening, assessment, plan and monitoring should be communicated to other health care professionals, when the patient is transferred, either back to the community or to another institution[7-9].
In order to give priority to nutritional intervention for relevant patients, nutrition screening methods have been developed and validated. These screening methods regard nutritional status and acute disease. However, there is still no clear consensus agreement on a uniform definition of undernutrition or agreement for a gold standard method of screening and identification of undernutrition[10]. This also applies for patients with CP and malnutrition which has been offered numerous definitions. Simply stated, malnutrition is a sub-optimal nutrient status appearing as a consequence of nutrient deficiencies, which change body composition and functional status. However, this definition neglects the numerous causes of malnutrition. An international guideline committee has recently proposed an aetiology-based approach that incorporates a current understanding of the inflammatory response, as seen in CP and many other patients. The committee proposed the following nomenclature for nutrition diagnosis in adults in the clinical practice setting: “Starvation-related malnutrition”, for chronic starvation without inflammation i.e., anorexia nervosa, “chronic disease-related malnutrition”, when inflammation is chronic and of mild to moderate degree i.e., CP, and “acute disease or injury-related malnutrition”, i.e., CP with a serious complication in the intensive ward, that is, when inflammation is acute and of severe degree[11,12].
The causes of malnutrition in patients are thus included in its definition. As such, screening tools, which neglect to include relevant disease related parameters such as chronic or acute inflammation, may be less efficacious in identifying malnutrition risk.
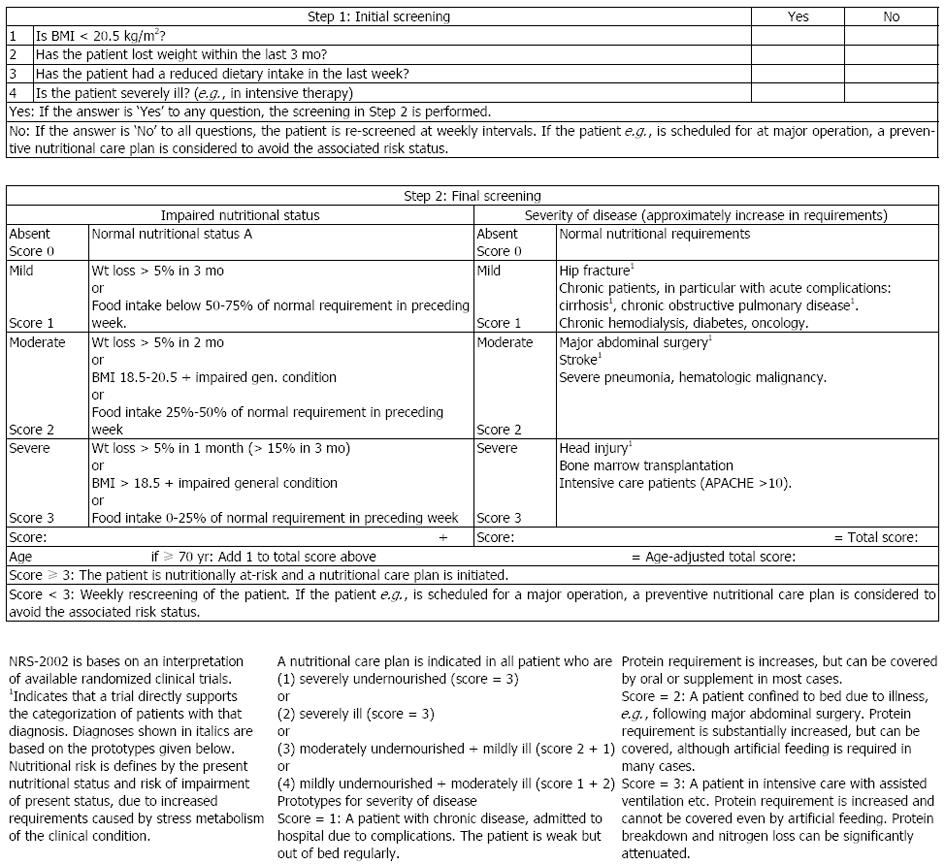
Nutritional screening should be a simple and rapid process, which can be carried out by busy admitting nursing and medical staff. Most screening tools address four basic questions: recent weight loss, recent food intake, current body mass index and disease severity or some other measure of predicting risk of malnutrition. In 2003, ESPEN published guidelines for nutrition screening in the community, in the hospital and among elderly in institutions. The Nutrition Risk Screening 2002 (NRS 2002) seems to be the best validated screening tool, in terms of predictive validity, i.e., that clinical outcome improves when patients identified to be at risk are treated, however the evidence is sparse[3,5,6,9].
For adult patients in hospital it is thus suggested to use the NRS 2002 (Figure 2)[9]. A score equal to or greater than 3 generates a nutrition plan in all cases. If the patient is at risk, but metabolic or functional problems prevent a standard plan being carried out, or if there is doubt as to whether the patient is at risk, a referral should be made to an expert or expert team for a more detailed assessment.
According to the definition, the severity of malnutrition is correlated with two major factors: depletion of nutrients (alcoholism and pain) and malabsorption causes impaired nutritional status and increased metabolic activity due to the inflammatory component of CP (severity of disease). Generally patients at nutritional risk have an increased number of complications and a poorer outcome, but specific studies investigating this issue in CP are however not available[2,13,14].
A persistent alcohol intake, pain after a meal and maldigestion are the main causes of weight loss, and weight loss is strongly associated with maldigestion of fat[1,14,15].
It is widely acknowledged that patients with CP are often undernourished; however few studies have assessed this. In a medical rehabilitation clinic setting, 32% had a BMI < 20 kg/m2; 57% chronic diarrhea and 24% steatorrhea[2].
Others have found that patients are malnourished prior to surgery and that the problem remains after surgery in a substantial proportion of patients with CP[16]. Furthermore, lean body mass and fat mass are found to be decreased both in patients with or without residual pancreatic function[17]. Decreased lean body mass may lead to decreased functional capacity as found in 34% of patients with moderate to severe weight loss. This also affected quality of life[18]. In this study fatigue, found in 46%, also had a major impact on quality of life. In a cohort of ambulatory patients with CP (60 patients), we found 28% at nutritional risk. These patients had a lower fat- and muscle mass and a tendency to lower handgrip strength. Furthermore, handgrip strength was associated with muscle- and fat mass[19].
It has been found that 30%-50% of patients with CP have increased resting energy expenditure[20]. We found no difference between estimated (Harris-Benedict) and measured resting energy expenditure (indirect calorimetric) in a cohort of ambulatory CP patients. However, when adjusted for fat free mass and height, we found higher resting energy expenditure (around 20%) in patients with low BMI (< 20 kg/m2)[19].
Daily intake of carbohydrates is about 300 grams corresponding to about half the caloric intake per day. About half of the caloric intake is carbohydrates and 30% are sucrose. Pancreatic alfa-amylase is the only enzyme for carbohydrate digestion that is produced by the pancreas. The final digestion of sugars take place at the brushborder of enterocytes where a range of disaccharidases produce the three sugars glucose, galactose and fructose that can be absorbed. In exocrine pancreatic insufficiency, carbohydrate digestion is maintained for a long time by salivary amylase and brush-border oligosaccharidoses. The loss of endocrine function leads to glucose intolerance in 40%-90% of patients with severe CP. Furthermore, in 20%-30% of patients an insulin-dependent diabetes develops associated with impaired glucagon and pancreatic polypeptide regulation (discussed in other article).
Daily protein intake in the Western world lies between 70 and 100 g and gastrointestinal secretions contain further 50-60 g of proteins per day. Protein digestion is initiated by intragastric proteolytic activity and continued by intestinal brush-border peptidases. Luminal proteolytic activity is maintained even in the absence of pancreatic peptidases and azotorrhea is therefore a very late and rare symptom in CP[21]. Trypsinogen is the inactive precursor for trypsin, which is the key enzyme for activation of all proteolytic enzymes in the duodenum. Trypsinogen becomes activated by enteropeptidase that is secreted by the brush-border of duodenal enterocytes. Proelastase is produced by the pancreas and belong to the chymotrypsin-like elastase family (elastase-2A, elastase-2B, elastase-3A, and elastase-3B). The elastase-3A isoform can be measured and quantified by the faecal elastase test. About 40% of proteins are digested to free amino acids. Brush border peptidases continue the digestion of peptides longer than three amino acids. Dipeptides, tripeptides, and free amino acids are then absorbed by the enterocytes by different transport mechanisms.
In Western diet up to 40% of the daily caloric intake derives from lipids, although 30% is recommended. Lipids are insoluble in water and need to be transferred to water-soluble micelles formed by bile acids, phospholipids, cholesterol and other products. This facilitates hydrolysis by lipase. In the stomach lingual lipase and gastric lipase hydrolyse triglycerides to glycerol and free fatty acids. Lipases from the gastric and salivary glands have a minor role in digestion of triglycerides and cannot compensate an insufficient pancreatic fat digestion. Thus, luminal lipid digestion within the small intestine depends on the action of pancreatic lipase and cofactors such as colipase and bile acids. There are no triglyceride-digestion enzyme systems within the intestinal brush-border membrane. Consequently, lipid digestion is decreased by insufficient lipase secretion and reduced luminal bile acid concentration. Because bicarbonate secretion is also diminished in CP and postprandial intraduodenal pH may fall < 4, luminal lipase degradation occurs more rapidly than that of other enzymes due to its greater instability. Gastric lipase only partly compensates for the lack of pancreatic lipase (discussed in other article).
A deficiency in vitamins A, D, E, K correlates with the severity of steatorrhoea in patients with CP, but may be caused by several different mechanisms including, suboptimal dietary intakes, increased losses, increased requirements, impaired binding of nutrients, antioxidant activity, and fat malabsorption. E vitamin deficiency may be seen more often than deficiencies of vitamin A, D, and K[22-24]. However, osteopathy (osteoporosis, osteomalacia, osteopenia) may occur in at least 25% of CP patients[22]. The adjusted hazard ratio (HR) for any fracture was 1.7 in patients with CP (95%CI: 1.6-1.8) in a recently Danish study[25]. Due to inadequate protease secretion by the pancreas, vitamin B12 deficiency can occur[26]. Zink deficiency may be seen especially in association with diabetes[27]. Also, deficiencies in calcium, magnesium, thiamine and folic acids have been reported. The functional meaning of such deficiencies are however undetermined and in our cohort of ambulatory patients with CP we did not find any correlation between micronutrient deficiencies and handgrip strength as measured by bioimpedance (unpublished data). On the other hand we recently demonstrated that the fibrotic changes as well as atrophy and ductal-related parameters were associated with exocrine insufficiency such as reflected in vitamin D deficiency, and future studies should explore this in further details[28].
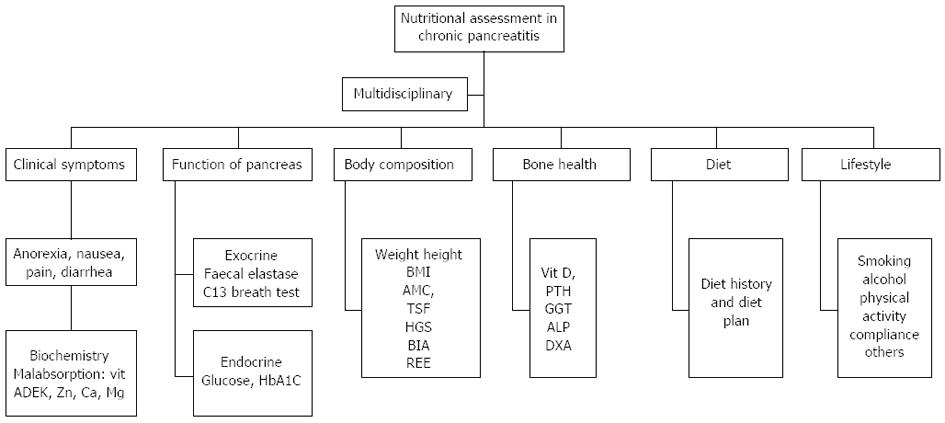
Assessment of nutrition status should include a multidisciplinary approach[14], including assessment of clinical symptoms, exocrine and endocrine pancreatic function, body composition, bone health, dietary evaluation and lifestyle as illustrated in Figure 3.
Clinical symptoms should include routine medical history and psychical examination with special emphasis on nutrition related symptoms and risk factors (nausea, anorexia, pain, alcohol, smoking). Micronutrient status should be measured 1-2 times a year including malabsorption of fat-soluble vitamins, minerals and trace elements. Function of pancreas concerning the exocrine- and endocrine part will not be discussed in this article.
Because both weight and BMI do not take body composition into account and may be misinterpreted as a result of fluid changes including ascites and edema, further investigation and assessment may be made with anthropometrics and bioimpedance measurements, both for baseline and follow-up (i.e., every 3-6 mo), because even patients with a normal BMI may have a decreased muscle mass that further might decrease function and lead to a higher morbidity, i.e., higher incidence of postoperative complications after surgery for CP[16].
Anthropometric measurements as mid-arm circumference estimating lean body mass and triceps skinfold estimating subcutaneous fat stores can be compared with age- and gender-specific centiles and are useful especially in patients with edema or ascites and as a long-term follow-up for nutritional status[14].
Bioimpedance is easy, non-invasive, and relatively inexpensive, and can be performed in almost any subject because it is portable. ESPEN guidelines[29-31], report results for fat-free mass body fat, body cell mass total body water, extracellular water and intracellular water from various studies in healthy and ill subjects. The data suggests that bioimpedance works well in healthy subjects and in patients with stable water and electrolytes balance with a validated bioimpedance equation that is appropriate with regard to age, sex and race. Clinical use of bioimpedance in subjects at extremes of BMI ranges or with abnormal hydration cannot be recommended for routine assessment of patients until further validation has proven for bioimpedance algorithm to be accurate in such conditions. Multi-frequency- and segmental-bioimpedance may have advantages over single-frequency bioimpedance in these conditions, but further validation is necessary. Longitudinal follow-up of body composition by bioimpedance is possible in subjects with BMI 16-34 kg/m2 without abnormal hydration, but must be interpreted with caution.
Since muscle function correlates closely with whole body protein, body cell mass, anthropometrically measured arm muscle mass, and even with BMI, loss of weight or muscle mass invariably results in decreased muscle strength which is reflected in deteriorating function tests as well as in prominently altered muscle morphology[32]. Reduced muscle strength is in turn associated with loss of physical functionality and with negative impact on recovery of health after illness or surgery, which partly explains the high predictive power of muscle function tests[2]. Hence, various studies have shown a close correlation between muscle strength and outcome in acute and chronic disease[32-34]. Just as measuring body composition offers a qualitative aspect of nutritional status, muscle function represents a dynamic indicator of muscle mass. Measurement of muscle function as indicator of functional as well as nutritional status has therefore gained considerable attention in the past years. Although hand grip strength correlates well with other muscle function tests such as knee extension strength or peak expiratory flow, it cannot be used as surrogate for muscle function of lower extremities when evaluating physical performance. Short term effects of nutritional therapy as e.g., refeeding of acute malnutrition are seen earlier by muscle function than by changes in body composition. Long-term nutritional therapy should result in both changes of body composition and muscle function, which should be paralleled by improvements of physical status[31,35].
Reference values for age- and gender-specific percentiles exist[36].
Resting energy expenditure (REE): Institution of appropriate nutritional therapy necessitates accurate determination of energy requirements. Data on measured REE in CP are very limited, but has shown, that weight loss is accompanied by hypermetabolism, and that between 30% and 50% of patients with CP have increased REE[20]. It may help us predict the energy level necessary to promote weight restoration and optimize nutritional rehabilitation preventing severe medical complications such as the refeeding syndrome[37]. Unfortunately, this technology is not available in the majority of the hospitals, because it requires skilled technicians and sophisticated methodologies that are costly and difficult to apply in standard clinical settings.
Predictive formulas of REE may be used as an alternative to indirect calorimetric that may be utilized by clinicians. The most cited and used predictive formula is the Harris-Benedict equation, which includes age, stature, and body weight to estimate REE[38]. However, studies are needed to validate predictive formulas vs measured resting energy expenditure by indirect calorimetric.
Bone health: Despite the reported high incidence of osteopathy in CP, no disease specific guideline exits for CP. However, extrapolating from guidelines for comparable malabsorptive diseases, calcium and vitamin D supplementation as well as regular monitoring of bone health should be an integral part of the nutrition management of CP, hence biochemistry and DXA scan should be performed regularly (i.e., once a year).
Diet: A detailed assessment of current and habitual dietary intakes should be made by a dietician for all patients at nutritional risk as indicated by the NRS 2002 screening tool. Nutrient intake may be measured by a 24-h recall interview or a diet history, and analyzed using specialized software providing detailed information about energy- and protein intake as well as fat and micronutrients intake. For evaluating specific food items a food frequency questionnaire can be used[39]. A specific diet plan should be made and follow-up visits evaluating intake compared to recommended energy- and protein intake should be employed according to clinical monitoring practice. It has been shown, that both dietary counseling and oral supplements for CP patients at nutritional risk can improve weight, BMI and decrease fecal fat excretion[40].
Lifestyle: To minimize nutritional risk factors in patients with CP, an effort should be made to investigate and eliminate possible individual barriers such as smoking and alcohol abuse. Furthermore, to assure patient compliance regarding medical treatment (i.e., treatment with enzymes) and to evaluate pain treatment (discussed in another article). Physical activity should be encouraged alongside nutritional therapy for optimal result[32-34].
Maldigestion of macronutrients is the major cause of progressive nutritional and metabolic impairment in patients with CP. Nutritional interventions depend on the degree of maldigestion and the nutritional status.
The main goals for nutritional interventions are to ensure sufficient macro- and micronutrients intake, to decrease maldigestion, malabsorption and other risk factors in order to prevent or treat malnutrition.
The treatment of exocrine deficiency begins with dietary recommendations and pancreatic enzyme supplementation. About 80% of patients can be managed by a combination of analgesics, dietary recommendations and pancreatic enzyme supplements, while 10%-15% need oral nutritional supplements, 5% need enteral tube feeding and around 1% require parenteral nutrition[21,41,42].
Dietary recommendations begin with total abstinence from alcohol. In addition, an adequate number of calories should be taken. Estimation of REE [or measurement in patients with a low BMI (< 20 kg/m2)] is essential in all patients to calculate the adequate caloric intake because of risk of increased resting energy expenditure. Frequent small meals (4-8 times a day) should be given. The carbohydrate intake might be limited when an overt diabetes mellitus is present (described in more detail in other article).
A protein diet of 1.0-1.5 g/kg body weight/d is generally sufficient and well tolerated. Usually, if 30%-40% of the calories are given as fat this is well tolerated, especially when the foods are rich in vegetable fats.
If weight gain is insufficient and/or steatorrhea persists, medium chain triglycerides (MCT) can be tried to increase fat absorption. MCT are absorbed directly across the small bowel into the portal vein, even in the absence of lipase, co-lipase and bile salts. However, MCTs have low energy density and unpalatable taste, and a maximum of about 50 g/d might be given. Higher doses may be ketogenic and are associated with side effects such as cramps, nausea and diarrhea. Fat soluble vitamins (A, D, E and K), vitamin B12 and other micronutrients should be supplemented if serum levels indicate deficiencies.
In general, a low fibre diet is recommended, because fibre may absorb enzymes and delay the absorption of nutrients. An adequate quantity of exogenous pancreatic enzymes is necessary to correct protein and lipid maldigestion[1,43-45]. In 10%-15% of patients oral supplements can help to attenuate weight loss and delay the use of enteral tube feeding[42,46].
The best clinical follow-up parameters for monitoring therapeutic success of dietary counseling are improvement of the patient’s general condition and weight gain.
The cause of inadequate caloric intake in CP can be anatomical (due to pyloro-duodenal-stenosis or cyst compression) or inflammatory with acute complications (new attack of acute pancreatitis or development of fistulas). Patients suffering from serious insufficient caloric intake may benefit by oral supplements or enteral nutrition. To test if enteral nutrition is tolerated and increases nutritional status it is recommended to give the nutrition via a naso-jejunal tube. However, for long-term therapy feeding (exceeding 2-3 wk) a percutaneous endoscopic gastrostomy with a jejunal tube extension is more convenient. Continuous overnight delivery of the nutrients is suitable and entails more easily the patient’s nutritional goal. From a theoretical point of view a semi-elemental diet can be recommended, but there are no studies showing improvement in the nutritional status compared to regular enteral nutritional formulas.
Owing to the fact that CP patients are frequently undernourished, nutritional support before pancreatic surgery may be beneficial. Data from patients undergoing general abdominal surgery have provided evidence that preoperative enteral or oral nutritional support improves outcome compared to undernourished patients by reducing postoperative morbidity and the length of hospital stay[47]. Thus, it should be emphasized that nutritional therapy should go alongside surgery, and that surgery for pain or any obstruction in the GI-channel should be a primary indication.
The potential to modulate the activity of the immune system by interventions with specific nutrients is termed immunonutrition. This concept is normally applied to any situation where nutritional formulas are supplemented with specific nutrients such as arginine, glutamine, omega-3 fatty acids, nucleotides and others. Meta-analysis of randomized controlled trials giving a combination of arginine and omega-3 fatty acids at home for 5-7 d before surgery reduced both postoperative infection rate and length of hospital stay in patients who underwent elective major GI surgery[48,49]. Specific trials in patients with chronic pancreatitis are not available. A recent RCT giving supplemental immunonutrition preoperatively in well-nourished and mal-nourished gastrointestinal surgery patients showed, that LOS and costs were reduced especially in mal-nourished patients[50].
Another recent study showed that enteral immunonutrition given post-operatively vs a standard enteral nutrition showed a reduction of infectious complications, anastomotic leak rate and LOS as well as an improved immunologic outcome[51].
We therefore highly recommend a week with preoperative immunonutrition to patients going to elective surgery for chronic pancreatitis. Early postoperative enteral nutrition is feasible and may additionally improve outcome after surgery.
Parenteral nutrition is infrequently used in patients with chronic pancreatitis. Enteral nutrition preserves immune function and mucosal architecture and decreases the possibility for hyperglycemia while parenteral nutrition also increases the risk of catheter infections and sepsis complications. Parenteral nutrition is therefore only indicated when it is impossible to use enteral nutrition[52]. This means if the patients do not reach their requirements because gastric emptying is blocked, the patient needs gastric decompression, it is impossible to introduce a tube into the jejunum, or a complicated fistula is present. Parenteral nutrition is mainly performed over a short term period, e.g., in apparent severe malnutrition prior to pancreatic surgery if enteral feeding is incomplete and may thus be used as a supplement to fulfill their requirements. There are no reported studies of patients with chronic pancreatic insufficiency who have been treated with parenteral nutrition for a long period.
Future studies evaluating a systematic approach for assessment and treatment of CP patients at nutritional risk should be made to further elucidate the nutritional course of these patients. Special emphasis should be on body composition, absorption of nutrients and metabolism, as well as measuring micronutrient deficiencies. Furthermore, nutritional interventions should be tested in randomized controlled trials with relevant clinical outcomes, i.e., morbidity, quality of life, physical function and mortality.
P- Reviewers: Keck T, Kochhar R S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Sikkens EC, Cahen DL, van Eijck C, Kuipers EJ, Bruno MJ. Patients with exocrine insufficiency due to chronic pancreatitis are undertreated: a Dutch national survey. Pancreatology. 2012;12:71-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Armbrecht U. [Chronic pancreatitis: weight loss and poor physical performance - experience from a specialized rehabilitation centre]. Rehabilitation (Stuttg). 2001;40:332-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 3. | Sorensen J, Kondrup J, Prokopowicz J, Schiesser M, Krähenbühl L, Meier R, Liberda M. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr. 2008;27:340-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 317] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 4. | Rasmussen HH, Kondrup J, Staun M, Ladefoged K, Kristensen H, Wengler A. Prevalence of patients at nutritional risk in Danish hospitals. Clin Nutr. 2004;23:1009-1015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Johansen N, Kondrup J, Plum LM, Bak L, Nørregaard P, Bunch E, Baernthsen H, Andersen JR, Larsen IH, Martinsen A. Effect of nutritional support on clinical outcome in patients at nutritional risk. Clin Nutr. 2004;23:539-550. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Kondrup J, Allison SP, Elia M, Vellas B, Plauth M. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22:415-421. [PubMed] [Cited in This Article: ] |
| 7. | Jie B, Jiang ZM, Nolan MT, Zhu SN, Yu K, Kondrup J. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition. 2012;28:1022-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 8. | Weekes CE, Spiro A, Baldwin C, Whelan K, Thomas JE, Parkin D, Emery PW. A review of the evidence for the impact of improving nutritional care on nutritional and clinical outcomes and cost. J Hum Nutr Diet. 2009;22:324-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Kondrup J, Rasmussen HH, Hamberg O, Stanga Z. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321-336. [PubMed] [Cited in This Article: ] |
| 10. | Soeters PB, Reijven PL, van Bokhorst-de van der Schueren MA, Schols JM, Halfens RJ, Meijers JM, van Gemert WG. A rational approach to nutritional assessment. Clin Nutr. 2008;27:706-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Jensen GL, Mirtallo J, Compher C, Dhaliwal R, Forbes A, Grijalba RF, Hardy G, Kondrup J, Labadarios D, Nyulasi I. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J Parenter Enteral Nutr. 2010;34:156-159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 12. | Jensen GL. Inflammation: an expanding universe. Nutr Clin Pract. 2008;23:1-2. [PubMed] [Cited in This Article: ] |
| 13. | Botta D, Gauthier AP. [Indications of enteral nutrition in pancreatic disorders]. Ann Gastroenterol Hepatol (Paris). 1988;24:335-338. [PubMed] [Cited in This Article: ] |
| 14. | Duggan S, O’Sullivan M, Feehan S, Ridgway P, Conlon K. Nutrition treatment of deficiency and malnutrition in chronic pancreatitis: a review. Nutr Clin Pract. 2010;25:362-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Affronti J. Chronic pancreatitis and exocrine insufficiency. Prim Care. 2011;38:515-37; ix. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Riediger H, Adam U, Fischer E, Keck T, Pfeffer F, Hopt UT, Makowiec F. Long-term outcome after resection for chronic pancreatitis in 224 patients. J Gastrointest Surg. 2007;11:949-59; discussion 959-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Haaber AB, Rosenfalck AM, Hansen B, Hilsted J, Larsen S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int J Pancreatol. 2000;27:21-27. [PubMed] [Cited in This Article: ] |
| 18. | Fitzsimmons D, Kahl S, Butturini G, van Wyk M, Bornman P, Bassi C, Malfertheiner P, George SL, Johnson CD. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol. 2005;100:918-926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Holst M, Schou-Olesen S, Kohler M, Drewes AM, Rasmussen HH. Nutritional assessment in ambulatory patients with chronic pancreatitis. Clin Nutr Suppl. 2013;32 Suppl 1:47. [Cited in This Article: ] |
| 20. | Hébuterne X, Hastier P, Péroux JL, Zeboudj N, Delmont JP, Rampal P. Resting energy expenditure in patients with alcoholic chronic pancreatitis. Dig Dis Sci. 1996;41:533-539. [PubMed] [Cited in This Article: ] |
| 21. | Meier RF, Beglinger C. Nutrition in pancreatic diseases. Best Pract Res Clin Gastroenterol. 2006;20:507-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 51] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 22. | Dujsikova H, Dite P, Tomandl J, Sevcikova A, Precechtelova M. Occurrence of metabolic osteopathy in patients with chronic pancreatitis. Pancreatology. 2008;8:583-586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Kalvaria I, Labadarios D, Shephard GS, Visser L, Marks IN. Biochemical vitamin E deficiency in chronic pancreatitis. Int J Pancreatol. 1986;1:119-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Nakamura T, Tando Y. [Study on pancreatic steatorrhea in patients with chronic pancreatitis]. Nihon Shokakibyo Gakkai Zasshi. 2000;97:1347-1354. [PubMed] [Cited in This Article: ] |
| 25. | Bang UC, Benfield T, Bendtsen F, Hyldstrup L, Beck Jensen JE. The Risk of Fractures Among Patients With Cirrhosis or Chronic Pancreatitis. Clin Gastroenterol Hepatol. 2013;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Glasbrenner B, Malfertheiner P, Büchler M, Kuhn K, Ditschuneit H. Vitamin B12 and folic acid deficiency in chronic pancreatitis: a relevant disorder? Klin Wochenschr. 1991;69:168-172. [PubMed] [Cited in This Article: ] |
| 27. | Quilliot D, Walters E, Bonte JP, Fruchart JC, Duriez P, Ziegler O. Diabetes mellitus worsens antioxidant status in patients with chronic pancreatitis. Am J Clin Nutr. 2005;81:1117-1125. [PubMed] [Cited in This Article: ] |
| 28. | Frokjaer J, Olesen SS, Drewes AM. Fibrosis, atrophy and ductal pathology in chronic pancreatitis are associated with pancreatic function but independent of symptoms. Pancreas. 2013;In press. [Cited in This Article: ] |
| 29. | Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23:1226-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1523] [Cited by in F6Publishing: 1549] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 30. | Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, Lilienthal Heitmann B, Kent-Smith L, Melchior JC, Pirlich M. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23:1430-1453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1224] [Cited by in F6Publishing: 1301] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 31. | Norman K, Pirlich M, Sorensen J, Christensen P, Kemps M, Schütz T, Lochs H, Kondrup J. Bioimpedance vector analysis as a measure of muscle function. Clin Nutr. 2009;28:78-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, van Loon LJ. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:713-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 367] [Cited by in F6Publishing: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 33. | Candow DG, Forbes SC, Little JP, Cornish SM, Pinkoski C, Chilibeck PD. Effect of nutritional interventions and resistance exercise on aging muscle mass and strength. Biogerontology. 2012;13:345-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Geirsdottir OG, Arnarson A, Briem K, Ramel A, Jonsson PV, Thorsdottir I. Effect of 12-week resistance exercise program on body composition, muscle strength, physical function, and glucose metabolism in healthy, insulin-resistant, and diabetic elderly Icelanders. J Gerontol A Biol Sci Med Sci. 2012;67:1259-1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Norman K, Stobäus N, Gonzalez MC, Schulzke JD, Pirlich M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. 2011;30:135-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 538] [Cited by in F6Publishing: 576] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 36. | Schlüssel MM, dos Anjos LA, de Vasconcellos MT, Kac G. Reference values of handgrip dynamometry of healthy adults: a population-based study. Clin Nutr. 2008;27:601-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 37. | Słodkowski M, Rubinsztajn R, Cebulski W, Krasnodebski IW. [A case report of severe hypophosphatemia in the course of refeeding syndrome]. Pol Merkur Lekarski. 2004;17:638-639. [PubMed] [Cited in This Article: ] |
| 38. | Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci USA. 1918;4:370-373. [PubMed] [Cited in This Article: ] |
| 39. | Streppel MT, de Vries JH, Meijboom S, Beekman M, de Craen AJ, Slagboom PE, Feskens EJ. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden Longevity Study. Nutr J. 2013;12:75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 40. | Singh S, Midha S, Singh N, Joshi YK, Garg PK. Dietary counseling versus dietary supplements for malnutrition in chronic pancreatitis: a randomized controlled trial. Clin Gastroenterol Hepatol. 2008;6:353-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Meier R, Ockenga J, Pertkiewicz M, Pap A, Milinic N, Macfie J, Löser C, Keim V. ESPEN Guidelines on Enteral Nutrition: Pancreas. Clin Nutr. 2006;25:275-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 162] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 42. | Plauth M, Cabré E, Campillo B, Kondrup J, Marchesini G, Schütz T, Shenkin A, Wendon J. ESPEN Guidelines on Parenteral Nutrition: hepatology. Clin Nutr. 2009;28:436-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 43. | Nakajima K, Oshida H, Muneyuki T, Kakei M. Pancrelipase: an evidence-based review of its use for treating pancreatic exocrine insufficiency. Core Evid. 2012;7:77-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Domínguez-Muñoz JE. Chronic pancreatitis and persistent steatorrhea: what is the correct dose of enzymes? Clin Gastroenterol Hepatol. 2011;9:541-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Domínguez-Muñoz JE, Iglesias-García J. Oral pancreatic enzyme substitution therapy in chronic pancreatitis: is clinical response an appropriate marker for evaluation of therapeutic efficacy? JOP. 2010;11:158-162. [PubMed] [Cited in This Article: ] |
| 46. | Lochs H, Dejong C, Hammarqvist F, Hebuterne X, Leon-Sanz M, Schütz T, van Gemert W, van Gossum A, Valentini L, Lübke H. ESPEN Guidelines on Enteral Nutrition: Gastroenterology. Clin Nutr. 2006;25:260-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 47. | Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, Jauch KW, Kemen M, Hiesmayr JM, Horbach T. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr. 2006;25:224-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 736] [Cited by in F6Publishing: 639] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 48. | Cerantola Y, Hübner M, Grass F, Demartines N, Schäfer M. Immunonutrition in gastrointestinal surgery. Br J Surg. 2011;98:37-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 49. | Marik PE, Zaloga GP. Immunonutrition in high-risk surgical patients: a systematic review and analysis of the literature. JPEN J Parenter Enteral Nutr. 2010;34:378-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 50. | Barker LA, Gray C, Wilson L, Thomson BN, Shedda S, Crowe TC. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: a randomised controlled trial. Eur J Clin Nutr. 2013;67:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Marano L, Porfidia R, Pezzella M, Grassia M, Petrillo M, Esposito G, Braccio B, Gallo P, Boccardi V, Cosenza A. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol. 2013;20:3912-3918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Seres DS, Valcarcel M, Guillaume A. Advantages of enteral nutrition over parenteral nutrition. Therap Adv Gastroenterol. 2013;6:157-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |