Published online Jan 21, 2012. doi: 10.3748/wjg.v18.i3.279
Revised: August 12, 2011
Accepted: October 14, 2011
Published online: January 21, 2012
AIM: To investigate the role of prophylactic antibiotics in the reduction of mortality of severe acute pancreatitis (SAP) patients, which is highly questioned by more and more randomized controlled trials (RCTs) and meta-analyses.
METHODS: An updated meta-analysis was performed. RCTs comparing prophylactic antibiotics for SAP with control or placebo were included for meta-analysis. The mortality outcomes were pooled for estimation, and re-pooled estimation was performed by the sensitivity analysis of an ideal large-scale RCT.
RESULTS: Currently available 11 RCTs were included. Subgroup analysis showed that there was significant reduction of mortality rate in the period before 2000, while no significant reduction in the period from 2000 [Risk Ratio, (RR) = 1.01, P = 0.98]. Funnel plot indicated that there might be apparent publication bias in the period before 2000. Sensitivity analysis showed that the RR of mortality rate ranged from 0.77 to 1.00 with a relatively narrow confidence interval (P < 0.05). However, the number needed to treat having a minor lower limit of the range (7-5096 patients) implied that certain SAP patients could still potentially prevent death by antibiotic prophylaxis.
CONCLUSION: Current evidences do not support prophylactic antibiotics as a routine treatment for SAP, but the potentially benefited sub-population requires further investigations.
- Citation: Jiang K, Huang W, Yang XN, Xia Q. Present and future of prophylactic antibiotics for severe acute pancreatitis. World J Gastroenterol 2012; 18(3): 279-284
- URL: https://www.wjgnet.com/1007-9327/full/v18/i3/279.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i3.279
Acute pancreatitis (AP) is still a common pancreatic disease, with an increasing incidence rate during the past two decades[1]. In the United States, AP accounts for more than 220 000 hospital admissions annually[2]. Severe acute pancreatitis (SAP) composes about 20% of AP, with a high mortality rate around 20%[3]. For several decades, the administration of prophylactic antibiotics has been one of the great controversies worldwide about the management of SAP.
Totally, the mortality rate for SAP is 10% with sterile and increased to 25% with infected pancreatic necrosis[4]. Hospitalization for patients with SAP may frequently extend beyond 2 wk and often involves an intensive care unit stay and increased infection rate[4]. Up to the late period of last century, complications of infection account for 80% of deaths from AP[5]. Currently, 30%-50% of the dead cases were due to infectious complications for 2 wk from onset[6]. Therefore, theoretically, no antibiotics are indicated in mild cases, but antibiotics were considered to play an important role in either therapeutic or prophylactic intention for SAP. The recognition and exploration of antibiotic prophylaxis for SAP experienced more than a half of a century. However, there was still a gap between theory and truth, and the proper role of antibiotics in SAP remains controversial[7].
Why pancreatologists keep dwelling on this controversy? In this review, we critically estimated the currently available evidence to find out the gap between theory and clinical practice. Moreover, through our hypothesis and calculation, we predicted what would occur in antibiotic prophylaxis for SAP if robust evidence was available in the future.
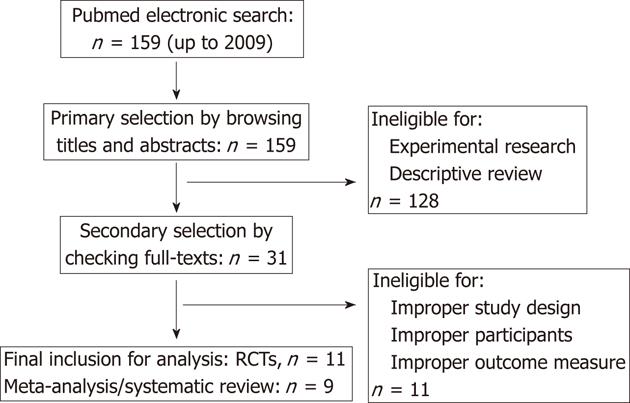
We searched the electronic databases of PubMed up to 2009. The reference lists from relevant articles, containing meta-analysis, systematic reviews or clinical trials, were screened for potential eligible studies. There was no limitation of publication date and language. The following strings were used in the search strategy for PubMed: “pancreatitis” (MeSH Terms) or “pancreatitis” (All Fields); “anti-bacterial agents” (MeSH Terms) or “anti-bacterial” (All Fields); “agents” (All Fields) or “anti-bacterial agents” (All Fields) or “antibiotics” (All Fields) or “anti-bacterial agents” (Pharmacological Action); “randomized controlled trial” (Publication Type) or “randomized controlled trials as topic” (MeSH Terms) or “randomized controlled trials” (All Fields) or “clinical trial” (Publication Type) or “clinical trials” (MeSH Terms) or “clinical trial” (All Fields); “meta-analysis” (Publication Type) or “meta-analysis” (MeSH Terms) or “meta-analysis” (All Fields); and “review literature” (MeSH Terms) or “systematic review” (All Fields).
The currently available meta-analyses and randomized controlled trials were analyzed by meta-analysis. The patients were all diagnosed as having SAP. The intervention group received prophylactic antibiotics. The control group received placebo or none-treatment. All potentially eligible meta-analyses or trials should report the mortality rate of each group. There were no limitations for race, age or gender. If any conditions did not conform to the above criteria or the essential data could not be extracted, the meta-analyses of trials were excluded.
All procedures were reviewed by two independent reviewers: (1) for descriptive review of available meta-analyses, the publication year, the number of included trials, and the effect sizes of mortality or infected necrosis were extracted. The effect sizes involved risk ratio (RR), odds ratio (OR), absolute risk reduction (ARR) and their 95% confidence interval (CI); (2) for updating meta-analysis, the general information including publication year, sample size, study design, general characteristics of patients, and intervention details were extracted. The dichotomous data for the mortality were extracted, including total number of participants and events for each group. The number of events was calculated by the reported percentage if possible; and (3) For the predication of the future meta-analysis, the synthesized mortality rate of each group was extracted by our updated meta-analysis.
Meta-analysis: Outcomes of eligible studies were statistically synthesized by Reviewer Manager 5.0 (The Nordic Cochrane Center, Cochrane Collaboration, Copenhagen, Denmark). The statistical method was referred to the Cochrane Handbook for Systematic Review of Intervention. The pooled statistics were calculated using a fixed effects model initially. The RR was reported for dichotomous data. The 95% CI was also calculated. The Mantel-Haenszel method was used to test significance of dichotomous data. P value less than 0.05 was considered statistically significant. Heterogeneity between comparable studies was tested in all analyses using a standard χ2 test for between-study statistical heterogeneity and considered significant at P < 0.1. If heterogeneity existed, the random effects model was used for analysis.
Sample size calculation: The format for equivalency estimate of rates between two arms is shown below.
Expected sample size in each group = (μα + μβ)2[p1 (1 - p1) + p2 (1-p2)]/(Δ -|p1-p2|)2; Limitation: Δ > |p1-p2|
(Δ: threshold of difference value; α: possibility of type I error; β: possibility of type II error; μα: critical value corresponding to α; μβ: critical value corresponding to β; p1: possibility of mortality in prophylactic antibiotics group; p2: possibility of mortality in placebo or blank control group; |p1-p2|: absolute value of difference between two groups.)
Hypothesis test: SPSS 13.0 (SPSS, Chicago, IL, United States) was used for statistical analysis. For dichotomous data, the χ2 test was used to compare frequencies of mortality. Linear correlation between accumulated sample size and time (year) was analyzed by the Pearson correlation test. P value of less than 0.05 (two-sided) was considered significant.
Published meta-analysis: Recently, there have been several RCTs on prophylactic antibiotic for SAP[8-14], and therefore several meta-analyses on this topic have already published (Table 1, Figure 1). For the primary outcome in mortality rate, only one meta-analysis showed a significant reduction by antibiotic prophylaxis. Moreover, only two other meta-analyses showed a significant preventive effect on infected necrosis for SAP by antibiotic prophylaxis. More than a half of the meta-analyses did not recommend the administration of antibiotic prophylaxis, while the others suggested that the effectiveness of antibiotic prophylaxis was still controversial.
| Meta-analysis | Year | No. of RCTs | Mortality | Infected necrosis | Recommendation | ||
| Effect size | 95% CI | Effect size | 95% CI | ||||
| Jafri et al[15] | 2009 | 8 | RR = 0.76 | (0.49, 1.16) | RR = 0.79 | (0.56, 1.11) | Unfavorable |
| Xu et al[16] | 2008 | 8 | RR = 0.76 | (0.50, 1.18) | RR = 0.69 | (0.50, 0.95)a | Pending |
| Bai et al[17] | 2008 | 7 | RR = 0.70 | (0.42, 1.17) | RR = 0.81 | (0.54, 1.22) | Unfavorable |
| Hart et al[18] | 2008 | 7 | OR = 0.71 | (0.41, 1.23) | OR = 0.72 | (0.45, 1.16) | Unfavorable |
| de Vries et al[19] | 2007 | 6 | ARR = 0.058 | (-0.017, 0.134) | ARR = 0.055 | (-0.084, 0.194) | Pending |
| Dambrauskas et al[20] | 2007 | 10 | RR = 0.76 | (0.586, 0.976) | RR = 0.57 | (0.418, 0.784)a | Pending |
| Villatoro et al[21] | 2006 | 5 | OR = 0.37 | (0.17, 0.83)a | OR = 0.62 | (0.35, 1.09) | Pending |
| Mazaki et al[22] | 2006 | 6 | RR = 0.78 | (0.44, 1.39) | RR = 0.77 | (0.54, 1.12) | Unfavorable |
| Xiong et al[23] | 2006 | 6 | RR = 0.54 | (0.28, 1.04) | RR = 0.77 | (0.48, 1.24) | Unfavorable |
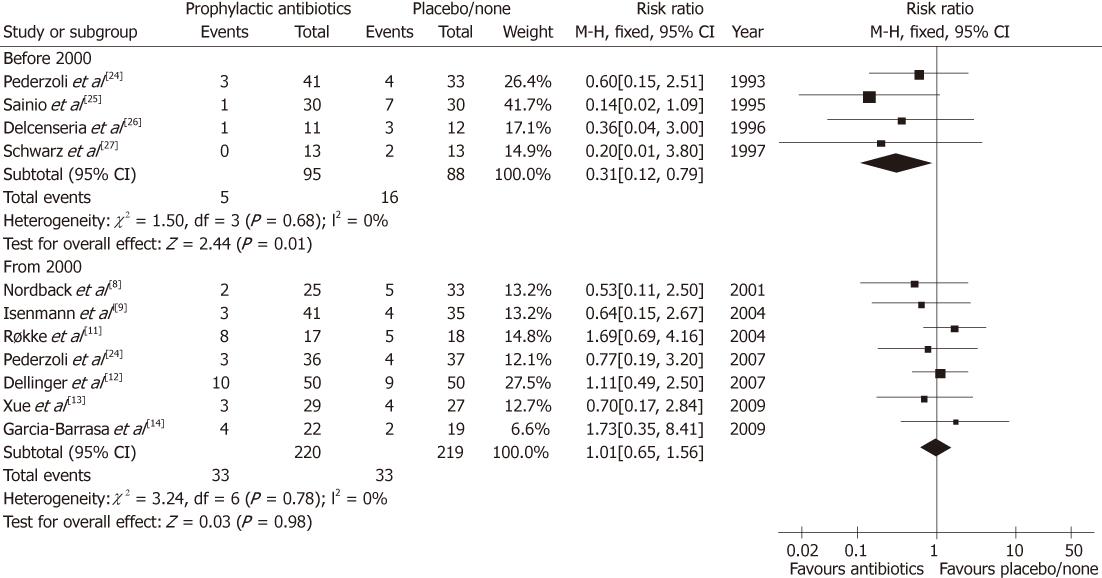
Update on meta-analysis: To improve the robustness of evidence, the meta-analysis was updated in present review. We comprehensively searched the PubMed database to identify available RCTs on the comparison between prophylactic antibiotics and placebo/none-treatment for SAP. There were 11 eligible RCTs (Figure 1)[8-14,24-27], which were re-pooled to update the meta-analysis (Figure 2). There were two newly published RCTs in 2009[13,14], which was different from previous meta-analysis[15].
Interestingly, we found that before 2000 the pooling estimate of 4 RCTs (183 patients) showed a significant benefit to reduce the mortality of SAP (RR = 0.31, 95% CI: 0.12-0.79, P = 0.01)[24-27]. The mortality rates were 5.26% (5/95) and 18.18% (16/88) in prophylactic antibiotics and placebo/none-treatment groups, respectively. The number needed to treat (NNT) was one of 8 treated patients potentially being benefited to prevent death by prophylactic antibiotics (Table 2).
However, since 2000, seven RCTs (439 patients) have been identified and pooled to meta-analysis[8-14]. Differently, the result indicated that there was no benefit of preventing death in the prophylactic antibiotics group (RR = 1.01, 95% CI: 0.65-1.56, P = 0.98). The mortality rates were 15.00% (33/220) and 15.07% (33/219) in the prophylactic antibiotics and placebo/none-treatment groups, respectively. Sadly, the NNT was one of 1429 treated patients potentially being benefited (Table 2), and it was indeed a negative evidence to support administration of prophylactic antibiotics.
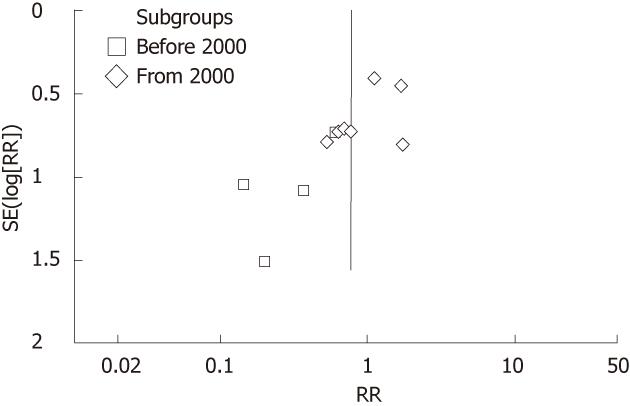
In the funnel plot, the asymmetric distribution of RCTs before 2000 implied that there might be apparent publication bias (Figure 3). Moreover, the distribution of RCTs from 2000 was relatively symmetric.
What a scale does a trial require? To the best of our knowledge, the key to end the controversy is to conduct a large-scale RCT so as to control the random sampling error. To perform an ideal RCT, we have to calculate the required sample size by statistical approach. The mortality rates were calculated based on the pooling estimate from 2000, and the results of calculation are shown in Table 3.
| Δ | α | β | μα | μβ | p1 | p2 | |p1-p2| | Group | Study |
| 0.10 | 0.05 | 0.10 | 1.9600 | 1.2816 | 0.1500 | 0.1507 | 0.0007 | 272 | 544 |
| 0.05 | 0.05 | 0.05 | 1.9600 | 1.9600 | 0.1500 | 0.1507 | 0.0007 | 1615 | 3230 |
If the difference between mortality rates was no more than 10% as an acceptable threshold of equivalence, at least 544 patients would be required for a single robust trial (Table 3), while the difference was limited to no more than 5%, 3230 patients would be demanded in a single trial (Table 3).
In practice, it is hard to conduct a single-center randomized trial of such large scale in the study of SAP treatment. A multi-center trial might be a way out of this corner, but difficulties in quality control and possible performance bias might occur. Among the included RCTs in the above updated meta-analysis, the absolute value of difference between mortality rates |p1-p2| was 6.7% ± 5.9% (range, 2.0%-19.3%), and the mean was more than 5%. Therefore, the threshold of 10% and the sample size of 544 patients could be rational. It would take more than two years to complete a large-scale multi-center RCT.
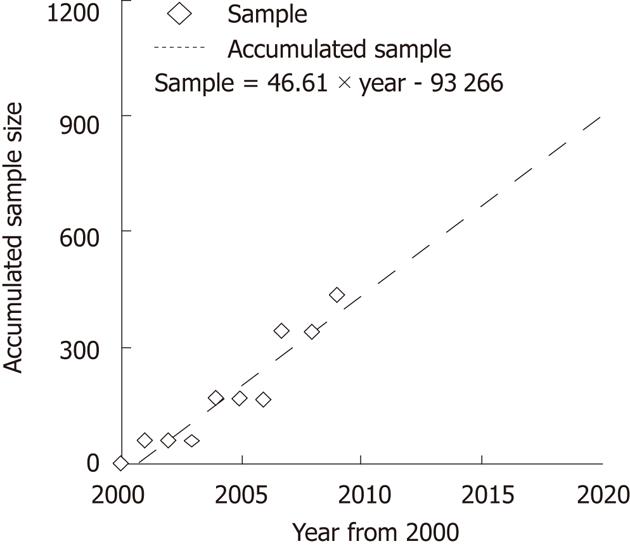
The above meta-analysis showed a minor difference between mortality rates, |p1-p2| = 0.0007. Thus, we have to choose the lower threshold 5% to calculate the required accumulated sample size, and the calculation result indicated that the overall accumulated sample size need 3230 patients. The linear regression test showed the accumulated sample size is positively correlated with the time period (year by year from 2000) (Figure 4). If there were no larger-scale RCTs, it would take a long time to achieve the statistical goal, and the controversy would continue.
An assumed large-scale trial (sensitivity analysis): If years later a large-scale RCT is completed, how will it contribute to addressing the controversy on prophylactic antibiotics for SAP? Thus, we carried out a sensitivity analysis to estimate the margin of potential benefit from prophylactic antibiotics (Table 4). It was performed by re-pooling both the 7 RCTs from 2000 and an assumed large-scale RCT containing 544 patients for meta-analysis[8-14]. The theoretical mortality rates of assumed RCT were evaluated as follows.
| Antibiotics | Placebo/none-treatment | |||
| Mortality rate | No. of events | Mortality rate | No. of events | |
| By best/worst data1 | 7.32% | 40/544 | 27.78% | 151/544 |
| Meta-analysis3 | RR = 0.76, 95% CI: 0.38-1.53, P = 0.44 (random effect model) | |||
| By pooling data2 | 15.00% | 82/544 | 15.07% | 82/544 |
| Meta-analysis3 | RR=1.00, 95% CI: 0.79-1.27, P = 0.99 (fixed effect model) | |||
Given there was obvious random sampling error, the best-worst method was used to calculate the best marginal benefit (Table 4). Since there was between-study heterogeneity (P = 0.0003), the meta-analysis was performed by random effect model. The result of sensitivity analysis showed no significant benefit of antibiotic prophylaxis for SAP (RR = 0.76, P = 0.44), which was similar with the previous meta-analyses (forest plot not shown).
If there was no random sampling error, the mortality rates would be equal to the pooling data from above updated meta-analysis (Table 4). The heterogeneity of repooling estimate was not significant (P = 0.86), so fixed effect model was used. The result of sensitivity analysis showed nearly equivalent efficacy between antibiotic prophylaxis and non-antibiotic treatment (RR = 1.00, P = 0.99) (forest plot not shown).
Therefore, we can assume that even if a large-scale RCT was completed, the RR of the mortality rate would only range from 0.77 to 1.00 with a relatively narrow confidence interval (P < 0.05). It means, as a whole, that antibiotic prophylaxis is not effective for SAP patients. Moreover, by the sensitivity analysis, the NNT ranged from 7 to 5069 patients.
As the mortality of SAP is obviously associated with the complications of infection, prophylactic antibiotics have been administrated for SAP patients for several decades, which seemed to play an important role in the treatment of SAP. However, currently, its role is highly questioned by more and more RCTs and meta-analyses, and the controversies continue due to insufficient evidence.
Among the existing meta-analyses, only one meta-analysis showed a significant reduction in the mortality rate by antibiotic prophylaxis, and most of the meta-analyses did not recommend the administration of antibiotic prophylaxis. Therefore, the current academic opinion obviously trends to be unfavorable for antibiotic prophylaxis for SAP. However, antibiotic prophylaxis has not been given up in clinical practice in the treatment of SAP. Why do physicians often go reversely in aspect of the decision-making on antibiotic prophylaxis for SAP?
Practice of evidence-based medicine is a procedure of integrating the best available external clinical evidence with clinical expertise and patient needs[28]. There should be a balance among these three aspects. If the power of current available evidence is not robust enough, the influence of clinical expertise will be inevitably enhanced. Thus, physicians are quite cautious to the available evidence due to the weakness in the meta-analyses. Firstly, the eligible RCTs are fairly small-sized and the accumulated sample size is also limited. Secondly, the validity of some RCTs in earlier period is affected by the absence of blinding method.
In the funnel plot of our updated meta-analysis, the asymmetric distribution of RCTs before 2000 implied that there might be apparent publication bias. At that period, the positive results of trials tended to be accepted for publication more easily. Therefore, most of the scholars believed that the evidence before 2000 would be weak to validate. Another critical reason is that blinding method was not used in these trials, which may result in performance and observation biases. Thus, positive results might be more easily to reach under that condition. Since the year of 2000, the improved methodology of RCTs has made the pooling estimate non-significant.
By now, physicians have become more conservative and suspicious about the administration of prophylactic antibiotics for SAP. The effectiveness of prophylactic antibiotics seemed to be equal to the placebo or blank control. Whether the evidence obtained in the current decade is robust enough to make a mandatory recommendation to quit the administration of prophylactic antibiotics for SAP? As small-sized RCTs inevitably result in the random sampling error. Thus, there must be a long way to go to answer this question.
Through our hypothesis and calculation, we predict that antibiotic prophylaxis would not be effective as a whole in reducing the mortality of SAP patients, even if a large enough RCT was completed. However, the minor lower limit of NNT range implies that certain SAP patients might potentially be benefited by antibiotic prophylaxis. Therefore, if possible, the individual patient data analysis will be meaningful to identify potential candidates who can gain survival benefit from antibiotic prophylaxis.
The mortality of severe acute pancreatitis (SAP) is obviously associated with the infectious complications, so prophylactic antibiotics have been administrated for SAP patients for several decades. However, the role of prophylactic antibiotics in reduction of mortality of SAP patients has been highly questioned by more and more randomized controlled trials (RCTs) and meta-analyses.
Evidence-based medicine is frequently used in clinical practice. Although there have been several meta-analyses on the administration of prophylactic antibiotics for SAP, the conclusion is still not confirmed. By now, some new reports on this topic have become available for updated review and analysis.
An updated meta-analysis on the mortality of SAP patients was performed. Prophylactic antibiotics for SAP was compared with control or placebo. Subgroup analysis showed that there was significant reduction of mortality rate in the period before 2000, while no significant reduction in the period from 2000. Sensitivity analysis by assuming an ideal large-scale RCT was performed to prove the results, and found that SAP patients did benefit from prophylactic antibiotics. In addition, current evidences do not support administration of prophylactic antibiotics as a routine treatment for SAP.
Although the administration of prophylactic antibiotics for SAP in general practice is controversial, there is still a potentially high-risk sub-population who could benefit from prophylactic antibiotics.
Prophylactic antibiotics, is administrated to the sterile SAP patients to prevent the potential and even fatal complications, involving both peri-pancreatic and systematic infection.
The paper investigates the role of prophylactic antibiotics on mortality of severe acute pancreatitis. The statistical analysis used in the study is appropriate and the results are reliable.
Peer reviewer: Naoaki Sakata, MD, PhD, Division of Hepato-Biliary Pancreatic Surgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8574, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Xiong L
| 1. | Jiang K, Chen XZ, Xia Q, Tang WF, Wang L. Early nasogastric enteral nutrition for severe acute pancreatitis: a systematic review. World J Gastroenterol. 2007;13:5253-5260. [PubMed] [Cited in This Article: ] |
| 2. | DeFrances CJ, Hall MJ, Podgornik MN. Advance Data from Vital and Health Statistics, no 359. Hyattsville, MD: National Center for Health Statistics; 2005; . [Cited in This Article: ] |
| 3. | Heinrich S, Schäfer M, Rousson V, Clavien PA. Evidence-based treatment of acute pancreatitis: a look at established paradigms. Ann Surg. 2006;243:154-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Swaroop VS, Chari ST, Clain JE. Severe acute pancreatitis. JAMA. 2004;291:2865-2868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Bradley EL. Antibiotics in acute pancreatitis. Current status and future directions. Am J Surg. 1989;158:472-477; discussion 472-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 83] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Carnovale A, Rabitti PG, Manes G, Esposito P, Pacelli L, Uomo G. Mortality in acute pancreatitis: is it an early or a late event? JOP. 2005;6:438-444. [PubMed] [Cited in This Article: ] |
| 7. | Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 494] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 8. | Nordback I, Sand J, Saaristo R, Paajanen H. Early treatment with antibiotics reduces the need for surgery in acute necrotizing pancreatitis--a single-center randomized study. J Gastrointest Surg. 2001;5:113-118; discussion 113-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Isenmann R, Rünzi M, Kron M, Kahl S, Kraus D, Jung N, Maier L, Malfertheiner P, Goebell H, Beger HG. Prophylactic antibiotic treatment in patients with predicted severe acute pancreatitis: a placebo-controlled, double-blind trial. Gastroenterology. 2004;126:997-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 346] [Cited by in F6Publishing: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Spicak J, Hejtmankova S, Cech P, Hoskovec D, Kostka R, Leffler J, Kasalicky M, Svoboda P, Bartova J. Antibiotic prophylaxis in large pancreatic necrosis: multicenter randomized trial with ciprofloxacin and metronidazole or meropenem (abstract). Gastroenterology. 2004;126:A229. [Cited in This Article: ] |
| 11. | Røkke O, Harbitz TB, Liljedal J, Pettersen T, Fetvedt T, Heen LØ, Skreden K, Viste A. Early treatment of severe pancreatitis with imipenem: a prospective randomized clinical trial. Scand J Gastroenterol. 2007;42:771-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Dellinger EP, Tellado JM, Soto NE, Ashley SW, Barie PS, Dugernier T, Imrie CW, Johnson CD, Knaebel HP, Laterre PF. Early antibiotic treatment for severe acute necrotizing pancreatitis: a randomized, double-blind, placebo-controlled study. Ann Surg. 2007;245:674-683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Xue P, Deng LH, Zhang ZD, Yang XN, Wan MH, Song B, Xia Q. Effect of antibiotic prophylaxis on acute necrotizing pancreatitis: results of a randomized controlled trial. J Gastroenterol Hepatol. 2009;24:736-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | García-Barrasa A, Borobia FG, Pallares R, Jorba R, Poves I, Busquets J, Fabregat J. A double-blind, placebo-controlled trial of ciprofloxacin prophylaxis in patients with acute necrotizing pancreatitis. J Gastrointest Surg. 2009;13:768-774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Jafri NS, Mahid SS, Idstein SR, Hornung CA, Galandiuk S. Antibiotic prophylaxis is not protective in severe acute pancreatitis: a systematic review and meta-analysis. Am J Surg. 2009;197:806-813. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Xu T, Cai Q. Prophylactic antibiotic treatment in acute necrotizing pancreatitis: results from a meta-analysis. Scand J Gastroenterol. 2008;43:1249-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Bai Y, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot reduce infected pancreatic necrosis and mortality in acute necrotizing pancreatitis: evidence from a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2008;103:104-110. [PubMed] [Cited in This Article: ] |
| 18. | Hart PA, Bechtold ML, Marshall JB, Choudhary A, Puli SR, Roy PK. Prophylactic antibiotics in necrotizing pancreatitis: a meta-analysis. South Med J. 2008;101:1126-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | de Vries AC, Besselink MG, Buskens E, Ridwan BU, Schipper M, van Erpecum KJ, Gooszen HG. Randomized controlled trials of antibiotic prophylaxis in severe acute pancreatitis: relationship between methodological quality and outcome. Pancreatology. 2007;7:531-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Dambrauskas Z, Gulbinas A, Pundzius J, Barauskas G. Meta-analysis of prophylactic parenteral antibiotic use in acute necrotizing pancreatitis. Medicina (Kaunas). 2007;43:291-300. [PubMed] [Cited in This Article: ] |
| 21. | Villatoro E, Bassi C, Larvin M. Antibiotic therapy for prophylaxis against infection of pancreatic necrosis in acute pancreatitis. Cochrane Database Syst Rev. 2006;18:CD002941. [PubMed] [Cited in This Article: ] |
| 22. | Mazaki T, Ishii Y, Takayama T. Meta-analysis of prophylactic antibiotic use in acute necrotizing pancreatitis. Br J Surg. 2006;93:674-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Xiong GS, Wu SM, Wang ZH. Role of prophylactic antibiotic administration in severe acute pancreatitis: a meta-analysis. Med Princ Pract. 2006;15:106-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Pederzoli P, Bassi C, Vesentini S, Campedelli A. A randomized multicenter clinical trial of antibiotic prophylaxis of septic complications in acute necrotizing pancreatitis with imipenem. Surg Gynecol Obstet. 1993;176:480-483. [PubMed] [Cited in This Article: ] |
| 25. | Sainio V, Kemppainen E, Puolakkainen P, Taavitsainen M, Kivisaari L, Valtonen V, Haapiainen R, Schröder T, Kivilaakso E. Early antibiotic treatment in acute necrotising pancreatitis. Lancet. 1995;346:663-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 324] [Cited by in F6Publishing: 259] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Delcenserie R, Yzet T, Ducroix JP. Prophylactic antibiotics in treatment of severe acute alcoholic pancreatitis. Pancreas. 1996;13:198-201. [PubMed] [Cited in This Article: ] |
| 27. | Schwarz M, Isenmann R, Meyer H, Beger HG. [Antibiotic use in necrotizing pancreatitis. Results of a controlled study]. Dtsch Med Wochenschr. 1997;122:356-361. [PubMed] [Cited in This Article: ] |