- 1Department of Neurology, Vanderbilt University Medical Center, Nashville, TN, United States
- 2Department of Pediatrics, Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, United States
Tics are the hallmark feature of Tourette syndrome (TS), but psychiatric and sensory symptoms are widely prevalent and increasingly recognized as core manifestations of the disorder. Accumulating evidence suggests that these psychiatric and sensory symptoms exert greater influence on quality of life (QOL) than tics themselves. However, much remains uncertain about determinants of QOL in TS due to the complexity of the clinical presentation. Here, we sought to clarify the association between health-related QOL (HRQOL) and common psychiatric and sensory symptoms in adults with TS and other chronic tic disorders. To do so, we prospectively recruited 52 patients from a tertiary care clinic to complete self-report measures assessing HRQOL (Gilles de la Tourette-Quality of Life Scale, GTS-QOL), depression (Patient Health Questionnaire-9, PHQ-9), anxiety (Generalized Anxiety Disorder Scale-7, GAD-7), obsessive-compulsive symptoms (Dimensional Obsessive-Compulsive Scale, DOCS), attention deficit hyperactivity disorder symptoms (Adult ADHD Self-Report Screening Scale for DSM-5, ASRS-V), and premonitory urge (Premonitory Urge to Tic Scale, PUTS). All participants were also administered the Yale Global Tic Severity Scale (YGTSS) to quantify tic severity. Using correlational analysis and multivariable linear regression modeling, we found that GTS-QOL score was significantly associated with scores from all other rating scales, with the exception of the PUTS. GTS-QOL was most strongly associated with PHQ-9, followed by ASRS-V, GAD-7, DOCS, and YGTSS total tic score. The regression model including these five independent variables, as well as sex, explained 79% of GTS-QOL score variance [F(6,40) = 29.6, p < 0.001]. Specific psychiatric symptoms differentially impacted physical, psychological, and cognitive HRQOL. Systematic assessment of psychiatric comorbidities is imperative for clinical care and clinical research efforts directed at improving QOL in adults with chronic tic disorders.
Introduction
Tourette syndrome (TS) is a multi-faceted neurodevelopmental disorder affecting 0.3–1% of school-aged children (1, 2), and one-third of these patients continue to experience bothersome tics into adulthood (3). Tics are the defining characteristic of TS, but psychiatric and sensory symptoms are increasingly recognized as core manifestations. Ninety-percent of TS patients have at least one comorbid psychiatric diagnosis, and 60% have two or more (4). Obsessive-compulsive disorder (OCD) and attention deficit hyperactivity disorder (ADHD) are the two most common psychiatric comorbidities in TS, with respective prevalence ranges of 10–50% and 30–60% (4–7). Features of OCD and/or ADHD are also frequently evident among TS patients not fulfilling diagnostic criteria for these disorders (8). Depression and anxiety are common, occurring in 30% and 36% of TS patients, respectively (4). The prevalence of several other psychiatric conditions, including impulse control disorder and autism spectrum disorder, is elevated in TS samples relative to the general population (4, 6, 9, 10). In addition to psychiatric comorbidities, 90% of adult and adolescent patients endorse pre-monitory urges, which are uncomfortable sensory disturbances preceding tics (11, 12).
Psychiatric and sensory symptoms of TS undermine patient quality of life (QOL) (12–16). In children, ADHD (15, 17–19), OCD (15, 17, 18), and depression (15) are each linked with worse QOL. In adults, depression appears to be the predominant determinant of poor QOL (20–23), though OCD (20), anxiety (24), and premonitory urge (11) are also implicated. QOL is lowest in those patients afflicted with multiple psychiatric comorbidities (19). Mounting data suggests that psychiatric (15, 19, 20, 24, 25) and sensory (26) symptoms exert greater influence on QOL than tics themselves. Studies exploring QOL in TS vary considerably in their clinical assessments; QOL measures [generic (15, 17, 18, 20–23, 25, 27) vs. disorder-specific QOL (11, 24)]; sample populations [treatment-seeking (11, 15, 17, 18, 20, 21, 23, 24) vs. non-treatment-seeking (22, 25, 27), children (15, 17, 18, 24) vs. adults (11, 20–23, 25, 27)]; and statistical methodologies. QOL research in adults with TS has focused on the roles of depression (11, 19–21, 23, 24), anxiety (11, 19, 20, 23, 24), and OCD (11, 19, 20, 23), with lesser attention devoted to ADHD (11, 19), other psychiatric diagnoses (19), and premonitory urge (11, 24). Given their frequent co-occurrence and co-variance, neglecting one or more of these symptoms in an analysis could inflate or deflate the statistical relevance of factors under consideration, leading to potentially inaccurate conclusions (28). Thus, despite the expanding literature on QOL in TS, important ambiguities remain, in large part due to the complexity of the TS phenotype and the multidimensionality of the QOL construct. When gauging the QOL impact of health or disease, researchers typically consider health-related QOL (HRQOL), conceptualized as the perceived influence of physical, emotional, and social health on an individual's overall satisfaction with life (29). Enhanced understanding of HRQOL determinants in TS is vital in order to direct attention and treatment to those factors most distressing to patients.
In this study, we sought to clarify the association between HRQOL and common psychiatric and sensory symptoms in adults with TS and other chronic tic disorders. To do so, we prospectively recruited patients from a specialty TS clinic to undergo clinical rating of tic severity and to complete a battery of self-report measures assessing depression, anxiety, obsessive-compulsive symptoms, ADHD symptoms, premonitory urge, and HRQOL.
Materials and Methods
Participants
From April 2019 through September 2020 we consecutively recruited adults with TS or other chronic tic disorders from Vanderbilt University Medical Center (VUMC) Tourette Syndrome Clinic and from institutional research registries. Inclusion criteria included diagnosis of TS, chronic motor tic disorder, or chronic vocal tic disorder based on Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) criteria, age ≥ 18 years, capacity to provide informed consent, and ability to speak English. For the purposes of this article, the term “chronic tic disorder” is used to encompass TS, chronic motor tic disorder, and chronic vocal tic disorder. Participants gave electronic informed consent prior to performing study-specific procedures. The study was conducted in accordance with the Declaration of Helsinki and was approved by the VUMC Institutional Review Board.
Measures
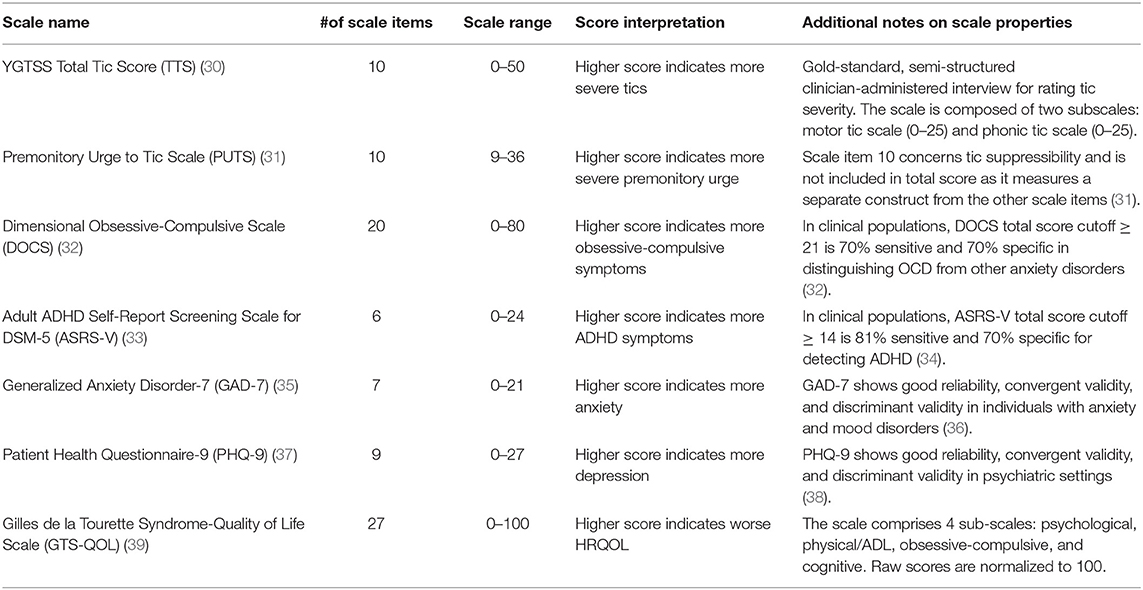
An experienced movement disorders neurologist (D.I.) administered the YGTSS to all participants. Subsequently, participants completed a battery of validated self-report instruments assessing common psychiatric and sensory symptoms in TS, including depression, anxiety, obsessive-compulsive symptoms, ADHD symptoms, and premonitory urge. Table 1 summarizes properties of these scales. In addition, participants completed the Gilles de la Tourette-Quality of Life Scale (GTS-QOL), a disorder-specific scale developed to assess QOL domains most impacted by TS (39). The GTS-QOL contains 27 items, each with a five-point Likert scale ranging from 0 (“no problem”) to 4 (“extreme problem”). As previously identified by exploratory factor analysis, the scale is composed of four sub-scales: Psychological (11 items), Physical/Activities of Daily Living (Physical/ADL) (7 items), Obsessive-Compulsive (OC) (5 items), and Cognitive (4 items) (39). Item scores within each subscale are summed and then normalized to 100 to generate the subscale score. The four subscale scores are then summed and normalized to 100 to yield the total score. The GTS-QOL has undergone rigorous development and validation, demonstrating satisfactory internal consistency, test-retest reliability, construct validity, and convergent and discriminant validity (39).
Self-report measures were administered in Research Electronic Data Capture (REDCap), a secure, web-based platform for collecting patient-reported information and storing research data (40, 41). Participants were permitted to complete online self-report scales in clinic or at home, as per their preference. Median time between administration of the YGTSS and completion of the self-report scale battery was 6.5 days (interquartile range 0–13.25 days).
Statistical Analysis
Measures of central tendency were calculated for relevant clinical variables. Spearman's rank correlation was used to assess degree of relationship between clinical measures. Significance thresholds were Bonferroni-adjusted to correct for multiple comparisons in the correlational analysis, resulting in a pre-specified acceptable Type 1 error rate of 0.001 (45 total comparisons in correlational analysis).
To determine the independent association of specific clinical variables with GTS-QOL total score, we conducted a hierarchical linear regression analysis with backwards elimination. Clinical variables that did not significantly correlate with GTS-QOL total score were not included in this regression analysis. As such, sex, YGTSS total tic score (TTS), DOCS score, ASRS-V score, PHQ-9 score, and GAD-7 score were included as independent variables in the first regression model of the analysis. The sample size was inadequate to permit inclusion of interaction terms. To identify influential outliers, we calculated Cook's D values for all observations in the first model; observations with Cook's D-value > 4/n were excluded from the regression analysis. Regression diagnostics performed at each step of the analysis included assessment for multicollinearity (conservatively defined as variance inflation factor > 2.5), normality of residuals (with Shapiro-Wilk-test of model residuals), heteroskedasticity (with Breusch-Pagan-test), model explanatory power (indexed by adjusted R2), and model quality [by Akaike information criteria, with lower values indicating superior model quality (42)]. At each step in the hierarchical analysis, we identified and removed the independent variable with the largest standardized coefficient. F-statistics were calculated to compare change in non-adjusted R2 following stepwise removal of independent variables. The acceptable Type 1 error rate for explanatory power of the models was set at 0.01 to account for multiple comparisons (5 models were constructed with GTS-QOL total score as the dependent variable). Post-hoc t-tests were performed to compare each independent variable in the model to GTS-QOL total; significance thresholds for post-hoc tests were Bonferroni-adjusted to correct for multiple comparisons. Both non-standardized and standardized independent variable coefficients (β) are reported.
To determine the independent association of clinical variables with GTS-QOL sub-scales, we performed separate multivariable linear regression analyses. Only those variables significantly associated with GTS-QOL total score, as per the hierarchical regression analysis, were included. The aforementioned regression diagnostics were undertaken for each model. The acceptable Type 1 error rate for model explanatory power was set at 0.025 to account for multiple comparisons (2 models were constructed for each GTS-QOL subscale).
Data was complete for all measures except GAD-7. One patient omitted answers to two items for that scale. Missing values were imputed with predictive mean matching. Statistical analyses were conducted in STATA.
Results
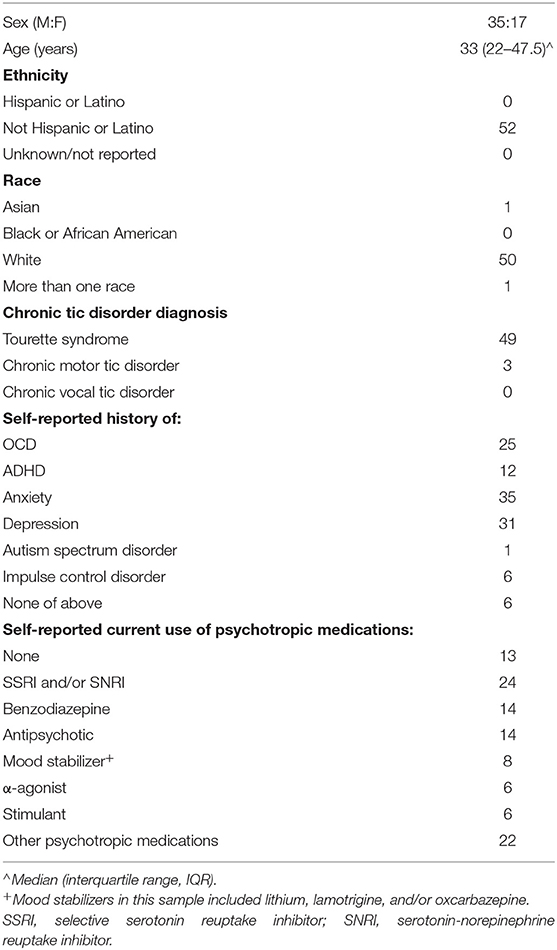
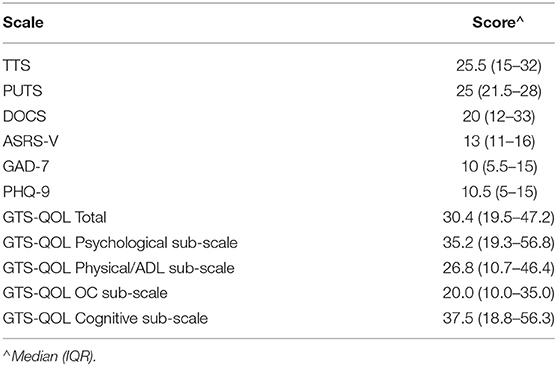
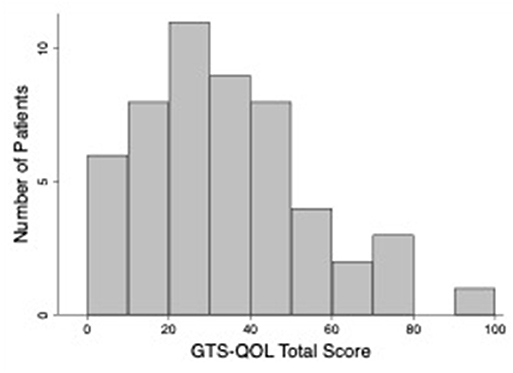
Table 2 presents clinical characteristics of the study population. The sample included three participants with chronic motor tic disorder; all other participants met criteria for TS. Eighty-nine percent of participants reported an established diagnosis of at least one of the following: depression, anxiety, OCD, ADHD, autism spectrum disorder, or impulse control disorder; 71% of participants reported 2 or more of these diagnoses. Forty-eight percent screened positive for ADHD, and the same percentage screened positive for OCD, based on validated scale cutoffs (see Table 1). Table 3 displays measures of central tendency for each rating scale. Figure 1 depicts the distribution of GTS-QOL total scores within the study population.
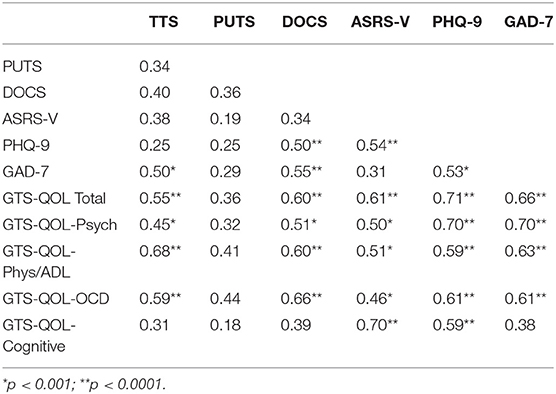
The correlation matrix for the study rating scales is presented in Table 4. After correcting for multiple comparisons, GTS-QOL total positively correlated with scores from all other scales except PUTS. GTS-QOL total correlated most strongly with PHQ-9 and GAD-7, followed by ASRS-V, DOCS, and TTS. GTS-QOL Psychological, Physical/ADL, and Obsessive-Compulsive sub-scale scores likewise positively correlated with scores from all measures except PUTS. GTS-QOL Cognitive sub-scale score correlated only with PHQ-9 and ASRS-V scores.
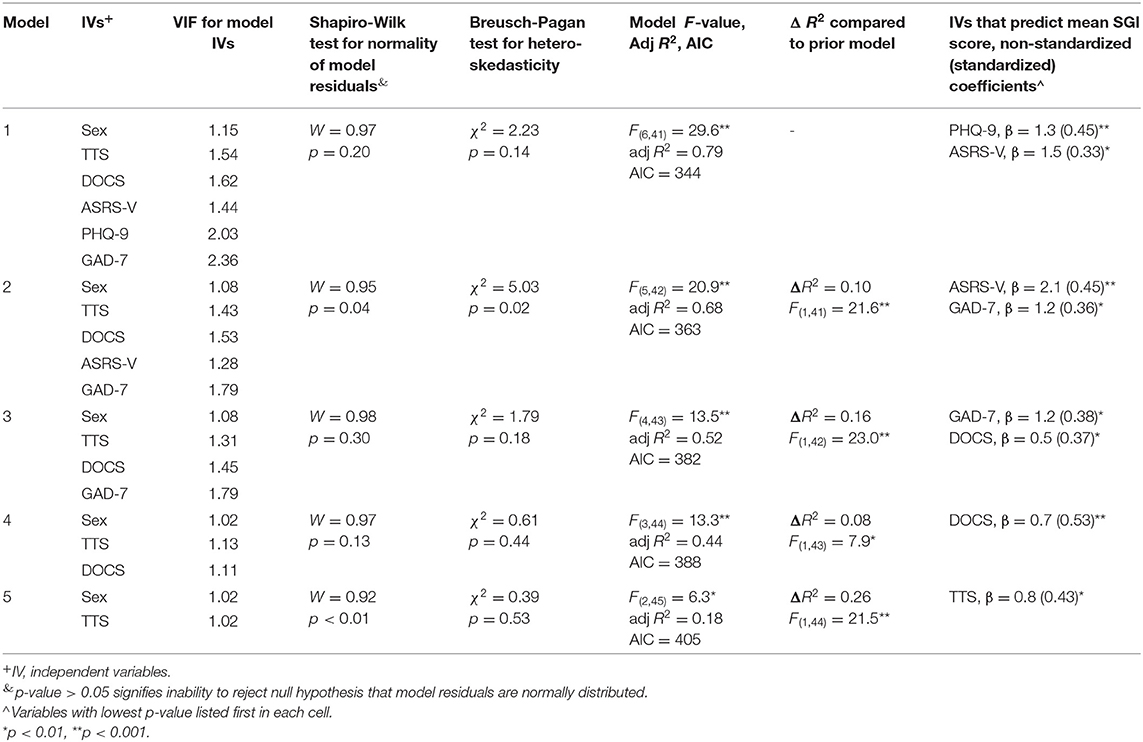
Table 5 provides details of the models from the hierarchical regression analysis for GTS-QOL total. Following construction of the first model, observations from four participants were identified as outliers and excluded from the analysis. PHQ-9 and ASRS-V were independently associated with GTS-QOL total, even after controlling for sex and severity of tics, anxiety, and obsessive-compulsive symptoms (Model 1). With PHQ-9 and ASRS-V eliminated from the model, GAD-7 and DOCS were each independently associated with GTS-QOL (Model 3). Only after removal of PHQ-9, ASRS-V, GAD-7, and DOCS from the model was TTS significantly associated with GTS-QOL total (Model 5). PHQ-9, ASRS-V, GAD-7, DOCS, and TTS all contributed to model explanatory power, as evidenced by significant decrease in unadjusted R2 following stepwise elimination of these variables. Notably, Model 2 exhibited heteroskedasticity, and residuals in Models 2 and 5 were not normally distributed, indicating linear regression assumptions were partially violated in these two nested models.
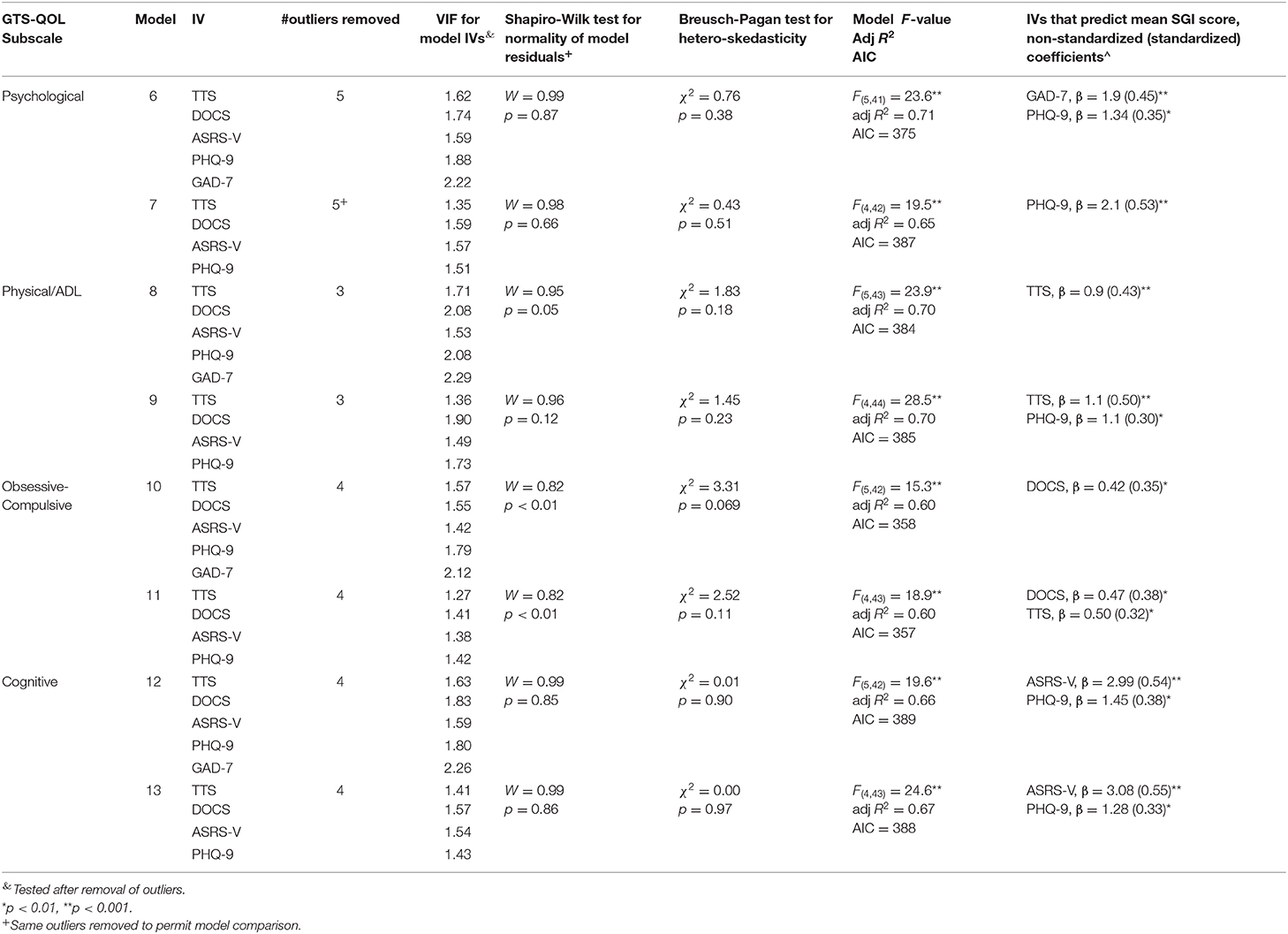
In the separate regression analysis with respective GTS-QOL subscales as the dependent variable, sex was excluded as an independent variable since it was not significantly associated with GTS-QOL total at any step of the hierarchical regression analysis. GAD-7 exhibited mild multicollinearity (VIF between 2 and 2.5) in regression models with GTS-QOL subscales as the dependent variable. To address this, models with and without GAD-7 were constructed (see Table 6). Clinical measures were differentially associated with specific GTS-QOL subscales: GAD-7 and PHQ-9 with GTS-QOL Psychological; TTS with GTS-QOL Physical/ADL; and ASRS-V and PHQ-9 with GTS-QOL Cognitive. Residuals for the linear models of GTS-QOL Obsessive-Compulsive were not normally distributed, raising concern for model validity.
Discussion
In this study, we examined the association of HRQOL with motor, psychiatric, and sensory symptoms in adults with chronic tic disorders. Our main finding is that psychiatric symptoms appear to be the predominant driver of HRQOL in this population. Novel findings include the significant association of ADHD with poor HRQOL and the differential impact of psychiatric symptoms on distinct HRQOL domains. We sequentially discuss these results and their ramifications for clinical care and research.
In this treatment-seeking sample of adults with chronic tic disorder, depression was most strongly linked with poor HRQOL, in accord with prior studies (20–22). The magnitude of depression's association with GTS-QOL total was twice as great as any other clinical factor. Anxiety, obsessive-compulsive symptoms, and ADHD symptoms were also independently associated with worse HRQOL. The relationship of the former two symptoms to HRQOL has been previously established (20, 23, 24); however, most investigations exploring QOL in adults with TS have not incorporated ADHD measures (20, 21, 23, 24). We were only able to find a single study with results suggesting that ADHD may negatively impact HRQOL in adults with chronic tic disorder (19). Methodologic limitations raise concerns regarding some of that study's conclusions: the sample population included both children and adults but the analysis was not stratified by age; and the analytic approach relied solely on reported diagnosis of ADHD (dichotomous variable) rather than severity of current ADHD symptoms (continuous variable) (19). Most investigations exploring QOL in adults with TS have not incorporated ADHD measures (20, 21, 23, 24). While inattention and hyperactivity symptoms tend to diminish with age in TS populations, half of patients meeting criteria for ADHD in childhood still meet criteria for the disorder as they enter adulthood (43). In a large international cohort study of 6,805 individuals with tic disorders, 61% of children and 39% of adults were diagnosed with ADHD (44). Outside the context of tic disorders, ADHD is increasingly recognized as a lifelong condition, with persistence of symptoms into adulthood for as many as two-thirds of individuals (45, 46). These adults with ADHD experience higher rates of workplace difficulties (47–49) and unemployment (47, 48), greater home life dysfunction (47, 48), and poor HRQOL (48). In children with tic disorders, the deleterious effect of ADHD on cognitive performance (50–53), psychosocial functioning (50, 54–56), and QOL (57, 58) has been repeatedly demonstrated, with several studies indicating ADHD exerts substantially greater impact on these domains than tics (50–55, 57). In adults with both ADHD and tic disorder, diagnosis of the former but not the latter accounts for impaired current and past global functioning (59). In our sample of adults with tic disorders, half of patients screened positive for ADHD, and ADHD symptoms were strongly associated with HRQOL, even after accounting for severity of depression, anxiety, and obsessive-compulsive symptoms. Patients with more severe ADHD symptoms endorsed poorer cognitive HRQOL, whereas no significant relationship emerged between ADHD symptoms and psychological, physical, or obsessive-compulsive HRQOL domains. Additional research is needed to further examine the course and role of ADHD symptomatology in adults with tic disorders, both in regards to QOL and functional impairment.
Study findings replicate prior work demonstrating QOL is more strongly associated with psychiatric symptoms than tics (20, 27). Greater tic severity did correspond with worse physical HRQOL, consistent with existing literature showing the detrimental physical toll of certain types of tics, particularly those causing pain (13, 16, 25). Severity of premonitory urge correlated with GTS-QOL total score in our study, but the significance of this relationship did not survive correction for multiple comparisons. Premonitory urge is a bothersome symptom for many patients (11), but our results indicate HRQOL is more tightly intertwined with psychiatric symptoms than pre-monitory urge.
While depression, anxiety, obsessive-compulsive symptoms, ADHD symptoms, and tics were each independently associated with overall HRQOL, their impact varied by HRQOL domain. Anxiety and depression were most robustly associated with psychological HRQOL, tic severity with physical HRQOL, and ADHD symptoms and depression with cognitive HRQOL. Given the lack of a valid linear model for the obsessive-compulsive domain of the GTS-QOL, no firm conclusions can be drawn from that particular regression analysis. It is notable that, in the correlational analysis, obsessive-compulsive symptoms were the clinical factor most closely corresponding with GTS-QOL Obsessive-Compulsive score. The association of specific psychiatric symptoms with specific HRQOL domains further validates the dimensional structure of the GTS-QOL scale.
Collectively, the battery of scales employed in this study explained 79% of the variance in GTS-QOL total score. Replication of these findings in another adult sample with chronic tic disorder is necessary to validate the regression model, but results nonetheless emphasize the importance of systematically evaluating for common psychiatric comorbidities given their individual and combined influence on HRQOL in this population. Consistent with this, recent practice guidelines prioritize recognition and management of psychiatric comorbidities, in addition to treatment of tics (60, 61). Given the multifaceted nature of the TS phenotype, disease-specific HRQOL measures, such as the GTS-QOL, are valuable means of quantifying patient-perceived overall burden from various aspects of the disorder. The GTS-QOL is specifically designed to measure the QOL impact of distinct motor and neuropsychiatric symptoms prevalent in tic disorder populations (16, 39, 62, 63). Generic QOL scales, on the other hand, tend to be less sensitive and/or comprehensive in their survey of the domains central to tic disorders (16, 62, 63). Strong consideration should be given to incorporating HRQOL scales, such as the GTS-QOL, as endpoints in clinical care and clinical trials. Longitudinal studies including such measures will be critical to elucidate risk factors for poor HRQOL in both children and adults with chronic tic disorders. The detrimental impact of psychiatric symptoms and tics on HRQOL in patients of all ages is evident (63), but additional work should also investigate broader psychosocial determinants of and potential protective factors for HRQOL, such as family dynamics, peer relationships, and school/work environment (13).
Our study has several notable limitations. First, participants were recruited from a tertiary care clinic and were predominantly white, limiting generalizability of study findings to other populations. Second, we relied on self-report measures of psychiatric symptoms rather than gold standard, clinician-administered rating scales. Third, the current sample size precluded more sophisticated modeling approaches, such as inclusion of interaction terms and medication status. Recruitment of a larger sample is in progress. Fourth, despite the scope of the assessment battery, we did not evaluate for symptoms specific to schizophrenia, autism spectrum disorder, or less frequent psychiatric conditions in TS. The fact that the constructed multivariable linear regression model explains the vast majority of GTS-QOL variance in this study suggests that no substantial variable omission occurred; however, unmeasured symptoms remain potential confounds that warrant consideration in future studies.
In conclusion, depression, anxiety, ADHD symptoms, and obsessive-compulsive symptoms are more strongly associated with HRQOL than tics in adults with chronic tic disorder. Systematic assessment of psychiatric comorbidities is imperative for optimizing clinical care and clinical research.
Data Availability Statement
The dataset discussed in this article is not readily available as the authors do not have current IRB approval to share de-identified data from this study. Requests to access the dataset should be directed to david.a.isaacs@vumc.org.
Ethics Statement
The studies involving human participants were reviewed and approved by Vanderbilt University Medical Center Human Research Protections Program. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
DI conceived and designed the study, implemented the protocol, and drafted the manuscript. HR and DC provided critical aid to study design and realization and provided substantive critique of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by Award No. K24AG064114-01A1 from the National Institute on Aging and the CTSA Award No. UL1 TR002243 from the National Center for Advancing Translational Sciences.
Disclaimer
Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Institute on Aging, the National Center for Advancing Translational Sciences, nor the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to extend their appreciation to Ms. Ashley Volk and Ms. Jacqueline Harris for their efforts in participant recruitment and data collection.
References
1. Cohen SC, Leckman JF, Bloch MH. Clinical assessment of Tourette syndrome and tic disorders. Neurosci Biobehav Rev. (2013) 37:997–1007. doi: 10.1016/j.neubiorev.2012.11.013
2. Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord. (2015) 30:221–8. doi: 10.1002/mds.26089
3. Bloch MH, Leckman JF. Clinical course of Tourette syndrome. J Psychosom Res. (2009) 67:497–501. doi: 10.1016/j.jpsychores.2009.09.002
4. Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. (2015) 72:325. doi: 10.1001/jamapsychiatry.2014.2650
5. Kurlan R, Como PG, Miller B, Palumbo D, Deeley C, Andresen EM, et al. The behavioral spectrum of tic disorders: a community-based study. Neurology. (2002) 59:414–20. doi: 10.1212/WNL.59.3.414
6. Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. (2000) 42:436–47. doi: 10.1017/S0012162200000839
7. Sambrani T, Jakubovski E, Müller-Vahl KR. New insights into clinical characteristics of Gilles de la Tourette syndrome: findings in 1032 patients from a single German Center. Front Neurosci. (2016) 10:415. doi: 10.3389/fnins.2016.00415
8. Huisman-van Dijk HM, Schoot R van de, Rijkeboer MM, Mathews CA, Cath DC. The relationship between tics, OC, ADHD and autism symptoms: a cross- disorder symptom analysis in Gilles de la Tourette syndrome patients and family-members. Psychiatry Res. (2016) 237:138–46. doi: 10.1016/j.psychres.2016.01.051
9. Burd L, Li Q, Kerbeshian J, Klug MG, Freeman RD. Tourette syndrome and comorbid pervasive developmental disorders. J Child Neurol. (2009) 24:170–5. doi: 10.1177/0883073808322666
10. Darrow SM, Grados M, Sandor P, Hirschtritt ME, Illmann C, Osiecki L, et al. Autism spectrum symptoms in a Tourette's disorder sample. J Am Acad Child Adolesc Psychiatry. (2017) 56:610–7.e1. doi: 10.1016/j.jaac.2017.05.002
11. Crossley E, Eugenio Cavanna A. Sensory phenomena: clinical correlates and impact on quality of life in adult patients with Tourette syndrome. Psychiatry Res. (2013) 209:705–10. doi: 10.1016/j.psychres.2013.04.019
12. Houghton DC, Capriotti MR, Conelea CA, Woods DW. Sensory phenomena in Tourette syndrome: their role in symptom formation and treatment. Curr Dev Disord Rep. (2014) 1:245–51. doi: 10.1007/s40474-014-0026-2
13. Eapen V, Cavanna AE, Robertson MM. Comorbidities, social impact, and quality of life in Tourette syndrome. Front Psychiatry. (2016) 7:97. doi: 10.3389/fpsyt.2016.00097
14. Cox JH, Seri S, Cavanna AE. Sensory aspects of Tourette syndrome. Neurosci Biobehav Rev. (2018) 88:170–6. doi: 10.1016/j.neubiorev.2018.03.016
15. Eddy CM, Cavanna AE, Gulisano M, Agodi A, Barchitta M, Calì P, et al. Clinical correlates of quality of life in Tourette syndrome. Mov Disord. (2011) 26:735–8. doi: 10.1002/mds.23434
16. Evans J, Seri S, Cavanna AE. The effects of Gilles de la Tourette syndrome and other chronic tic disorders on quality of life across the lifespan: a systematic review. Eur Child Adolesc Psychiatry. (2016) 25:939–48. doi: 10.1007/s00787-016-0823-8
17. Eddy CM, Cavanna AE, Gulisano M, Calì P, Robertson MM, Rizzo R. The effects of comorbid obsessive-compulsive disorder and attention-deficit hyperactivity disorder on quality of life in Tourette syndrome. J Neuropsychiatry Clin Neurosci. (2012) 24:458–62. doi: 10.1176/appi.neuropsych.11080181
18. Rizzo R, Gulisano M, Calì PV, Curatolo P. Long term clinical course of Tourette syndrome. Brain Dev. (2012) 34:667–73. doi: 10.1016/j.braindev.2011.11.006
19. Eapen V, Snedden C, Crnčec R, Pick A, Sachdev P. Tourette syndrome, co-morbidities and quality of life. Aust New Zeal J Psychiatry. (2016) 50:82–93. doi: 10.1177/0004867415594429
20. Huisman-van Dijk HM, Matthijssen SJMA, Stockmann RTS, Fritz AV, Cath DC. Effects of comorbidity on Tourette's tic severity and quality of life. Acta Neurol Scand. (2019) 140:390–8. doi: 10.1111/ane.13155
21. Müller-Vahl K, Dodel I, Müller N, Münchau A, Reese JP, Balzer-Geldsetzer M, et al. Health-related quality of life in patients with Gilles de la Tourette's syndrome. Mov Disord. (2010) 25:309–14. doi: 10.1002/mds.22900
22. Jalenques I, Galland F, Malet L, Morand D, Legrand G, Auclair C, et al. Quality of life in adults with Gilles de la Tourette syndrome. BMC Psychiatry. (2012) 12:109. doi: 10.1186/1471-244X-12-109
23. Elstner K, Selai CE, Trimble MR, Robertson MM. Quality of life (QOL) of patients with Gilles de la Tourette's syndrome. Acta Psychiatr Scand. (2001) 103:52–9. doi: 10.1111/j.1600-0447.2001.00147.x
24. Silvestri PR, Chiarotti F, Baglioni V, Neri V, Cardona F, Cavanna AE. Health-related quality of life in patients with Gilles de la Tourette syndrome at the transition between adolescence and adulthood. Neurol Sci. (2016) 37:1857–60. doi: 10.1007/s10072-016-2682-y
25. Conelea CA, Woods DW, Zinner SH, Budman CL, Murphy TK, Scahill LD, et al. The impact of Tourette syndrome in adults: results from the Tourette syndrome impact survey. Community Ment Health J. (2013) 49:110–20. doi: 10.1007/s10597-011-9465-y
26. Cavanna AE, Black KJ, Hallett M, Voon V. Neurobiology of the premonitory urge in Tourette's syndrome: pathophysiology and treatment implications. J Neuropsychiatry Clin Neurosci. (2017) 29:95–104. doi: 10.1176/appi.neuropsych.16070141
27. Lewin AB, Storch EA, Conelea CA, Woods DW, Zinner SH, Budman CL, et al. The roles of anxiety and depression in connecting tic severity and functional impairment. J Anxiety Disord. (2011) 25:164–8. doi: 10.1016/j.janxdis.2010.08.016
28. Rao P. Some notes on misspecification in multiple regressions. Am Stat. (1971) 25:37. doi: 10.2307/2686082
29. Theofilou P. Quality of life: definition and measurement. Eur J Psychol. (2013) 9:150–62. doi: 10.5964/ejop.v9i1.337
30. Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The yale global tic severity scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. (1989) 28:566–73. doi: 10.1097/00004583-198907000-00015
31. Woods D, Piacentini J, Himle M, Chang S. Premonitory urge for tics scale (PUTS). J Dev Behav Pediatr. (2005) 26:397–403. doi: 10.1097/00004703-200512000-00001
32. Abramowitz JS, Deacon BJ, Olatunji BO, Wheaton MG, Berman NC, Losardo D, et al. Assessment of obsessive-compulsive symptom dimensions: development and evaluation of the Dimensional Obsessive-Compulsive Scale. Psychol Assess. (2010) 22:180–98. doi: 10.1037/a0018260
33. Ustun B, Adler LA, Rudin C, Faraone SV, Spencer TJ, Berglund P, et al. The world health organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry. (2017) 74:520. doi: 10.1001/jamapsychiatry.2017.0298
34. van de Glind G, van den Brink W, Koeter MWJ, Carpentier P-J, van Emmerik-van Oortmerssen K, Kaye S, et al. Validity of the adult ADHD self-report scale (ASRS) as a screener for adult ADHD in treatment seeking substance use disorder patients. Drug Alcohol Depend. (2013) 132:587–96. doi: 10.1016/j.drugalcdep.2013.04.010
35. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med. (2006) 166:1092. doi: 10.1001/archinte.166.10.1092
36. Rutter LA, Brown TA. Psychometric properties of the generalized anxiety disorder scale-7 (GAD-7) in outpatients with anxiety and mood disorders. J Psychopathol Behav Assess. (2017) 39:140–6. doi: 10.1007/s10862-016-9571-9
37. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
38. Beard C, Hsu KJ, Rifkin LS, Busch AB, Björgvinsson T. Validation of the PHQ-9 in a psychiatric sample. J Affect Disord. (2016) 193:267–73. doi: 10.1016/j.jad.2015.12.075
39. Cavanna AE, Schrag A, Morley D, Orth M, Robertson MM, Joyce E, et al. The Gilles de la Tourette syndrome-quality of life scale (GTS-QOL): development and validation. Neurology. (2008) 71:1410–6. doi: 10.1212/01.wnl.0000327890.02893.61
40. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. doi: 10.1016/j.jbi.2019.103208
41. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
42. Akaike H. Information theory and an extension of the maximum likelihood principle. In: Second International Symposium on Information Theory. (1998). p. 199–213.
43. Groth C, Mol Debes N, Rask CU, Lange T, Skov L. Course of Tourette syndrome and comorbidities in a large prospective clinical study. J Am Acad Child Adolesc Psychiatry. (2017) 56:304–12. doi: 10.1016/j.jaac.2017.01.010
44. Freeman RD. Tic disorders and ADHD: answers from a world-wide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. (2007) 16:15–23. doi: 10.1007/s00787-007-1003-7
45. Kooij SJJ, Bejerot S, Blackwell A, Caci H, Casas-Brugué M, Carpentier PJ, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry. (2010) 10:67. doi: 10.1186/1471-244X-10-67
46. Sibley MH, Swanson JM, Arnold LE, Hechtman LT, Owens EB, Stehli A, et al. Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. J Child Psychol Psychiatry. (2017) 58:655–62. doi: 10.1111/jcpp.12620
47. Stein MA. Impairment associated with adult ADHD. CNS Spectr. (2008) 13:9–11. doi: 10.1017/S1092852900003187
48. Able SL, Johnston JA, Adler LA, Swindle RW. Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychol Med. (2007) 37:97–107. doi: 10.1017/S0033291706008713
49. Barkley RA, Murphy KR. Impairment in occupational functioning and adult ADHD: the predictive utility of executive function (EF) ratings vs. EF tests. Arch Clin Neuropsychol. (2010) 25:157–73. doi: 10.1093/arclin/acq014
50. Brand N. Brief report: cognitive functioning in children with Tourette's syndrome with and without comorbid ADHD. J Pediatr Psychol. (2002) 27:203–8. doi: 10.1093/jpepsy/27.2.203
51. Roessner V, Becker A, Banaschewski T, Rothenberger A. Executive functions in children with chronic tic disorders with/without ADHD: new insights. Eur Child Adolesc Psychiatry. (2007) 16:36–44. doi: 10.1007/s00787-007-1005-5
52. Shin M-S, Chung S-J, Hong K-EM. Comparative study of the behavioral and neuropsychologic characteristics of tic disorder with or without attention-deficit hyperactivity disorder (ADHD). J Child Neurol. (2001) 16:719–26. doi: 10.1177/088307380101601003
53. Rizzo R, Curatolo P, Gulisano M, Virzì M, Arpino C, Robertson MM. Disentangling the effects of Tourette syndrome and attention deficit hyperactivity disorder on cognitive and behavioral phenotypes. Brain Dev. (2007) 29:413–20. doi: 10.1016/j.braindev.2006.12.003
54. Hoekstra PJ, Steenhuis M-P, Troost PW, Korf J, Kallenberg CGM, Minderaa RB. Relative contribution of attention-deficit hyperactivity disorder, obsessive-compulsive disorder, and tic severity to social and behavioral problems in tic disorders. J Dev Behav Pediatr. (2004) 25:272–9. doi: 10.1097/00004703-200408000-00007
55. Carter AS, O'Donnell DA, Schultz RT, Scahill L, Leckman JF, Pauls DL. Social and emotional adjustment in children affected with Gilles de la Tourette's syndrome: associations with ADHD and family functioning. J Child Psychol Psychiatry. (2000) 41:S0021963099005156. doi: 10.1111/1469-7610.00602
56. Debes N, Hjalgrim H, Skov L. The presence of attention-deficit hyperactivity disorder (ADHD) and obsessive-compulsive disorder worsen psychosocial and educational problems in Tourette syndrome. J Child Neurol. (2010) 25:171–81. doi: 10.1177/0883073809336215
57. Bernard BA, Stebbins GT, Siegel S, Schultz TM, Hays C, Morrissey MJ, et al. Determinants of quality of life in children with Gilles de la Tourette syndrome. Mov Disord. (2009) 24:1070–3. doi: 10.1002/mds.22487
58. Cutler D, Murphy T, Gilmour J, Heyman I. The quality of life of young people with Tourette syndrome. Child Care Health Dev. (2009) 35:496–504. doi: 10.1111/j.1365-2214.2009.00983.x
59. Spencer TJ, Biederman J, Faraone S, Mick E, Coffey B, Geller D, et al. Impact of Tic disorders on ADHD outcome across the life cycle: findings from a large Group of adults with and without ADHD. Am J Psychiatry. (2001) 158:611–7. doi: 10.1176/appi.ajp.158.4.611
60. Pringsheim T, Okun MS, Müller-Vahl K, Martino D, Jankovic J, Cavanna AE, et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. (2019) 92:896–906. doi: 10.1212/WNL.0000000000007466
61. Cath DC, Hedderly T, Ludolph AG, Stern JS, Murphy T, Hartmann A, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part I: assessment. Eur Child Adolesc Psychiatry. (2011) 20:155–71. doi: 10.1007/s00787-011-0164-6
62. Su MT, McFarlane F, Cavanna AE, Termine C, Murray I, Heidemeyer L, et al. The english version of the Gilles de la Tourette syndrome-quality of life scale for children and adolescents (C&A-GTS-QOL). J Child Neurol. (2017) 32:76–83. doi: 10.1177/0883073816670083
Keywords: Tourette syndrome, tic disorder, psychiatric comorbidities, premonitory urge, quality of life, health related quality of life
Citation: Isaacs DA, Riordan HR and Claassen DO (2021) Clinical Correlates of Health-Related Quality of Life in Adults With Chronic Tic Disorder. Front. Psychiatry 12:619854. doi: 10.3389/fpsyt.2021.619854
Received: 21 October 2020; Accepted: 15 February 2021;
Published: 10 March 2021.
Edited by:
Kerstin Jessica von Plessen, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
A. Cavanna, Birmingham and Solihull Mental Health NHS Foundation Trust, United KingdomNatalia Szejko, Yale University, United States
Copyright © 2021 Isaacs, Riordan and Claassen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David A. Isaacs, david.a.isaacs@vumc.org
 David A. Isaacs
David A. Isaacs Heather R. Riordan1,2
Heather R. Riordan1,2 Daniel O. Claassen
Daniel O. Claassen