Relationship Between Cancer Related Fatigue, Physical Activity Related Health Competence, and Leisure Time Physical Activity in Cancer Patients and Survivors
- 1Institute of Sport Science, Heidelberg University, Heidelberg, Germany
- 2Public Health Sciences, Penn State College of Medicine, Hershey, PA, United States
- 3Working Group Exercise Oncology, Division Medical Oncology, National Center for Tumordiseases Heidelberg and Heidelberg University Hospital, Heidelberg, Germany
- 4Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands
Background: A large body of evidence supports the positive effects of leisure time physical activity (LTPA) and exercise on cancer survivors. However, only a fraction of survivors manages to attain international PA recommendations. This can be attributed to several external and internal barriers toward PA those patients seem to encounter, with cancer related fatigue (CRF) being the most reported internal barrier. Nevertheless, self-efficacy and knowledge about the utilization of LTPA can serve as facilitators of PA, which also correspond to certain constituents of physical activity related health competence (PAHCO). Since PAHCO is not investigated in cancer survivors we investigated if PAHCO can mediate the negative relationship between CRF and LTPA.
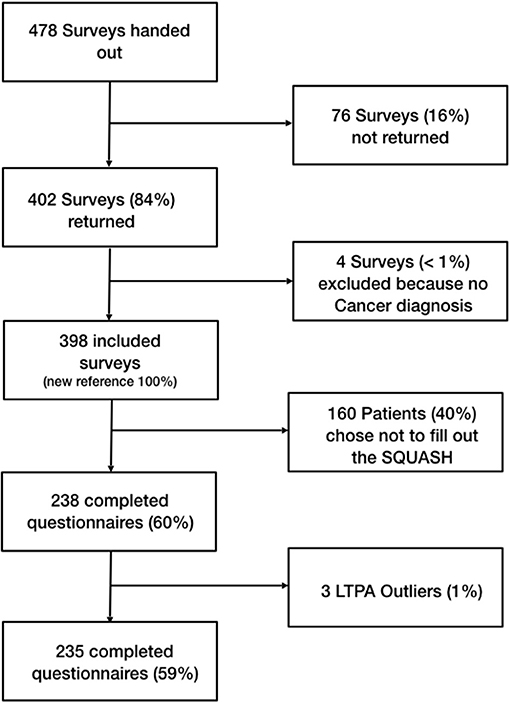
Methods: We surveyed 398 cancer survivors with different cancer types and therapy status. The patients completed the EORTC QLQ-FA12 (EORTC FA12) to assess CRF, the PAHCO questionnaire to assess PAHCO and the SQUASH to assess LTPA. We followed a two-step structural equation modeling approach. The first step established the measurement model, in the second step we fitted the mediation model. Since 163 patients chose not to answer the SQUASH, the mediation model was only fitted to the data of the remaining 235 participants.
Results: The proposed measurement model of the PAHCO offered an excellent fit. We found small to moderate positive associations between the PAHCO dimensions and the LTPA, and negative moderate relationships between the PAHCO and the EORTC FA12 dimensions. We did not observe a relationship between the EORTC FA12 dimensions and the LTPA (p > 0.05). The hypothesized mediation models did not display an appropriate fit.
Conclusion: The PAHCO confirmed its factorial validity; furthermore, it appears to have a positive relationship to LTPA. Therefore, integrating psycho-educational aspects can be beneficial in order to increase the PAHCO in exercise interventions. Because of the cross-sectional character of this study, causal conclusions are not suitable, therefore the longitudinal relationships of LTPA, CRF, and PAHCO require further investigation.
Introduction
A large body of evidence supports the positive effects of physical activity (PA) and exercise for cancer patients all along the cancer trajectory (Fong et al., 2012; Furmaniak et al., 2016; Christensen et al., 2018). Exercise has shown to increase physical fitness (Strasser et al., 2013; Scott et al., 2018; Sweegers et al., 2019), health related quality of life (Mishra et al., 2012; Buffart et al., 2017) and improvement in cancer-related fatigue (CRF; Mustian et al., 2017; Van Vulpen et al., 2020). Prior to initial treatment, exercise can improve the fitness of cancer patients and reduce the likelihood of certain complications (Vermillion et al., 2018). During treatment, exercise has shown preventive health effects on cardio- and neurotoxicity (Chen et al., 2017; Kleckner et al., 2018); furthermore, it is closely associated with reducing cancer mortality and the probability of recurrence (Cormie et al., 2017; Friedenreich et al., 2020). Despite the benefits of regular PA, cancer patients tend to reduce their PA-levels after their diagnosis (Mason et al., 2013; Pugh et al., 2020) and only a minority of cancer survivors meet the PA guidelines (Blanchard et al., 2008; Lin et al., 2019). This can be contributed to particular internal as well as external barriers (e.g., the availability of programs and qualified facilities) cancer patients have to overcome, such as the frequently reported internal barrier of CRF (Brunet et al., 2013; Hardcastle et al., 2018).
CRF is one of the most common and distressing symptoms reported by cancer patients, produced by the cancer as well as its treatment (Bower, 2014; Berger et al., 2015). It is defined as a feeling intensive physical, emotional and cognitive exhaustion, without getting relieve after resting or sleeping (Weis, 2011). By the time cancer is diagnosed, 40% of patients already report signs of CRF (Rüffer, 2019). This proportion goes up to 60–100% during cancer treatments like chemotherapy and radiotherapy (Weis, 2011). CRF may even continue to impact the lives of long-term survivors for up to 10 years, as noted in 25–33% of cases (Bower, 2014). As of right now, there is no promising pharmacological treatment for CRF (Bower, 2014), with the most effective approach to mitigate CRF being PA (Mustian et al., 2017). This means cancer patients are often trapped in a vicious circle, since CRF prevents PA and thus increases CRF symptoms.
Patients need certain skills, knowledge and motivation (Schmid et al., 2020), i.e., they need competence, in order to positively engage in an active lifestyle. Thus, Pfeifer et al. (2013) developed the model of domain-specific physical activity-related health competence (PAHCO), inspired by the health model of Lenartz (2012), which focuses on promoting person-oriented competencies that enable a person to integrate an effective PA level into daily life. It contains three sub-competences (movement competence, control competence, and PA-specific self-regulation competence). Movement competence is a necessary condition that enables a person to realize the health benefits of exercise and everyday PA. Basically, movement competence comprises of motor skills and abilities. Control competence describes the degree a person is capable of utilizing their knowledge, based on the positive effects of exercise, how to implement health enhancing PA in their daily life, and how to control physical load in regards to their body signals, such as breathing, perceived exertion or heart rate. The third sub-competence, PA-specific self-regulation competence, encompasses the motivational and volitional abilities of a person to engage in health enhancing PA and implementing it into daily life (Sudeck and Pfeifer, 2016). Each of these sub-competencies contributes to regular health-promoting activity behavior. Sudeck and Pfeifer proposed a questionnaire to operationalize PAHCO, which predicts the PA-levels of patients undergoing medical rehabilitation (Sudeck and Pfeifer, 2016; Carl et al., 2020a).
A typical characteristic of CRF is its impairing effect on motivation (Brunet et al., 2013; Hardcastle et al., 2018); furthermore, CRF shows a positive relationship with depression (Bower et al., 2000; Brown et al., 2013). Considering that motivation and volition help using PA as constituents of the PAHCO model, PAHCO can play an important role in the correlation between CRF and LTPA. Since physical fatigue (PF) shows a certain sensitivity to PA (Van Vulpen et al., 2016), we would expect the relationships to be the strongest for this fatigue dimension. Because we were interested in the voluntary engagement of PA and exercise, in the hypotheses we focused on leisure time physical activity (LTPA). We therefore derived following five hypotheses.
• H1: The CRF dimensions display negative correlations with LTPA
• H2: The PAHCO dimensions display positive correlations with LTPA
• H3: The CRF dimensions display negative correlations with PAHCO dimensions
• H4: The PAHCO dimensions mediate the impact of CRF on LTPA
• H5: The relationships are strongest for PF.
We first investigated the factorial validity of the PAHCO questionnaire in an US based sample, since the PAHCO questionnaire has thus far only been investigated in German speaking populations (Sudeck and Pfeifer, 2016), afterwards we tested the hypotheses in a structural model.
Materials and Methods
Participants
Patients with any kind of cancer who were meeting the following inclusion criteria were eligible to participate in the study: ≥18 years, mobile enough to conduct exercise, and able to follow the study instructions. Patients were approached and asked to participate when they came to their treatment or follow-up.
Procedure
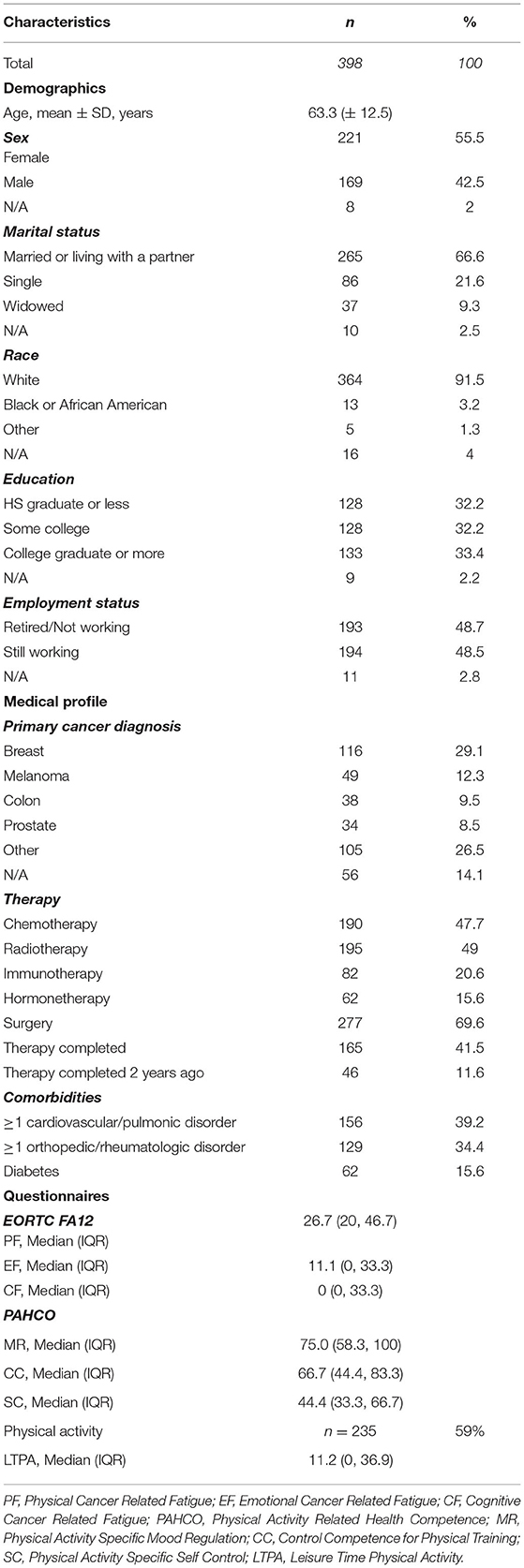
The current study followed a cross-sectional design conducted at the Penn State Cancer Institute in Hershey PA, USA. The study protocol was approved by the Penn State College of Medicine Internal Review Board (Study ID: HRP-591-PEXO, Clinical Trials.gov: NCT04328038). All participants signed a written informed consent form before completing the survey. Patients were recruited in the waiting rooms of the hospital (Infusion Suite, Radiation Oncology, Surgery), where they received and completed the paper-and-pencil survey. In only a few cases the questionnaires were taken home and returned at the patient's next visit. The survey consisted of demographics and medical information, the PAHCO questionnaire (Sudeck and Pfeifer, 2016), the Emotion Thermometers (Mitchell et al., 2010), the Perception of Health Scale (Diamond et al., 2007), the BRIEF Health Literacy Screening Tool (Haun et al., 2009), the EORTC QLQ-C30 (Fayers et al., 2002), EORTC QLQ_FA12 (EORTC FA12) (Weis et al., 2017) and the short questionnaire to assess health-enhancing physical activity (SQUASH) (Wendel-Vos et al., 2003).
In this analysis, the PAHCO questionnaire, EORTC FA12 and SQUASH are reported for being of particular interest while the other questionnaires only served as material for missing data imputation. The time to complete the survey ranged between 15 and 20 min.
Measures and Materials
The EORTC QLQ-FA12
The EORTC FA12 is a multidimensional self-reporting screening tool for assessing the extend of cancer-related fatigue. The tool was developed by the EORTC quality of life group and is used in conjunction with the EORTC QLQ-C30 (Weis et al., 2017). The questionnaire divides the CRF into three subscales using a total of 12 items: physical CRF (PF) (five items), emotional CRF (EF) (three items), and cognitive CRF (CF) (two items). The remaining two items serve as global indicators for impairment in performing daily life activities as well as the social sequelae of CRF, but they do not belong to a single subscale. All items were answered according to a four-stage Likert scale [from “not at all” (1) to “very much” (4)]. Reported Cronbach's alpha were good for all three dimensions with 0.88–0.90 for PF, 0.87–0.88 for EF and 0.79–0.82 for CF (Weis et al., 2017).
The Physical Activity Related Health Competence Questionnaire
The questionnaire is based on the PAHCO-Model outlined above and supposed to assess specific facets of the PAHCO, specifically addressing an individual's aptitude to effectively utilize physical activity to optimize their overall health. The questionnaire consists of 13 items comprised of three latent factors: PA-specific mood regulation (MR) (four items), control competence for physical training (CC) (six items) and PA-specific self-control (SC) (three items) (Sudeck and Pfeifer, 2016). In contrast to the PAHCO-Model, the PAHCO Questionnaire has no items to assess movement competence but instead focuses on the implementation (SC) and utilization (MR) of health enhancing PA, the control of physical load via body signals, as well as knowledge about the effects (CC). All items were answered on a four-stage Likert scale with possible responses ranging from “disagree completely” (1) to “agree completely” (4). Cronbach's alpha was good for all three dimensions with 0.89 for MR, 0.84 for CC and 0.78 for SC in patients undergoing exercise therapy and 0.88 for MR, 0.80 for CC and 0.80 for SC in people participating in health sports (Sudeck and Pfeifer, 2016). The original PAHCO questionnaire was developed and validated on two German samples (Sudeck and Pfeifer, 2016). However, the authors provided a version translated into English in their original publication. We used this translation and had it back translated into German by a member of the Penn State College of Medicine Internal Review Board who is both an English and German native speaker.
The Short Questionnaire to Assess Health-Enhancing Physical Activity
The SQUASH, a commonly used instrument to assess PA behavior in adults compares the physical activity levels of individuals and evaluates compliance to physical activity guidelines (Nicolaou et al., 2016). The SQUASH was developed by the Dutch National Institute of Public Health and the Environment (Wendel-Vos et al., 2003). It relies on self-reports, assessing 4 main domains: (a) commuting activities, (b) leisure time activities, (c) household activities, and (d) activities at work and school, which are evaluated based on an average week. The participants rate the amount of time they spent on each domain using three main queries: days per week, average time per day, and intensity (effort). To quantify the intensity of the activity, a metabolic equivalent task (MET) value, based on Ainsworth's compendium of PA (Ainsworth et al., 2000) is assigned to the activities, depending on the effort reported, the activities receive an intensity score and total score.
Data Analysis
Descriptive statistics (i.e., means, standard deviations, inter quartile ranges, skewness, and kurtosis) were calculated. Linearity and collinearity between the single items were assessed by comparing bivariate Pearson-, Spearman-, and Kendall-tau-b correlation coefficients; in addition, optical bivariate scatter plots where we added to the Likert-scale items to increase interpretability (Gelman and Hill, 2007).
Questionnaires
The analysis of the hypothesized mediation model involved a two-step process. In the first step a confirmatory factor analysis (CFA) was conducted to investigate the factorial validity of the proposed measurement models. In the second step the structural model was established (Anderson and Gerbing, 1988).
Items from both questionnaires show a good agreement regarding the three correlation coefficients and therefore indicate linearity in their relationships, which is supported by the scatter plots. The normality assumption was tested via Shapiro–Wilk-Test for univariate normality and Mardia-Test for multidimensional normality. Univariate as well as multivariate normality was violated for all items in both questionnaires (p < 0.001).
Leisure Time Physical Activity
The univariate normality of the LTPA data was tested with the Shapiro–Wilk Test. For the outlier identification we conducted a qualitative approach by visually examining the LTPA distribution. Three values beyond 150 MET-h/week were identified which did not fit into the remaining distribution and thus were omitted from the data set. The remaining LTPA-data violated the assumption of normality (p < 0.001). Because of the violation of the normality assumption, we conducted a Box-Cox transformation by the power of 1/6 with the LTPA-data, resulting in an improved approximation of the normal distribution (p = 0.055). The raw data and the transformed data showed a Pearson-correlation of r = 0.89.
Missing Data
We excluded cases when the entire questionnaire was missing and acknowledged one missing entry for age and eight missing entries for gender. In the remaining data set 2–7 (0.5–1.7%) answers for the PAHCO items were missing and for the EORTC-FA 12 1–2 (0.25–0.5%) answers were missing. The data showed a general missing data pattern. We investigated the appropriateness of the variables for multiple imputations via influx and outflux diagrams (Van Buuren, 2018). All variables except of LTPA were appropriate for the imputation procedure. The comparison of the original and imputed variables via Mann–Whitney-U-Test found no evidence for differences between the original and the imputed data sets (Range of p = [0.79, 1.0]).
Measurement Model Estimation and Model Fit
The measurement models were estimated with the variance of the latent factors set to 1.0, this allowed us to compare the observed factor loadings to the ones reported in the original publications. Because of the violation of the normality assumption, we applied the maximum likelihood approach with a 10,000-replication bootstrap with 95% bias corrected confidence interval as well as the DWLS estimation, treating the items of the questionnaires as ordinal. Evaluation of the model fit was based on chi-squared (χ2) tests, the chi-square-degrees of freedom ratio (χ2/df), the Root Means Square Error of Approximation (RMSEA), the standardized Root Mean Square Residual (SRMR) as well as the Comparative Fit Index (CFI) and the Tucker Lewis Index (TLI). For the χ2/df a value <3 was considered as acceptable (Iacobucci, 2010). For CFI and TLI values >0.90 were considered acceptable and >0.95 considered as good, for RMSEA and SRMR values <0.06 were considered as good and <0.08 as acceptable (Hu and Bentler, 1999). The measurement model which displayed the better fit was included into the structural model. Scale reliability was measured via McDonald's omega as an alternative to Cronbach's alpha, appreciating variability in factor loadings (Dunn et al., 2014). Discriminant validity between the factors was assessed using the Fornell–Larcker-Criterion. In this case the average variance extracted (AVE) should be larger than the highest squared inter-factor correlation corresponding to the variance shared by these two factors, i.e., the variance of the latent variable which can be attributed to its the indicators instead of measurement error (Fornell and Larcker, 1981; Rönkkö and Cho, 2020).
Structural Model
The three PAHCO dimensions as well as the three Fatigue dimensions were considered latent variables, as postulated in the literature (Sudeck and Pfeifer, 2016; Weis et al., 2017). The transformed LTPA was considered a manifest variable. To test the hypotheses we fitted three separate models for each CRF dimension where the relationship between CRF and LTPA is mediated by the three PAHCO (Figure 1).
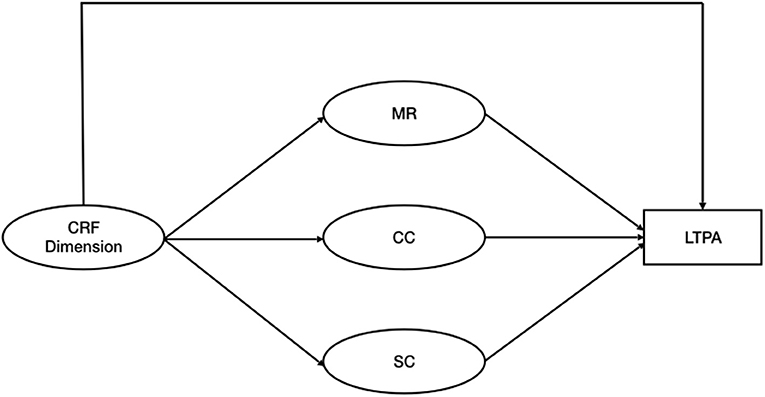
Figure 1. Hypothesized mediation model. CRF, Cancer Related Fatigue; MR, Physical Activity Specific Mood Regulation; CC, Control Competence for Physical Training; SC, Physical Activity Specific Self Control; LTPA, Leisure Time Physical Activity.
We calculated direct, indirect and total effects. To evaluate the model fit the same parameters and criteria as outlined for the measurement models were applied.
All analysis were conducted in R (Team, 2013). Multiple Imputation was done via the R-package mice (Van Buuren and Groothuis-Oudshoorn, 2011), CFA and structural model were conducted via R-package lavaan (Rosseel, 2012).
Results
Participants
In total a sample of 398 cancer patients (169 men and 221 women, 8 unknown) were surveyed. Initially 478 surveys were handed out, resulting in a return rate of 84%. The mean age of the participants was 63 years, with the age ranging from 22 to 89 years. The sample spanned over all levels of educational achievement. Approximately half of all participants reported treatment with chemotherapy, while half of the participants reported undergoing radiotherapy. Forty-two percent of participants have already finished their tumor therapy, of which 28% finished their therapy more than 2 years ago. Twenty-two percent of participants were still undergoing therapy at the time the survey was completed. Twenty-six percent did not provide information about their treatment. Twenty-nine percent of the sample were breast cancer survivors, and 14% of participants did not provide information about their cancer site. Two participants were excluded for having no history of cancer (Table 1; Figure 2). One hundred and sixty-three participants chose not to answer the SQUASH questionnaire. These patients displayed significant differences (p < 0.05) in four of the 13 PAHCO items (Item 1–3, loading on MR and Item 11 loading on SC) as well as five items (Item 1–3 loading on PF and Item 9–10 loading on CF) of the EORTC FA12. In the case of the PAHCO items, the proportion who completed the SQUASH showed higher scores in each of the four items, indicating a higher PAHCO. Simultaneously, those ones who did not complete the SQUASH showed higher scores in each of the five concerned EORTC FA12 items, indicating higher levels of CRF.
Measurement Models
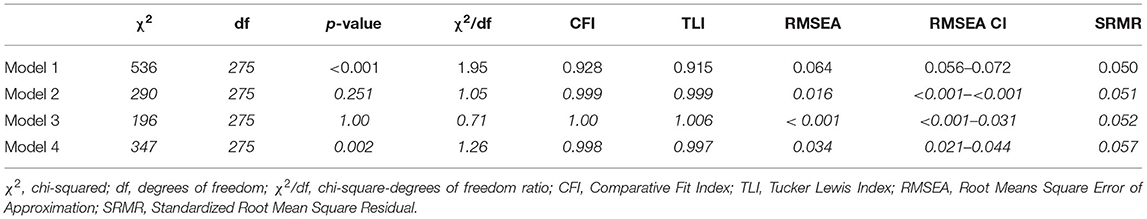
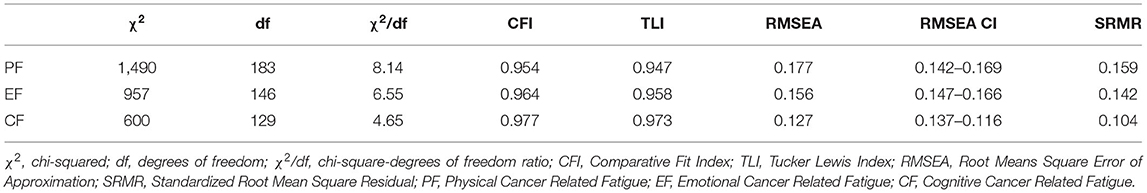
The confirmatory factor analysis measured three latent factors for the PAHCO (MR, CC, and SC) as well as the EORTC FA12 (PF, EF, and CF). All three PAHCO and EORTC factors showed good reliability with McDonald's omega of 0.94 for MR, 0.93 for CC and 0.92 for SC and 0.94 for PF, 0.89 for EF and 0.84 for CF. In order to assess discriminant validity we looked at the AVE from the indicators of 0.85 for MR, 0.67 for CC, and 0.87 for SC for the PAHCO as well as 0.79 for PF, 0.77 for EF, and 0.84 for CF for the EORTC FA12. In comparison the squared factor correlations between the latent variables is 0.23 for MR and SC, 0.33 for MR and CC, 0.55 for CC and SC, 0.56 for PF and EF, 0.41 for PF and CF and 0.60 for EF and CF in case of the EORTC FA12. Thus, the tallest shared variance between the factors appears to be smaller than the smallest AVE. Of the four different measurement models, the DWLS estimation treating the EORTC FA12 items as ordinal and the PAHCO items as continuous (Model 2) showed the best fit [ = 196, p = 1.00], with all fit criteria indicating good to excellent fit (Table 2). The second best fit is displayed by the model treating the PAHCO items as ordinal and the EORTC FA12 items as continuous (Model 3) [ = 290, p = 0.251] The model treating all items ordinal (Model 4)showed a slightly worse fit than Model 2 and 3. The least fitting model was Model 1 that treated all items continuous [ = 536, p = 0.251]. Thus, for all following analyses we opted for Model 2.
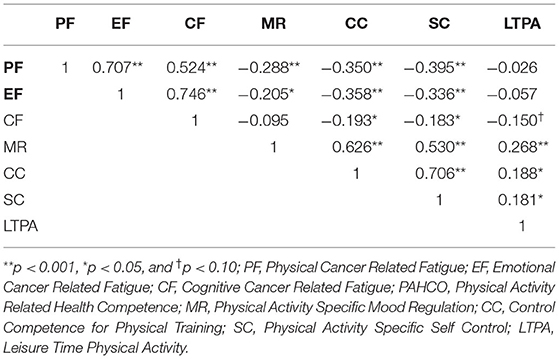
Latent factors within the same questionnaire showed moderate to strong positive associations with each other [0.5–0.7].
Structural Models
No association between LTPA and the CRF dimensions was observed (p > 0.05) with respect to the borderline significant negative association of −0.15 (p = 0.063) between CF and LTPA (Rejection of H1). The latent factors showed small to moderate negative correlations between the questionnaires [0.2–0.4], i.e., the CRF dimensions were negatively associated with the PAHCO dimensions (Confirmation of H3) (Figure 3). Small to moderate positive correlations [0.18–0.27] for all PAHCO dimensions and LTPA were observed (Table 3) (Confirmation of H2).
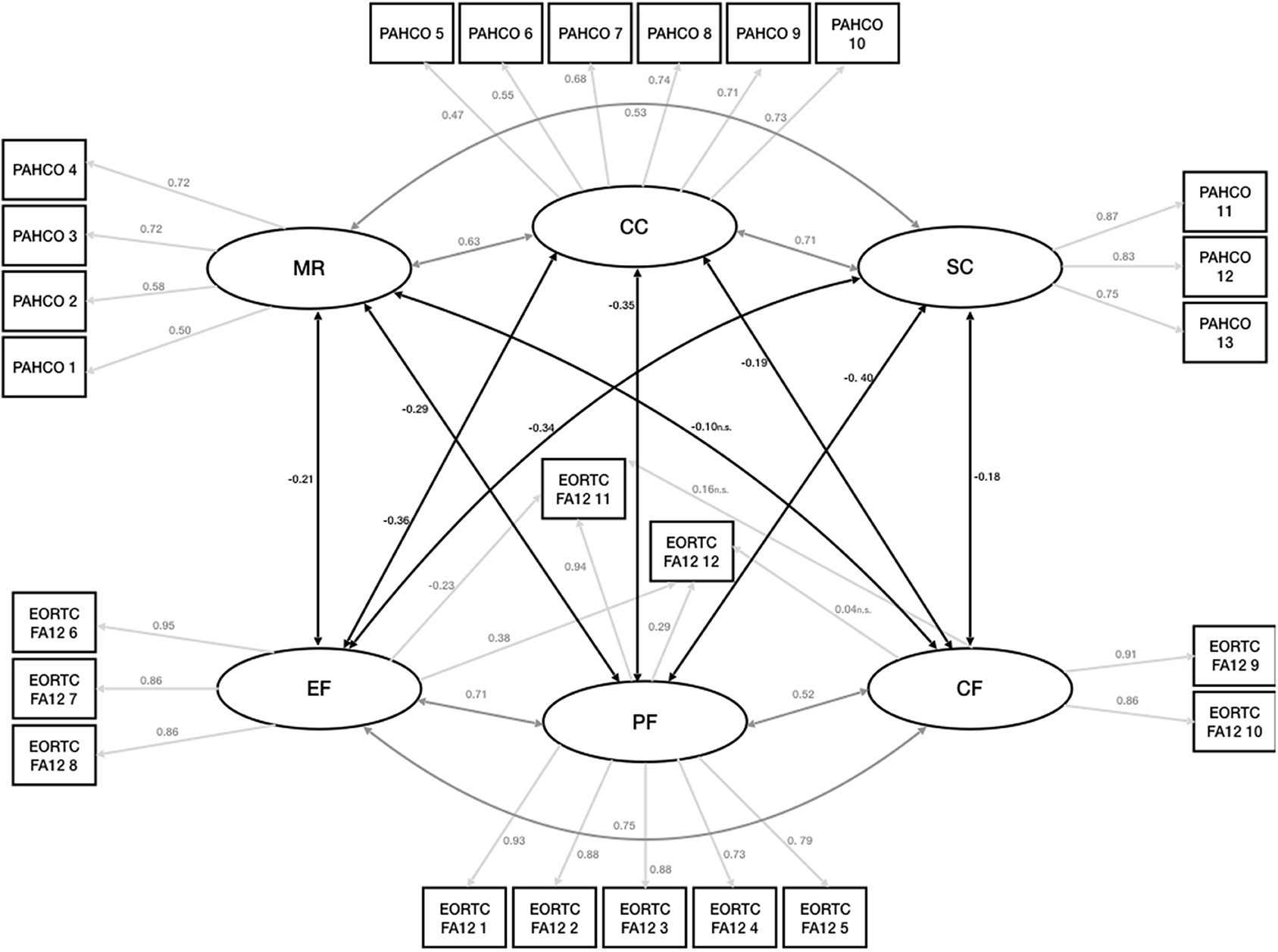
Figure 3. Measurement model and structural components. EORTC FA12, EORTC QLQ-FA12; PF, Physical Cancer Related Fatigue; EF, Emotional Cancer Related Fatigue; CF, Cognitive Cancer Related Fatigue; PAHCO, Physical Activity Related Health Competence; MR, Physical Activity Specific Mood Regulation; CC, Control Competence for Physical Training; SC, Physical Activity Specific Self Control.
The hypothesized models displayed an insufficient fit of the data regarding the mediation in all three CRF dimensions (PF, EF, and CF). Although the CFI and TLI showed acceptable to good fits, RMSEA, SRMR, chi2, and the chi/df-ratio did not meet any of the thresholds, therefore, the mediation models did not seem justified (Rejection of H4 and H5) (Table 4).
Discussion
This analysis was able to confirm the factorial validity for the American-English version of the PAHCO, involving US-American cancer patients and survivors. The loadings and correlations between the latent PAHCO variables in our model roughly coincides with the correlational structure of the German original publication, reporting slightly lower loadings on MR and CC as well as slightly higher loadings on SC. Furthermore, we identified a moderate positive association between PAHCO-dimensions and LTPA, which conforms to observations made in other populations and also confirms H2 (Sudeck and Pfeifer, 2016; Carl et al., 2020a). Of all three PAHCO dimensions, MR and LTPA showed the strongest association, while the association between LTPA and the other two remaining PAHCO dimensions had slightly smaller correlation coefficients. Ostensibly, the strong correlation between MR and LTPA can be attributed to the items loading on MR, addressing depression, stress and inner tension, which are elevated for cancer patients in comparison to the general public or even patients with other chronic diseases (Gil et al., 2012; Hartung et al., 2017; Rao et al., 2019). This may suggest that physical activity has an instrumental functionality when it comes to cancer patients since it helps them cope with psychological symptoms. It also goes in line with the recently published ACSM Roundtable on Physical Activity Guidelines for Cancer Patients (Campbell et al., 2019) which confirms a positive effect of exercise on depressive symptoms and anxiety. Nevertheless, the three correlation coefficients between LTPA and the PAHCO dimensions do not differ significantly from each other, considering their 95% confidence intervals, hence, conclusions must be drawn with caution and can be premature. According to H3, all PAHCO-dimensions displayed a negative relationship to the CRF-dimensions, however, MR showed the weakest correlation to either of the CRF dimensions, which are usually linked to mood disturbances and have a strong relation with depression (Bower et al., 2000; Brown et al., 2013).
A relationship between the CRF dimensions and LTPA has not been observed, which opposes the literature and H1, that exhibits a small to moderate negative associations between CRF and PA levels (Kummer et al., 2013; Galiano-Castillo et al., 2014; Romero et al., 2018). This could be partially explained by a possible selection bias due to a lower response rate to the SQUASH compared to the other questionnaires (about 59%). Pertaining the questionnaires, participants who chose not to answer the SQUASH scored significantly lower in three of the four items loading on MR, significantly higher in three of seven items loading on PF, and significantly higher on two of four items loading on CF. Thus, the patients who completed the SQUASH and were investigated for the final analysis presented a higher PAHCO and lower CRF than the others. Bearing in mind that the SQUASH items are more arduous to answer than the Likert-scaled questions of the other questionnaires, it appeared that people with a positive attitude toward PA in general and LTPA in particular would invest more effort into completing the survey.
We could not observe a ceiling effect or lack of variance in the LTPA-Data; though, a plausible correlation between the PAHCO-dimensions and PA as well as between the PAHCO dimensions and EORTC FA12 were established. Accordingly, we should have been able to identify a relevant association, despite the likelihood of selection bias.
A conceivable mediation for the effect of CRF on LTPA via PAHCO renders obsolete (H4 and H5) given the lack of association between the two variables. Considering the cross-sectional character of the study, derived conclusions should not indicate causation, and further investigations need to examine relationship between CRF, LTPA and the role of PAHCO in a longitudinal manner. Subsequently, the time-lagged cross-correlations between CRF and LTPA would reveal further information about the relationship between these variables along the cancer trajectory. Due to the decline in PA-levels patients experience following cancer diagnosis and treatment, finding the causes as well as potential interventions are of great interests. Besides its explanatory value, increasing PAHCO in patients could potentially increase adherence to given exercise programs and an enhanced active lifestyle. One of the most common reported intrinsic barriers of cancer patients toward LTPA is the lack of self-efficacy (Brunet et al., 2013; Phillips and McAuley, 2013; Ungar et al., 2016; Hardcastle et al., 2018). In a systematic review the perception of control over ones well-being was one of the most common facilitators of exercise (Clifford et al., 2018). Another review has shown a strong prediction of instrumental attitude and planning on LTPA levels (Vallance et al., 2008). In fact, all these constructs can be considered constituents of PAHCO.
For patient care, PAHCO could be used to assess the level of support patients require to initiate an exercise routine and LTPA. By stratifying patients in accordance with their PAHCO levels, exercise therapists would be able to provide educational instructions tailored particularly to every patient's needs. By increasing PAHCO, patients would presumably feel empowered to engage in exercise and LTPA independently. In regards to health literacy, cancer patients with lower levels of knowledge seemed to necessitate expert support more than patients with higher levels, who were also more likely to perform research themselves (Morris et al., 2013). Additionally, the stratification process may prevent the misallocation of resources by providing especially patients in need with the highest level of guidance. Consequently, PAHCO is a promising concept to promote PA in cancer patients, which would result in vital lifestyle changes and increased participation in exercise programs.
Limitations
This study seems to have a couple of limitations. First, the cross-sectional character of the study does not allow for causal conclusions. Therefore, the results from a mediation approach, which has an underlying causal preposition, will have to be interpreted with caution. An opposing relationship seems possible as well, meaning the therapeutic effect of LTPA on CRF can be utilized more efficiently by people with higher levels of PAHCO. Further research is necessary in order to investigate the relationship between LTPA, PAHCO and CRF with an experimental approach. Furthermore, movement competence, one of the three major sub-competences of the PAHCO model, was not assessed by the PAHCO questionnaire. Accordingly, the PAHCO has not been assessed in its entirety. More recent PAHCO instruments have already made suggestions to measure this dimension (Carl et al., 2020b) and should be applied to further research. Additionally, we encountered an immense loss of information due to incomplete PA-questionnaires, hence, less laborious but still reliable instruments, that assess physical activity in cancer patients, have been implemented without increasing study logistics and costs considerably, as it would be the case for accelerometers.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by PennState College of Medicine internal review board (Study ID: HRP-591-PEXO, Clincial Trials.gov: NCT04328038). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MK: data collection, conducting the statistical analysis, and writing the manuscript. CK: data entry, descriptive statistics, and writing the manuscript. RW and KS: study design, coordination of data collection, and critical review of the manuscript. JW: study design, conducting the statistical analysis, and critical review of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This survey was funded by the bi-national scholarship of the German Academic Exchange Service (DAAD). Open Access Fees are provided by the National Center for Tumor Diseases.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer SS declared a shared affiliation, with no collaboration, with the authors (CK and JW) to the handling editor at the time of review.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all patients who participated in this study. We appreciate the support of the physicians at the PennState Cancer Institute in approaching their patients. We gratefully acknowledge the support of Ilse Schilderinck and Eline DeKlerk for supporting the data collection. We would like to thank the reviewers for their thoughtful comments and efforts to improve our manuscript.
Abbreviations
AVE, Average Variance Extracted; CRF, Cancer Related Fatigue; χ2, chi-squared; χ2/df, chi-square-degrees of freedom ratio; CF, Cognitive Cancer Related Fatigue; CFI, Comparative Fit Index; CFA, Confirmatory Factor Analysis; CC, Control Competence for Physical Training; df, degrees of freedom; EF, Emotional Cancer Related Fatigue; LTPA, Leisure Time Physical Activity; MET, Metabolic Equivalent Task; MR, Physical Activity Specific Mood Regulation; SC, Physical Activity Specific Self Control; PA, Physical Activity; PAHCO, Physical Activity Related Health Competence; PF, Physical Cancer Related Fatigue; RMSEA, Root Means Square Error of Approximation; SRMR, Standardized Root Mean Square Residual; TLI, Tucker Lewis Index.
References
Ainsworth, B. E., Haskell, W. L., Whitt, M. C., Irwin, M. L., Swartz, A. M., Strath, S. J., et al. (2000). Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports Exerc. 32, S498–S504. doi: 10.1097/00005768-200009001-00009
Anderson, J. C., and Gerbing, D. W. (1988). Structural equation modeling in practice: a review and recommended two-step approach. Psychol. Bull. 103:411. doi: 10.1037/0033-2909.103.3.411
Berger, A.M., Mooney, K., Alvarez-Perez, A., Breitbart, W.S., Carpenter, K.M., Cella, D., et al. (2015). Cancer-related fatigue, version 2.2015. J. Natl. Compr. Cancer Netw. 13, 1012–1039. doi: 10.6004/jnccn.2015.0122
Blanchard, C. M., Courneya, K. S., and Stein, K. (2008). Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. J. Clin. Oncol. 26, 2198–2204. doi: 10.1200/JCO.2007.14.6217
Bower, J. E. (2014). Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 11:597. doi: 10.1038/nrclinonc.2014.127
Bower, J. E., Ganz, P. A., Desmond, K. A., Rowland, J. H., Meyerowitz, B. E., and Belin, T. R. (2000). Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J. Clin. Oncol. 18:743. doi: 10.1200/JCO.2000.18.4.743
Brown, L.F., Rand, K.L., Bigatti, S.M., Stewart, J.C., Theobald, D.E., Wu, J., et al. (2013). Longitudinal relationships between fatigue and depression in cancer patients with depression and/or pain. Health Psychol. 32:1199. doi: 10.1037/a0029773
Brunet, J., Taran, S., Burke, S., and Sabiston, C. M. (2013). A qualitative exploration of barriers and motivators to physical activity participation in women treated for breast cancer. Disabil. Rehabil. 35, 2038–2045. doi: 10.3109/09638288.2013.802378
Buffart, L.M., Kalter, J., Sweegers, M.G., Courneya, K.S., Newton, R.U., Aaronson, N.K., et al. (2017). Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 52, 91–104. doi: 10.1016/j.ctrv.2016.11.010
Campbell, K.L., Winters-Stone, K.M., Wiskemann, J., May, A.M., Schwartz, A.L., Courneya, K.S., et al. (2019). Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51, 2375–2390. doi: 10.1249/MSS.0000000000002116
Carl, J., Geidl, W., Lehbert, N., Wittmann, M., Pfeifer, K., and Schultz, K. (2020a). Körperliche Aktivität nach der pneumologischen Rehabilitation-Zur Rolle der Bewegungsbezogenen Gesundheitskompetenz (BGK). Pneumologie 74:P496. doi: 10.1055/s-0039-3403325
Carl, J., Sudeck, G., Geidl, W., Schultz, K., and Pfeifer, K. (2020b). Competencies for a healthy physically active lifestyle-Validation of an integrative model. Res. Q. Exerc. Sport 1–15. doi: 10.1080/02701367.2020.1752885. [Epub ahead of print].
Chen, J. J., Wu, P.-T., Middlekauff, H. R., and Nguyen, K.-L. (2017). Aerobic exercise in anthracycline-induced cardiotoxicity: a systematic review of current evidence and future directions. Am. J. Physiol. Heart Circ. Physiol. 312, H213–H222. doi: 10.1152/ajpheart.00646.2016
Christensen, J., Simonsen, C., and Hojman, P. (2018). Exercise training in cancer control and treatment. Compr. Physiol. 9, 165–205. doi: 10.1002/cphy.c180016
Clifford, B.K., Mizrahi, D., Sandler, C.X., Barry, B.K., Simar, D., Wakefield, C.E., et al. (2018). Barriers and facilitators of exercise experienced by cancer survivors: a mixed methods systematic review. Support. Care Cancer 26, 685–700. doi: 10.1007/s00520-017-3964-5
Cormie, P., Zopf, E. M., Zhang, X., and Schmitz, K. H. (2017). The impact of exercise on cancer mortality, recurrence, and treatment-related adverse effects. Epidemiol. Rev. 39, 71–92. doi: 10.1093/epirev/mxx007
Diamond, J. J., Becker, J. A., Arenson, C. A., Chambers, C. V., and Rosenthal, M. P. (2007). Development of a scale to measure adults' perceptions of health: preliminary findings. J. Community Psychol. 35, 557–561. doi: 10.1002/jcop.20164
Dunn, T. J., Baguley, T., and Brunsden, V. (2014). From alpha to omega: a practical solution to the pervasive problem of internal consistency estimation. Br. J. Psychol. 105, 399–412. doi: 10.1111/bjop.12046
Fayers, P., Bottomley, A., and Group, E. Q. O. L. (2002). Quality of life research within the EORTC-the EORTC QLQ-C30. Eur. J. Cancer 38, 125–133. doi: 10.1016/S0959-8049(01)00448-8
Fong, D.Y., Ho, J.W., Hui, B.P., Lee, A.M., Macfarlane, D.J., Leung, S.S., et al. (2012). Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ 344:e70. doi: 10.1136/bmj.e70
Fornell, C., and Larcker, D. F. (1981). Evaluating structural equation models with unobservable variables and measurement error. J. Market. Res. 18, 39–50. doi: 10.1177/002224378101800104
Friedenreich, C. M., Stone, C. R., Cheung, W. Y., and Hayes, S. C. (2020). Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectrum. 4:pkz080. doi: 10.1093/jncics/pkz080
Furmaniak, A. C., Menig, M., and Markes, M. H. (2016). Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 9:CD005001. doi: 10.1002/14651858.CD005001.pub3
Galiano-Castillo, N., Ariza-García, A., Cantarero-Villanueva, I., Fernández-Lao, C., Díaz-Rodríguez, L., and Arroyo-Morales, M. (2014). Depressed mood in breast cancer survivors: associations with physical activity, cancer-related fatigue, quality of life, and fitness level. Eur. J. Oncol. Nurs. 18, 206–210. doi: 10.1016/j.ejon.2013.10.008
Gelman, A., and Hill, J. (2007). Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge: Cambridge University Press.
Gil, F., Costa, G., Hilker, I., and Benito, L. (2012). First anxiety, afterwards depression: psychological distress in cancer patients at diagnosis and after medical treatment. Stress Health 28, 362–367. doi: 10.1002/smi.2445
Hardcastle, S. J., Maxwell-Smith, C., Kamarova, S., Lamb, S., Millar, L., and Cohen, P. A. (2018). Factors influencing non-participation in an exercise program and attitudes towards physical activity amongst cancer survivors. Support. Care Cancer 26, 1289–1295. doi: 10.1007/s00520-017-3952-9
Hartung, T., Brähler, E., Faller, H., Härter, M., Hinz, A., Johansen, C., et al. (2017). The risk of being depressed is significantly higher in cancer patients than in the general population: prevalence and severity of depressive symptoms across major cancer types. Eur. J. Cancer 72, 46–53. doi: 10.1016/j.ejca.2016.11.017
Haun, J., Noland-Dodd, V., Varnes, J., Graham-Pole, J., Rienzo, B., and Donaldson, P. (2009). Testing the BRIEF health literacy screening tool. Fed. Pract. 26, 24–31.
Hu, L. T., and Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Eq. Model. Multidiscipl. J. 6, 1–55. doi: 10.1080/10705519909540118
Iacobucci, D. (2010). Structural equations modeling: fit indices, sample size, and advanced topics. J. Consum. Psychol. 20, 90–98. doi: 10.1016/j.jcps.2009.09.003
Kleckner, I.R., Kamen, C., Gewandter, J.S., Mohile, N.A., Heckler, C.E., Culakova, E., et al. (2018). Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support. Care Cancer 26, 1019–1028. doi: 10.1007/s00520-017-4013-0
Kummer, F., Catuogno, S., Perseus, J. M., Bloch, W., and Baumann, F. T. (2013). Relationship between cancer-related fatigue and physical activity in inpatient cancer rehabilitation. Anticancer Res. 33, 3415–3422.
Lenartz, N. (2012). Gesundheitskompetenz und Selbstregulation: Modellbildung zur Gesundheitskompetenz unter besonderer Berücksichtigung selbstregulativer Kompetenzen. Göttingen: VandR unipress GmbH.
Lin, K.-Y., Edbrooke, L., Granger, C. L., Denehy, L., and Frawley, H. C. (2019). The impact of gynaecological cancer treatment on physical activity levels: a systematic review of observational studies. Braz. J. Phys. Therapy 23, 79–92. doi: 10.1016/j.bjpt.2018.11.007
Mason, C., Alfano, C.M., Smith, A.W., Wang, C.-Y., Neuhouser, M.L., Duggan, C., et al. (2013). Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol. Prev. Biomark. 22, 1153–1161. doi: 10.1158/1055-9965.EPI-13-0141
Mishra, S.I., Scherer, R.W., Geigle, P.M., Berlanstein, D.R., Topaloglu, O., Gotay, C.C., et al. (2012). Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 2012:CD007566. doi: 10.1002/14651858.CD007566.pub2
Mitchell, A. J., Baker-Glenn, E. A., Granger, L., and Symonds, P. (2010). Can the Distress Thermometer be improved by additional mood domains? Part I. Initial validation of the Emotion Thermometers tool. Psychooncology 19, 125–133. doi: 10.1002/pon.1523
Morris, N.S., Field, T.S., Wagner, J.L., Cutrona, S.L., Roblin, D.W., Gaglio, B., et al. (2013). The association between health literacy and cancer-related attitudes, behaviors, and knowledge. J. Health Commun. 18, 223–241. doi: 10.1080/10810730.2013.825667
Mustian, K.M., Alfano, C.M., Heckler, C., Kleckner, A.S., Kleckner, I.R., Leach, C.R., et al. (2017). Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 3, 961–968. doi: 10.1001/jamaoncol.2016.6914
Nicolaou, M., Gademan, M., Snijder, M., Engelbert, R., Dijkshoorn, H., Terwee, C., et al. (2016). Validation of the squash physical activity questionnaire in a multi-ethnic population: the helius study. PLoS One 11:e0161066. doi: 10.1371/journal.pone.0161066
Pfeifer, K., Geidl, W., Sudeck, G., and Tallner, A. (2013). Bewegungsförderung und Sport in der Neurologie: Kompetenzorientierung und Nachhaltigkeit. Neurol. Rehabil. 19, 7–19.
Phillips, S. M., and McAuley, E. (2013). Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology 22, 783–791. doi: 10.1002/pon.3074
Pugh, G., Hough, R., Gravestock, H., and Fisher, A. (2020). The health behaviour status of teenage and young adult cancer patients and survivors in the United Kingdom. Support. Care Cancer 28, 767–777. doi: 10.1007/s00520-019-04719-y
Rao, W.-W., Yang, M.-J., Cao, B.-N., You, Y.-Y., Zhang, Y.-Y., Liu, Y.-Y., et al. (2019). Psychological distress in cancer patients in a large Chinese cross-sectional study. J. Affect. Disord. 245, 950–956. doi: 10.1016/j.jad.2018.11.089
Romero, S. A., Jones, L., Bauml, J. M., Li, Q. S., Cohen, R. B., and Mao, J. J. (2018). The association between fatigue and pain symptoms and decreased physical activity after cancer. Support. Care Cancer 26, 3423–3430. doi: 10.1007/s00520-018-4203-4
Rönkkö, M., and Cho, E. (2020). An updated guideline for assessing discriminant validity. Organ. Res. Methods. 1–42. doi: 10.1177/1094428120968614 Available online at: https://journals.sagepub.com/doi/full/10.1177/1094428120968614
Rosseel, Y. (2012). Lavaan: an R package for structural equation modeling and more. Version 0.5-12 (BETA). J. Stat. Softw. 48, 1–36. doi: 10.18637/jss.v048.i02
Rüffer, J. U. (2019). CrF, ein Symptomkomplex mit vielen Implikationen. Im Fokus Onkol. 22, 73–77. doi: 10.1007/s15015-019-0719-9
Schmid, J., Haible, S., and Sudeck, G. (2020). Patterns of physical activity-related health competence: stability over time and associations with subjective health indicators. German J. Exerc. Sport Res. 50, 218–228. doi: 10.1007/s12662-020-00650-1
Scott, J.M., Zabor, E.C., Schwitzer, E., Koelwyn, G.J., Adams, S.C., Nilsen, T.S., et al. (2018). Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J. Clin. Oncol. 36:2297. doi: 10.1200/JCO.2017.77.5809
Strasser, B., Steindorf, K., Wiskemann, J., and Ulrich, C. M. (2013). Impact of resistance training in cancer survivors: a meta-analysis. Med. Sci. Sports Exerc. 45, 2080–2090. doi: 10.1249/MSS.0b013e31829a3b63
Sudeck, G., and Pfeifer, K. (2016). Physical activity-related health competence as an integrative objective in exercise therapy and health sports-conception and validation of a short questionnaire. Sportwissenschaft 46, 74–87. doi: 10.1007/s12662-016-0405-4
Sweegers, M.G., Altenburg, T.M., Brug, J., May, A.M., Van Vulpen, J.K., Aaronson, N.K., et al. (2019). Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: a meta-analysis of individual patient data. Br. J. Sports Med. 53:812. doi: 10.1136/bjsports-2018-099191
Ungar, N., Wiskemann, J., and Sieverding, M. (2016). Physical activity enjoyment and self-efficacy as predictors of cancer Patients' physical activity level. Front. Psychol. 7:898. doi: 10.3389/fpsyg.2016.00898
Vallance, J. K., Courneya, K. S., Plotnikoff, R. C., and Mackey, J. R. (2008). Analyzing theoretical mechanisms of physical activity behavior change in breast cancer survivors: results from the activity promotion (ACTION) trial. Ann. Behav. Med. 35, 150–158. doi: 10.1007/s12160-008-9019-x
Van Buuren, S. (2018). Flexible Imputation of Missing Data. Boca Raton, FL: CRC Press. doi: 10.1201/9780429492259
Van Buuren, S., and Groothuis-Oudshoorn, K. (2011). mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67. doi: 10.18637/jss.v045.i03
Van Vulpen, J.K., Sweegers, M.G., Peeters, P.H., Courneya, K.S., Newton, R.U., Aaronson, N.K., et al. (2020). Moderators of exercise effects on cancer-related fatigue: a meta-analysis of individual patient data. Med. Sci. Sports Exerc. 52, 303–314. doi: 10.1249/MSS.0000000000002154
Van Vulpen, J. K., Peeters, P. H., Velthuis, M. J., Van Der Wall, E., and May, A. M. (2016). Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: a meta-analysis. Maturitas 85, 104–111. doi: 10.1016/j.maturitas.2015.12.007
Vermillion, S.A., James, A., Dorrell, R.D., Brubaker, P., Mihalko, S.L., Hill, A.R., et al. (2018). Preoperative exercise therapy for gastrointestinal cancer patients: a systematic review. Syst. Rev. 7, 1–10. doi: 10.1186/s13643-018-0771-0
Weis, J. (2011). Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev. Pharmacoecon. Outcomes Res. 11, 441–446. doi: 10.1586/erp.11.44
Weis, J., Tomaszewski, K.A., Hammerlid, E., Ignacio Arraras, J., Conroy, T., Lanceley, A., et al. (2017). International psychometric validation of an EORTC quality of life module measuring cancer related fatigue (EORTC QLQ-FA12). J. Natl. Cancer Inst. 109. doi: 10.1093/jnci/djw273
Keywords: cancer, cancer related fatigue, physical activity, physical activity related health competence, physical activity promotion among women in difficult life situations
Citation: Koeppel M, Körbi C, Winkels RM, Schmitz KH and Wiskemann J (2021) Relationship Between Cancer Related Fatigue, Physical Activity Related Health Competence, and Leisure Time Physical Activity in Cancer Patients and Survivors. Front. Sports Act. Living 3:687365. doi: 10.3389/fspor.2021.687365
Received: 29 March 2021; Accepted: 12 July 2021;
Published: 05 August 2021.
Edited by:
Ansgar Thiel, University of Tübingen, GermanyReviewed by:
Johannes A. Carl, University of Erlangen Nuremberg, GermanySven Schneider, University of Heidelberg, Germany
Copyright © 2021 Koeppel, Körbi, Winkels, Schmitz and Wiskemann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joachim Wiskemann, joachim.wiskemann@nct-heidelberg.de
†These authors share last authorship
 Maximilian Koeppel
Maximilian Koeppel Carlotta Körbi
Carlotta Körbi Renate M. Winkels
Renate M. Winkels Kathryn H. Schmitz2†
Kathryn H. Schmitz2†  Joachim Wiskemann
Joachim Wiskemann