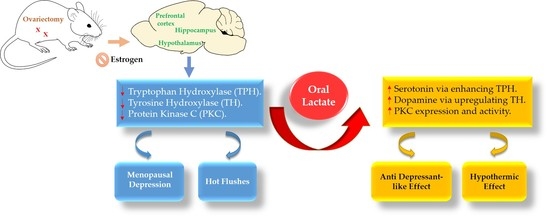
The Antidepressant-Like Effect of Lactate in an Animal Model of Menopausal Depression
Abstract
:1. Introduction
2. Experimental Section
2.1. Animal
2.2. Experiments Schedule
2.2.1. 1st Experiment
2.2.2. 2nd Experiment
2.3. Drug Administration
2.4. Ovariectomy Surgery (OVX)
2.5. Immobilization Stress
2.6. Tail Suspension Test (TST)
2.7. Forced Swimming Test (FST)
2.8. Cutaneous Body Temperature Measurement
2.9. Enzyme-Linked Immunosorbent Assay (ELISA) for Serotonin (5-HT) and Dopamine (DA) Measurements in Hypothalamus, Hippocampus, and Prefrontal Cortex
2.10. Immunohistochemistry for Tyrosine Hydroxylase (TH), Tryptophan Hydroxylase (TPH), and Protein Kinase C (PKC)
2.11. Statistical Analysis
3. Results
3.1. Oral Lactate Administration Induced Antidepressant-Like Effect in TST
3.2. Oral Lactate Administration Decreased Immobility Time in FST
3.3. Oral Lactate Administration Produced a Reduction in Cutaneous Body Temperature in Rats with OVX and Stress
3.4. Oral Lactate Administration Elevated 5-HT and DA Concentrations in the Hypothalamus, Hippocampus, and Prefrontal Cortex (PFC)
3.5. Oral Lactate Administration Enhanced TH Expression in Locus Coeruleus (LC)
3.6. Oral Lactate Administration Enhanced Immunohistochemistry Reaction of TPH in Hypothalamic Nuclei
3.7. Oral Lactate Administration Upregulated TPH Expression in Peduncular Part of the Lateral Hypothalamic (PLH)
3.8. Oral Lactate Administration Regulated PKC Expression in Hypothalamic Area
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. World Health Organization, 2017. Available online: http://www.who.int/iris/handle/10665/254610. License: CC BY-NC-SA 3.0 IGO (accessed on 14 February 2017).
- Dalal, P.K.; Agarwal, M. Postmenopausal syndrome. Indian J. Psychiatry 2015, 57, S222–S232. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.H.; Ninan, P.T. Depression or menopause? Presentation and management of major depressive disorder in perimenopausal and postmenopausal women. Prim. Care Companion J. Clin. Psychiatry 2010, 12, PCC 08r00747. [Google Scholar] [CrossRef]
- Low, D.A.; Davis, S.L.; Keller, D.M.; Shibasaki, M.; Crandall, C.G. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause 2008, 15, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronenberg, F.; Cote, L.J.; Linkie, D.M.; Dyrenfurth, I.; Downey, J.A. Menopausal hot flashes: Thermoregulatory, cardiovascular, and circulating catecholamine and LH changes. Maturitas 1984, 6, 31–43. [Google Scholar] [CrossRef]
- Freedman, R.R. Hot flashes: Behavioral treatments, mechanisms, and relation to sleep. Am. J. Med. 2005, 118, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.; Villringer, A.; Sacher, J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 2015, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Gundlah, C.; Alves, S.E.; Clark, J.A.; Pai, L.Y.; Schaeffer, J.M.; Rohrer, S.P. Estrogen receptor-beta regulates tryptophan hydroxylase-1 expression in the murine midbrain raphe. Biol. Psychiatry 2005, 57, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Zsarnovszky, A.; Scalise, T.J.; Horvath, T.L.; Naftolin, F. Estrogen effects on tyrosine hydroxylase-immunoreactive cells in the ventral mesencephalon of the female rat: Further evidence for the two cell hypothesis of dopamine function. Brain Res. 2000, 868, 363–366. [Google Scholar] [CrossRef]
- Cordey, M.; Gundimeda, U.; Gopalakrishna, R.; Pike, C.J. Estrogen activates protein kinase C in neurons: role in neuroprotection. J. Neurochem. 2003, 84, 1340–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, P. How protein kinase C activation protects nerve cells from oxidative stress-induced cell death. J. Neurosci. 2001, 21, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Coull, M.A.; Lowther, S.; Katona, C.L.; Horton, R.W. Altered brain protein kinase C in depression: A post-mortem study. Eur. Neuropsychopharmacol. 2000, 10, 283–288. [Google Scholar] [CrossRef]
- Abrial, E.; Etievant, A.; Betry, C.; Scarna, H.; Lucas, G.; Haddjeri, N.; Lambás-Señas, L. Protein kinase C regulates mood-related behaviors and adult hippocampal cell proliferation in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rivera, N.M.; Fernandez-Guasti, A.; Ramirez-Rodriguez, G.; Estrada-Camarena, E. Acute stress further decreases the effect of ovariectomy on immobility behavior and hippocampal cell survival in rats. Psychoneuroendocrinology 2013, 38, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Lasota, A.; Danowska-Klonowska, D. Experimental osteoporosis—Different methods of ovariectomy in female white rats. Rocz. Akad. Med. Bialymst. 2004, 49, 129–131. [Google Scholar] [PubMed]
- Nibuya, M.; Takahashi, M.; Russell, D.S.; Duman, R.S. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci. Lett. 1999, 267, 81–84. [Google Scholar] [CrossRef]
- Vyas, A.; Mitra, R.; Shankaranarayana Rao, B.S.; Chattarji, S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002, 22, 6810–6818. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, E.; Luarte, A.; Santibanez, M.; Varas-Godoy, M.; Toledo, J.; Diaz-Veliz, G.; Cavada, G.; Rubio, F.J.; Wyneken, U. Two Chronic Stress Models Based on Movement Restriction in Rats Respond Selectively to Antidepressant Drugs: Aldolase C As a Potential Biomarker. Int. J. Neuropsychopharmacol. 2015, 18, pyv038. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef] [PubMed]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Riedel, N.; Grittner, U.; Endres, M.; Banneke, S.; Emmrich, J.V. Body temperature measurement in mice during acute illness: implantable temperature transponder versus surface infrared thermometry. Sci. Rep. 2018, 8, 3526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xu, H.; Zhu, M.; Liu, K.; Lin, B.; Luo, R.; Chen, C.; Li, M. Stress inhibits tryptophan hydroxylase expression in a rat model of depression. Oncotarget 2017, 8, 63247–63257. [Google Scholar] [CrossRef] [PubMed]
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragniere, L.; Meylan, E.M.; Petit, J.M.; Fiumelli, H.; Magistretti, P.J.; Martin, J.L. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 2018, 23, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.; Tang, J.M.; Ludvig, N.; Bergold, P.J. Elevated lactate suppresses neuronal firing in vivo and inhibits glucose metabolism in hippocampal slice cultures. Brain Res. 2006, 1117, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.R. Menopausal hot flashes: mechanisms, endocrinology, treatment. J. Steroid Biochem. Mol. Biol. 2014, 142, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Newsom, D.M.; Bolgos, G.L.; Colby, L.; Nemzek, J.A. Comparison of body surface temperature measurement and conventional methods for measuring temperature in the mouse. Contemp. Top. Lab. Anim. Sci. 2004, 43, 13–18. [Google Scholar] [PubMed]
- Vianna, D.M.; Carrive, P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. Neuropsychopharmacol. 2005, 21, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- MacLeay, J.M.; Lehmer, E.; Enns, R.M.; Mallinckrodt, C.; Bryant, H.U.; Turner, A.S. Central and peripheral temperature changes in sheep following ovariectomy. Maturitas 2003, 46, 231–238. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Wehr, T.A.; Rosenthal, N.E.; Bartko, J.J.; Oren, D.A.; Luetke, C.; Murphy, D.L. Serotonin and thermoregulation. Physiologic and pharmacologic aspects of control revealed by intravenous m-CPP in normal human subjects. Neuropsychopharmacology 1995, 13, 105–115. [Google Scholar] [CrossRef]
- Lee, T.F.; Mora, F.; Myers, R.D. Dopamine and thermoregulation: an evaluation with special reference to dopaminergic pathways. Neurosci. Biobehav. Rev. 1985, 9, 589–598. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J. Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry 2008, 69, 4–7. [Google Scholar] [PubMed]
- Nutt, D.J. The role of dopamine and norepinephrine in depression and antidepressant treatment. J. Clin. Psychiatry 2006, 67, 3–8. [Google Scholar] [PubMed]
- Wang, Z.; Li, J.; Wang, Z.; Xue, L.; Zhang, Y.; Chen, Y.; Su, J.; Li, Z. l-Tyrosine improves neuroendocrine function in a mouse model of chronic stress. Neural. Regen. Res. 2012, 7, 1413–1419. [Google Scholar] [PubMed]
- Mateos, S.S.; Sanchez, C.L.; Paredes, S.D.; Barriga, C.; Rodriguez, A.B. Circadian levels of serotonin in plasma and brain after oral administration of tryptophan in rats. Basic Clin. Pharmacol. Toxicol. 2009, 104, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Devoto, P.; Flore, G.; Saba, P.; Fa, M.; Gessa, G.L. Stimulation of the locus coeruleus elicits noradrenaline and dopamine release in the medial prefrontal and parietal cortex. J. Neurochem. 2005, 92, 368–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devoto, P.; Flore, G. On the origin of cortical dopamine: Is it a co-transmitter in noradrenergic neurons? Curr. Neuropharmacol. 2006, 4, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Guiard, B.P.; El Mansari, M.; Merali, Z.; Blier, P. Functional interactions between dopamine, serotonin and norepinephrine neurons: An in-vivo electrophysiological study in rats with monoaminergic lesions. Int. J. Neuropsychopharmacol. 2008, 11, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Greene, R.W. CNS dopamine transmission mediated by noradrenergic innervation. J. Neurosci. 2012, 32, 6072–6080. [Google Scholar] [CrossRef] [PubMed]
- Kempadoo, K.A.; Mosharov, E.V.; Choi, S.J.; Sulzer, D.; Kandel, E.R. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. USA 2016, 113, 14835–14840. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.G.; Dupret, D. Two sources of dopamine for the hippocampus. Trends Neurosci. 2017, 40, 383–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borodovitsyna, O.; Flamini, M.D.; Chandler, D.J. Acute Stress Persistently Alters Locus Coeruleus Function and Anxiety-like Behavior in Adolescent Rats. Neuroscience 2018, 373, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Felten, D.L.; O’Banion, M.K.; Maida, M.S. 13–Telencephalon. In Netter’s Atlas of Neuroscience, 3rd ed.; Elsevier: Philadelphia, PA, USA, 2016; pp. 295–352. [Google Scholar]
- Gartside, S.E.; Cowen, P.J.; Sharp, T. Evidence that the large neutral amino acid l-valine decreases electrically-evoked release of 5-HT in rat hippocampus in vivo. Psychopharmacology 1992, 109, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, W.; Xiong, H.; Hoogenraad, C.C.; Krugers, H.J. Stress and excitatory synapses: From health to disease. Neuroscience 2013, 248, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.A.; Helmer-Matyjek, E.; Nairn, A.C.; Muller, T.H.; Haycock, J.W.; Greene, L.A.; Goldstein, M.; Greengard, P. Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc. Natl. Acad. Sci. USA 1984, 81, 7713–7717. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Faucon Biguet, N.; Mallet, J. Transcriptional and post-transcriptional regulation of tyrosine hydroxylase gene by protein kinase C. EMBO J. 1990, 9, 3707–3712. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.M.; Sakowski, S.A.; Geddes, T.J.; Wilkerson, C.; Haycock, J.W. Phosphorylation and activation of tryptophan hydroxylase 2: Identification of serine-19 as the substrate site for calcium, calmodulin-dependent protein kinase II. J. Neurochem. 2007, 103, 1567–1573. [Google Scholar] [CrossRef] [PubMed]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaif, N.A.; Jang, D.; Cho, D.; Kim, S.; Seo, D.B.; Shim, I. The Antidepressant-Like Effect of Lactate in an Animal Model of Menopausal Depression. Biomedicines 2018, 6, 108. https://doi.org/10.3390/biomedicines6040108
Shaif NA, Jang D, Cho D, Kim S, Seo DB, Shim I. The Antidepressant-Like Effect of Lactate in an Animal Model of Menopausal Depression. Biomedicines. 2018; 6(4):108. https://doi.org/10.3390/biomedicines6040108
Chicago/Turabian StyleShaif, Noof Abdullah, Daehyuk Jang, Donghyun Cho, Sunmi Kim, Dae Bang Seo, and Insop Shim. 2018. "The Antidepressant-Like Effect of Lactate in an Animal Model of Menopausal Depression" Biomedicines 6, no. 4: 108. https://doi.org/10.3390/biomedicines6040108