Osteoarthritis in the XXIst Century: Risk Factors and Behaviours that Influence Disease Onset and Progression
Abstract
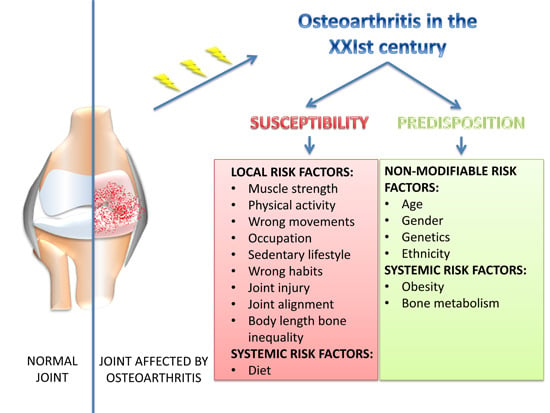
:1. Introduction

2. Predisposition

2.1. Genetic and Epigenetic Predisposition
2.2. Age, Cellular Senescence, Apoptosis and Lubricin
2.3. Gender
2.4. Ethnicity
3. Susceptibility: XXIst Century Lifestyles, Behaviors and OA
3.1. Diet and Obesity

3.1.1. ROS and Vitamins
3.1.2. Glucose Concentration
3.1.3. Adipokines


3.2. Movements and Physical Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Musumeci, G.; Szychlinska, M.A.; Mobasheri, A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: Molecular markers of senescent chondrocytes. Histol. Histopathol. 2015, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reynard, L.N.; Loughlin, J. Genetics and epigenetics of osteoarthritis. Maturitas 2012, 71, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D.; Dieppe, P.; Radin, E.L. Etiopathogenesis of osteoarthritis. Rheum. Dis. Clin. N. Am. 2008, 34, 531–559. [Google Scholar] [CrossRef]
- Ryder, J.J.; Garrison, K.; Song, F.; Hooper, L.; Skinner, J.; Loke, Y.; Loughlin, J.; Higgins, J.P.; MacGregor, A.J. Genetic associations in peripheral joint osteoarthritis and spinal degenerative disease: A systematic review. Ann. Rheum. Dis. 2008, 67, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Nordenvall, R.; Bahmanyar, S.; Adami, J.; Stenros, C.; Wredmark, T.; Felländer-Tsai, L. A population-based ationwide study of cruciate ligament injury in Sweden, 2001–2009: Incidence, treatment, and sex differences. Am. J. Sports Med. 2012, 40, 1808–1813. [Google Scholar] [CrossRef] [Green Version]
- Ramos, Y.F.; den Hollander, W.; Bovée, J.V.; Bomer, N.; van der Breggen, R.; Lakenberg, N.; Keurentjes, J.C.; Goeman, J.J.; Slagboom, P.E.; Nelissen, R.G.; et al. Genes involved in the osteoarthritis process identified through genome wide expression analysis in articular cartilage; the RAAK study. PLoS One 2014, 9, e103056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2014. [Google Scholar] [CrossRef]
- Musumeci, G.; Mobasheri, A.; Trovato, F.M.; Szychlinska, M.A.; Graziano, A.C.E.; Lo Furno, D.; Avola, R.; Mangano, S.; Giuffrida, R.; Cardile, R. Biosynthesis of collagen I, II, RUNX2 and lubricin at different time points of chondrogenic differentiation in a 3D in vitro model of human mesenchymal stem cells derived from adipose tissue. Acta Histochem. 2014, 116, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Lo Furno, D.; Loreto, C.; Giuffrida, R.; Caggia, S.; Leonardi, R.; Cardile, V. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp. Biol. Med. 2011, 236, 1333–1341. [Google Scholar] [CrossRef]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Archer, C.W.; Francis-West, P. The chondrocyte. Int. J. Biochem. Cell. Biol. 2003, 35, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Redman, S.N.; Williams, R.; Dowthwaite, G.P.; Oldfield, S.F.; Archer, C.W. The development of synovial joints. Curr. Top. Dev. Biol. 2007, 79, 1–36. [Google Scholar] [PubMed]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Styrkarsdottir, U.; Thorleifsson, G.; Helgadottir, H.T.; Bomer, N.; Metrustry, S.; Bierma-Zeinstra, S.; Strijbosch, A.M.; Evangelou, E.; Hart, D.; Beekman, M.; et al. Severe osteoarthritis of the hand associates with common variants within the ALDH1A2 gene and with rare variants at 1p31. Nat. Genet. 2014, 46, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.; Barter, M.J.; Scott, J.L.; Xu, Y.; Galler, M.; Reynard, L.N.; Rowan, A.D.; Young, D.A. cAMP response element-binding (CREB) recruitment following a specific CpGdemethylation leads to the elevated expression of the matrix metalloproteinase 13 in human articular chondrocytes and osteoarthritis. FASEB J. 2012, 26, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Otero, M.; Imagawa, K.; de Andrés, M.C.; Coico, J.M.; Roach, H.I.; Oreffo, R.O.; Marcu, K.B.; Goldring, M.B. Regulated transcription of human matrix metalloproteinase 13 (MMP13) and interleukin-1β (IL1B) genes in chondrocytes depends on methylation of specific proximal promoter CpG Sites. J. Biol. Chem. 2013, 288, 10061–10072. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Matta, C.; Zákány, R.; Musumeci, G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015, 80, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Intracellular determinants of cell aging. Mech. Ageing Dev. 1984, 28, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A. Applications of proteomics to osteoarthritis, a musculoskeletal disease characterized by aging. Front. Physiol. 2011, 2. [Google Scholar] [CrossRef]
- Hollander, A.P.; Pidoux, I.; Reiner, A.; Rorabeck, C.; Bourne, R.; Poole, A.R. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J. Clin. Investig. 1995, 96, 2859–2869. [Google Scholar] [CrossRef] [PubMed]
- Zamli, Z.; Sharif, M. Chondrocyte apoptosis: A cause or consequence of osteoarthritis? Int. J. Rheum. Dis. 2011, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging and osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Martinez, G. Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Strehin, I.; Elisseeff, J. OA cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histol. Histopathol. 2011, 26, 1265–1278. [Google Scholar] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Loreto, C.; Castorina, S.; Pichler, K.; Weinberg, A.M. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site. A morphological study. Int. J. Mol. Sci. 2013, 14, 15767–15784. [Google Scholar] [CrossRef] [PubMed]
- Zamli, Z.; Adams, M.A.; Tarlton, J.F.; Sharif, M. Increased chondrocyte apoptosis is associated with progression of osteoarthritis in spontaneous Guinea pig models of the disease. Int. J. Mol. Sci. 2013, 14, 17729–17743. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Mazzone, V.; Szychlinska, M.A.; Castorina, S.; Loreto, C. Histochemistry as a unique approach for investigating normal and osteoarthritic cartilage. Eur. J. Histochem. 2014, 58, 107–111. [Google Scholar] [CrossRef]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Leonardi, R.; Castorina, S.; Giunta, S.; Carnazza, M.L.; Trovato, F.M.; Pichler, K.; Weinberg, A.M. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J. Bone Miner. Metab. 2013, 31, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Coppolino, F.; Cardile, V.; Leonardi, R. Lubricin is expressed in chondrocytes derived from osteoarthritic cartilage encapsulated in poly (ethylene glycol) diacrylate scaffold. Eur. J. Histochem. 2011, 55. [Google Scholar] [CrossRef]
- Leonardi, R.; Rusu, M.C.; Loreto, F.; Loreto, C.; Musumeci, G. Immunolocalization and expression of lubricin in the bilaminar zone of the human temporomandibular joint disc. Acta Histochem. 2011, 114, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Musumeci, G.; Sicurezza, E.; Loreto, C. Lubricin in human temporomandibular joint disc: An immunohistochemical study. Arch. Oral Biol. 2012, 57, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, R.; Loreto, C.; Talic, N.; Caltabiano, R.; Musumeci, G. Immunolocalization of lubricin in the rat periodontal ligament during experimental tooth movement. Acta Histochem. 2012, 114, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Loreto, C.; Leonardi, R.; Szychlinska, M.A.; Castorina, S.; Mobasheri, A. Lubricin expression in human osteoarthritic knee meniscus and synovial fluid: A morphological, immunohistochemical and biochemical study. Acta Histochem. 2014, 116, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Teeple, E.; Elsaid, K.A.; Jay, G.D.; Zhang, L.; Badger, G.J.; Akelman, M.; Bliss, T.F.; Fleming, B.C. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. Am. J. Sports Med. 2011, 39, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.S.; Bremner, J.M.; Bier, F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and X-ray changes. Ann. Rheum. Dis. 1966, 25, 1–24. [Google Scholar] [PubMed]
- Tsai, C.L.; Liu, T.K. Osteoarthritis inwomen: Its relationship to estrogen and current trends. Life Sci. 1992, 50, 1737–1744. [Google Scholar] [PubMed]
- Anderson, J.J.; Felson, D.T. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am. J. Epidemiol. 1988, 128, 179–189. [Google Scholar] [PubMed]
- Jordan, J.M.; Linder, G.F.; Renner, J.B.; Fryer, J.G. The impact of arthritis in rural populations. Arthritis Care Res. 1995, 8, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.; Hochberg, M.C. Factors associated with hip osteoarthritis: Data from the first National Health and Nutrition Examination Survey (NHANES-I). Am. J. Epidemiol. 1993, 137, 1081–1088. [Google Scholar] [PubMed]
- Jordan, J.M.; Helmick, C.G.; Renner, J.B.; Luta, G.; Dragomir, A.D.; Woodard, J.; Fang, F.; Schwartz, T.A.; Abbate, L.M.; Callahan, L.F.; et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: The Johnston County Osteoarthritis Project. J. Rheumatol. 2007, 34, 172–180. [Google Scholar] [PubMed]
- Clark, A.G.; Jordan, J.M.; Vilim, V.V.; Renner, J.B.; Dragomir, A.D.; Luta, G.; Kraus, V.B. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: The Johnston County Osteoarthritis Project. Arthritis Rheumatol. 1999, 42, 2356–2364. [Google Scholar] [CrossRef]
- Sartori-Cintra, A.R.; Aikawa, P.; Cintra, D.E. Obesity versus osteoarthritis: Beyond the mechanical overload. Einstein (Sao Paulo) 2014, 12, 374–379. [Google Scholar] [CrossRef]
- Sanghi, D.; Mishra, A.; Sharma, A.C.; Raj, S.; Mishra, R.; Kumari, R.; Natu, S.M.; Agarwal, S.; Srivastava, R.N. Elucidation of dietary risk factors in osteoarthritis knee-a case-control study. J. Am. Coll. Nutr. 2015, 34, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Eymard, F.; Parsons, C.; Edwards, M.H.; Petit-Dop, F.; Reginster, J.Y.; Bruyère, O.; Richette, P.; Cooper, C.; Chevalier, X. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthr. Cartil. 2015. [Google Scholar] [CrossRef]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford) 2014. [Google Scholar] [CrossRef]
- Katz, J.D.; Agrawal, S.; Velasquez, M. Getting to the heart of the matter: Osteoarthritis takes its place as part of the metabolic syndrome. Curr. Opin. Rheumatol. 2010, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.; Karvonen-Gutierrez, C.A.; Palmieri-Smith, R.; Jacobson, J.A.; Jiang, Y.; Ashton-Miller, J.A. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheumatol. 2009, 61, 1328–1336. [Google Scholar] [CrossRef]
- Yoshimura, N.; Muraki, S.; Oka, H.; Kawaguchi, H.; Nakamura, K.; Akune, T. Association of knee osteoarthritis with the accumulation of metabolic risk factors such as overweight, hypertension, dyslipidemia, and impaired glucose tolerance in Japanese men and women: The ROAD study. J. Rheumatol. 2011, 38, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Reijman, M.; Pols, H.A.; Bergink, A.P.; Hazes, J.M.; Belo, J.N.; Lievense., A.M.; Bierma-Zeinstra, S.M. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: The Rotterdam study. Ann. Rheum. Dis. 2007, 66, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Razak, F.; Tso, P.; Davey, J.R.; Mahomed, N.N. Asian ethnicity and the prevalence of metabolic syndrome in the osteoarthritic total knee arthroplasty population. J. Arthroplast. 2010, 25, 416–419. [Google Scholar] [CrossRef]
- Jungmann, P.M.; Kraus, M.S.; Alizai, H.; Nardo, L.; Baum, T.; Nevitt, M.C.; McCulloch, C.E.; Joseph, G.B.; Lynch, J.A.; Link, T.M. Association of metabolic risk factors with cartilage degradation assessed by T2 relaxation time at the knee: Data from the osteoarthritis initiative. Arthritis Care Res. (Hoboken) 2013, 65, 1942–1950. [Google Scholar] [CrossRef]
- Engstrom, G.; Gerhardsson de Verdier, M.; Rollof, J.; Nilsson, P.M.; Lohmander, L.S. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthr. Cartil. 2009, 17, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Puenpatom, R.A.; Victor, T.W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: An analysis of NHANES III data. Postgrad. Med. 2009, 121, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Stanaitis, I. Osteoarthritis year in review 2014: Clinical. Osteoarthr. Cartil. 2014, 22, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Frei, B. Reactive oxygen species and antioxidant vitamins: Mechanisms of action. Am. J. Med. 1994, 97, 5S–13S. [Google Scholar] [CrossRef] [PubMed]
- Rathakrishnan, C.; Tiku, K.; Raghavan, A.; Tiku, M.L. Release of oxygen radicals by articular chondrocytes: A study of luminol-dependent chemoluminescence and hydrogen peroxide secretion. J. Bone Miner. Res. 1992, 7, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Deby-Dupont, G.; Deby, C.; de Bruyn, M.; Lamy, M.; Franchimont, P. Production of active oxygen species by isolated human chondrocytes. Br. J. Rheumatol. 1993, 32, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.A.; Moy, W.W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheumatol. 1979, 22, 251–259. [Google Scholar] [CrossRef]
- McCord, J.M. Free radicals and inflammation: Protection of synovial fluid by superoxide dismutase. Science 1974, 185, 529–531. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.E.; Jacques, P.; Zhang, Y.; Hannan, M.Y.; Aliabadi, P.; Weissman, B.; Rush, D.; Levy, D.; Felson, D.T. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheumatol. 1996, 39, 648–658. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Felson, D.; Zhang, Y. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann. Intern. Med. 1996, 125, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Booth, S.L.; Zhang, Y.Q.; Jacques, P.F.; Terkeltaub, R.; Aliabadi, P.; Felson, D.T. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheumatol. 2006, 54, 1255–1261. [Google Scholar] [CrossRef]
- Felson, D.T.; Niu, J.; Clancy, M.; Aliabadis, P.; Sack, B.; Guermazi, A.; Hunter, D.J.; Amin, S.; Rogers, G.; Booth, S.L. Low levels of vitamin D and worsening of knee osteoarthritis: Results of two longitudinal studies. Arthritis Rheumatol. 2007, 56, 129–136. [Google Scholar] [CrossRef]
- Lane, N.E.; Gore, L.R.; Cummings, S.R.; Lin, P.; Christiansen, L.; Nevitt, M.C. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: A longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheumatol. 1999, 42, 854–860. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Dawson-Huges, B.; Driban, J. Clinical trial of vitamin D to reduce pain and structural progression of knee osteoarthritis (OA). Arthritis Rheumatol. 2010, 62, S294. [Google Scholar]
- DeGroot, J.; Verzijl, N.; Bank, R.A.; Lafeber, F.P.; Bijlsma, J.W.; TeKoppele, J.M. Age-related decrease in proteoglycan synthesis of human articular chondrocytes: The role of nonenzymaticglycation. Arthritis Rheumatol. 1999, 42, 1003–1009. [Google Scholar] [CrossRef]
- Loeser, R.F.; Yammani, R.R.; Carlson, C.S.; Chen, H.; Cole, A.; Im, H.J.; Bursch, L.S.; Yan, S.D. Articular chondrocytes express the receptor for advanced glycation end products: Potential role in osteoarthritis. Arthritis Rheumatol. 2005, 52, 2376–2385. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Ben, Z.C.; Brau-Benjamin, O.; Maroudas, A.; Bank, R.A.; Mizrahi, J.; Schalkwijk, C.G.; Thorpe, S.R.; Baynes, J.W.; et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheumatol. 2002, 46, 114–123. [Google Scholar] [CrossRef]
- Cecil, D.L.; Johnson, K.; Rediske, J.; Lotz, M.; Schmidt, A.M.; Terkeltaub, R. Inflammation-induced chondrocyte hypertrophy is driven by receptor for advanced glycation end products. J. Immunol. 2005, 175, 8296–8302. [Google Scholar] [CrossRef] [PubMed]
- Yammani, R.R.; Carlson, C.S.; Bresnick, A.R.; Loeser, R.F. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheumatol. 2006, 54, 2901–2911. [Google Scholar] [CrossRef]
- Manninen, P.; Riihimaki, H.; Heliovaara, M.; Makela, P. Overweight, gender and knee osteoarthritis. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 595–597. [Google Scholar] [PubMed]
- Felson, D.T.; Zhang, Y.; Hannan, M.T.; Naimark, A.; Weissman, B.; Aliabadi, P.; Levy, D. Risk factors for incident radiographic knee osteoarthritis in the elderly: The Framingham study. Arthritis Rheumatol. 1997, 40, 728–733. [Google Scholar] [CrossRef]
- Carman, W.J.; Sowers, M.; Hawthorne, V.M.; Weissfeld, L.A. Obesity as a risk factor for osteoarthritis of the hand and wrist: A prospective study. Am. J. Epidemiol. 1994, 139, 119–129. [Google Scholar] [PubMed]
- Cicuttini, F.; Baker, J.; Spector, T. The association of obesity with osteoarthritis of the hand and knee in women: A twin study. J. Rheumatol. 1996, 23, 1221–1226. [Google Scholar] [PubMed]
- Margetic, S.; Gazzola, C.; Pegg, G.G.; Hill, R.A. Leptin: A review of its peripheral actions and interactions. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1407–1433. [Google Scholar] [CrossRef] [PubMed]
- Aspden, R.; Scheven, B.; Hutchison, J. Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism. Lancet 2001, 357, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Figenschau, Y.; Knutsen, G.; Shahazeydi, S.; Johansen, O.; Sveinbjornsson, B. Human articular chondrocytes express functional leptin receptors. Biochem. Biophys. Res. Commun. 2001, 287, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Dumond, H.; Presle, N.; Terlain, B.; Mainard, D.; Loeuille, D.; Netter, P.; Pottie, P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheumatol. 2003, 48, 3118–3129. [Google Scholar] [CrossRef]
- Van Beuningen, H.M.; Glansbeek, H.L.; van der Kraan, P.M.; van den Berg, W.B. Osteoarthritis-like changes in the murine knee joint resulting from intra-articular transforming growth factor-B injections. Osteoarthr. Cartil. 2000, 8, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Lago, R.; Lago, F.; Reino, J.J.; Gualillo, O. Signalling pathway involved in nitric oxide synthase type II activation in chondrocytes: Synergistic effect of leptin with interleukin-1. Arthritis Res. Ther. 2005, 7, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, G.; Loreto, C.; Imbesi, R.; Trovato, F.M.; di Giunta, A.; Lombardo, C.; Castorina, S.; Castrogiovanni, P. Advantages of exercise in rehabilitation, treatment and prevention of altered morphological features in knee osteoarthritis. A narrative review. Histol. Histopathol. 2014, 29, 707–719. [Google Scholar] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sports 2014. [Google Scholar] [CrossRef]
- Pichler, K.; Loreto, C.; Leonardi, R.; Reuber, T.; Weinberg, A.M.; Musumeci, G. In rat with glucocorticoid-induced osteoporosis, RANKL is downregulated in bone cells by physical activity (treadmill and vibration stimulation training). Histol. Histopathol. 2013, 28, 1185–1196. [Google Scholar] [PubMed]
- Mow, V.C.; Ratcliffe, A.; Poole, A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials 1992, 13, 67–97. [Google Scholar] [CrossRef] [PubMed]
- Macirowski, T.; Tepic, S.; Mann, R.W. Cartilage stresses in the human hip joint. J. Biomech. Eng. 1994, 116, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ateshian, G.A.; Wang, H.; Lai, W.M. The role of interstitial fluid pressurization and surface porosities on the boundary friction of articular cartilage. J. Tribol. 1998, 120, 241–251. [Google Scholar] [CrossRef]
- Soltz, M.A.; Ateshian, G.A. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J. Biomech. 1998, 31, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Akizuki, S.; Mow, V.C.; Muller, F.; Pita, J.C.; Howell, D.S. Tensile properties of human knee joint cartilage. II. Correlations between weight bearing and tissue pathology and the kinetics of swelling. J. Orthop. Res. 1987, 5, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.M.; Hou, J.S.; Mow, V.C. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J. Biomech. Eng. 1991, 113, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Leonardi, R.; Carnazza, M.L.; Cardile, V.; Pichler, K.; Weinberg, A.M.; Loreto, C. Aquaporin 1 (AQP1) expression in experimentally induced osteoarthritic knee menisci: An in vivo and in vitro study. Tissue Cell 2013, 45, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; Lo Castro, E.; Musumeci, G.; Loreto, F.; Rapisarda, G.; Rezzani, R.; Castorina, S.; Leonardi, R.; Rusu, M.C. Aquaporin 1 expression in human temporomandibular disc. Acta Histochem. 2012, 114, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Messier, S.P.; Leagult, C.; Mihalko, S.; Miller, G.D.; Loeser, R.F.; DeVita, P.; Lyles, M.; Eckstein, F.; Hunter, D.J.; Williamson, J.D.; et al. The Intensive Diet and Exercise for Arthritis (IDEA) trial: Design and rationale. BMC Muscul. Dis. 2009, 10. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Lane, L.E. Athletics and osteoarthritis. Am. J. Sports Med. 1997, 25, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Cardile, V.; Leonardi, R. Acute injury affects lubricin expression in knee menisci. An immunohistochemical study. Ann. Anat. 2013, 195, 151–158. [Google Scholar]
- Slauterbeck, J.; Kousa, P.; Clifton, B. Geographic mapping of meniscus and cartilage lesions associated with anterior cruciate ligament injuries. J. Bone Jt. Surg. Am. 2009, 91, 2094–2103. [Google Scholar] [CrossRef]
- Giunta, S.; Castorina, A.; Marzagalli, R.; Szychlinska, M.A.; Pichler, K.; Mobasheri, A.; Musumeci, G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int. J. Mol. Sci. 2015, 16, 5922–5944. [Google Scholar] [CrossRef]
- Di Rosa, M.; Szychlinska, M.A.; Tibullo, D.; Malaguarnera, L.; Musumeci, G. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur. J. Histochem. 2014, 58, 213–221. [Google Scholar] [CrossRef]
- Hadler, N.M.; Gillings, D.B.; Imbus, H.R.; Levitin, P.M.; Makuc, D.; Utsinger, P.D.; Yount, W.J.; Slusser, D.; Moskovitz, N. Hand structure and function in an industrial setting. Arthritis Rheumatol. 1978, 21, 210–220. [Google Scholar] [CrossRef]
- Jonsson, H.; Manolescu, I.; Stefansson, E.; Ingvarsson, T.; Jonsson, H.H.; Manolescu, A.; Gulcher, J.; Stefansson, K. The inheritance of hand osteoarthritis in Iceland. Arthritis Rheumatol. 2003, 48, 391–395. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst Century: Risk Factors and Behaviours that Influence Disease Onset and Progression. Int. J. Mol. Sci. 2015, 16, 6093-6112. https://doi.org/10.3390/ijms16036093
Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the XXIst Century: Risk Factors and Behaviours that Influence Disease Onset and Progression. International Journal of Molecular Sciences. 2015; 16(3):6093-6112. https://doi.org/10.3390/ijms16036093
Chicago/Turabian StyleMusumeci, Giuseppe, Flavia Concetta Aiello, Marta Anna Szychlinska, Michelino Di Rosa, Paola Castrogiovanni, and Ali Mobasheri. 2015. "Osteoarthritis in the XXIst Century: Risk Factors and Behaviours that Influence Disease Onset and Progression" International Journal of Molecular Sciences 16, no. 3: 6093-6112. https://doi.org/10.3390/ijms16036093