- Department of Neuropsychology and Psychopharmacology, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands
Depression is a major public health problem that affects approximately 4.4% of the global population. Since conventional pharmacotherapies and psychotherapies are only partially effective, as demonstrated by the number of patients failing to achieve remission, alternative treatments are needed. Mindfulness meditation (MM) and psilocybin represent two promising novel treatments that might even have complementary therapeutic effects when combined. Since the current literature is limited to theoretical and empirical underpinnings of either treatment alone, the present review aimed to identify possible complementary effects that may be relevant to the treatment of depression. To that end, the individual effects of MM and psilocybin, and their underlying working mechanisms, were compared on a non-exhaustive selection of six prominent psychological and biological processes that are well known to show impairments in patients suffering from major depression disorder, that is mood, executive functioning, social skills, neuroplasticity, core neural networks, and neuroendocrine and neuroimmunological levels. Based on predefined search strings used in two online databases (PubMed and Google Scholar) 1129 articles were identified. After screening title and abstract for relevance related to the question, 82 articles were retained and 11 were added after reference list search, resulting in 93 articles included in the review. Findings show that MM and psilocybin exert similar effects on mood, social skills, and neuroplasticity; different effects were found on executive functioning, neural core networks, and neuroendocrine and neuroimmune system markers. Potential mechanisms of MM’s effects are enhanced affective self-regulation through mental strategies, optimization of stress reactivity, and structural and functional adjustments of prefrontal and limbic areas; psilocybin’s effects might be established via attenuation of cognitive associations through deep personal insights, cognitive disinhibition, and global neural network disintegration. It is suggested that, when used in combination, MM and psilocybin could exert complementary effects by potentiating or prolonging mutual positive effects, for example, MM potentially facilitating psilocybin-induced peak experiences. Future placebo-controlled double-blind randomized trials focusing on psilocybin-assisted mindfulness-based therapy will provide knowledge about whether the proposed combination of therapies maximizes their efficacy in the treatment of depression or depressive symptomatology.
Introduction
Depression or major depressive disorder (MDD) is a common mood disorder and major cause of disability worldwide. Approximately 4.4% of the global population is affected by this condition, with wide-ranging variations across gender, age, and nationality (1). Typical symptoms include depressed mood, anhedonia, fatigue, feelings of worthlessness or guilt, changes in appetite, weight, and sleep, psychomotor retardation or agitation, executive deficits, and suicidal ideation (2). These are thought to originate from a complex interplay of psychological and biological factors (3).
Psychological factors that underlie the pathology of MDD comprise deficiencies on an emotional, cognitive, and social level (3). Negative thinking patterns paired with inadequate emotion regulation and excessive rumination have been implicated in the maintenance of depressed mood (4, 5). The aforementioned combination of these three psychological processes further promotes cognitive rigidity, as evident from underperformance in executive functioning tests measuring for example task-switching, working memory (WM), attention, and inhibitory control (4, 6–8). To exemplify, depressed patients take more time to adapt to new rules in the Wisconsin Card Sorting Test and show attentional and memory deficits predominantly in the context of positive affective stimuli (9, 10).
The emotional and cognitive deficiencies accompanying MDD have an impact on interpersonal competencies as well (11, 12). Not only do depressed people show differences in dispositional empathy compared to controls, with for example higher personal distress (13, 14), they also demonstrate shortcomings in communication skills, which might, for example, be expressed in an inanimate body language and bias toward negative facial expressions and conversational contents (12, 15). Additionally, they tend to seek excessively for approval and negative feedback, which may verify their negative self-image (12). Such poor social skills along with self-centered introversion provoke conflicts within the social environment, which pose stressors that crucially contribute to the perpetuation of depressive symptoms (16).
Biological factors that pertain to the characteristics of MDD range from neural imbalances to signaling dysregulations, partly grounded in genetic predispositions (3). Neuroplasticity, a crucial neural mechanism that entails structural and functional brain adaptations in response to altered environmental circumstances, is impaired in individuals with depression, as indicated by abnormally low levels of brain-derived neurotrophic factor (BDNF), the latter being related to hippocampal and prefrontal atrophy in MDD (17, 18). Deficiencies in MDD BDNF levels might originate from epigenetic factors, such as stress exposure (19, 20). A meta-analysis showed that clinical changes in depression were related to BDNF levels, and suggested a role for neuroplasticity in the improvement of symptoms (21)
Another biological disruption in MDD concerns the imbalances between functionally connected fronto-limbic and thalamo-cortical networks, which could further contribute to the maintenance of negative and rigid thinking patterns (22). More precisely, MDD is associated with hyper-connectivity within the default mode network (DMN), a system of brain areas engaged during rumination (22, 23). The DMN works in close accordance with the central executive network (CEN), a group of brain regions involved in WM and goal-directed behavior (24, 25), and the salience network (SN), which mediates the activity of the DMN and CEN according to the saliency of external or internal stimuli (26). In MDD, both the SN and CEN are intrinsically hypo-connected. In addition, the SN is generally hyper-connected to the DMN, while being over-responsive to negative emotional stimuli. This state relates to emotional over-reactivity in depressed patients. The CEN, on the other hand, is under-reactive to negative affective stimuli and its connections to the DMN and SN are weakened compared to that of healthy controls. This disrupted biological brain pattern is linked to deficits in executive functioning (27, 28).
MDD also features dysregulations within the hypothalamic-pituitary-adrenal (HPA) axis, a circuit within the neuroendocrine system that plays a central role in the regulation of stress and immune responses. Hypersecretion of cortisol and impaired negative feedback result in chronically elevated cortisol levels, which increase the vulnerability to stressors, cause disruptions in monoamine and immune systems, and ultimately promote the emergence of depressive symptoms (3, 18, 29). The inadequate HPA responsivity in MDD is further marked by a diminished cortisol awakening response (CAR), as opposed to healthy people who demonstrate steeply elevated cortisol levels within the first 30 min upon awakening (30, 31). Moreover, abnormally high levels of pro-inflammatory cytokines, such as interleukin 6 (IL-6), can be found in depressed patients (32), which is why theories link depression to inflammation (33). IL-6 has a stimulating effect on the HPA axis, and mediates BDNF levels (34, 35).
The outlined socio-cognitive and biological deficiencies can be categorized into six non-exhaustive broad factors, i.e., mood, executive functioning, social skills, neuroplasticity, neural core networks, and neuroendocrine and neuroimmunological factors. Although these factors seem to play a causal role in the symptomology of MDD to differing degrees, the precise etiology of depression is not known and cannot be delineated from the current evidence. The six individual factors presented here appear to influence each other in a circular, perpetuating manner, as illustrated in Figure 1. Thus, the modulation of one factor is expected to exert a net effect across other factors, and subsequently to affect the overall depressive symptomology. Of note, there are more psychological (e.g., cognitive biases) and biological factors (e.g., serotonin transporter genotype) that are known to be involved in depression (36, 37); this review is limited to the six selected factors.
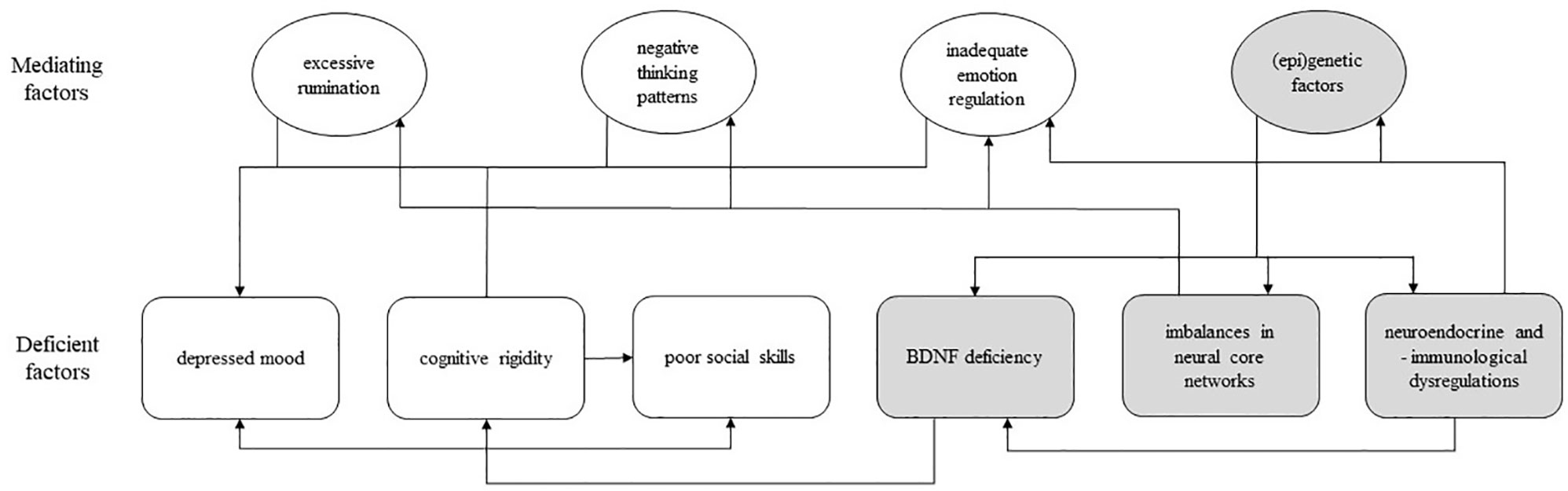
Figure 1 A model of psychological and biological deficiencies associated with major depressive disorder; rounded square-shaped box, deficient factor(s); oval-shaped box, mediating factor(s); white box, psychological factor; gray box, biological factor; arrow, unidirectional influence; BDNF, brain-derived neurotrophic factor.
The sum of deficits within these factors has been shown to result in profound impairments in daily functioning (38), a reduced quality of life (38, 39), an increased risk of suicide (40), and a substantial lack of productivity (41, 42). This also renders MDD costly on an economical level. Estimates of the financial burden that can be ascribed to occupational incapacity due to depression approximate 33 billion euro per year in the United States of America alone, excluding treatment costs (43). Taken together it is clear that there is a pressing need to come up with alternative treatments for depression, next to the conventional first-line psycho- and pharmaco-therapies.
Conventional Treatments of Depression
A wide array of biological (“pharmacotherapy”) and psychological (“psychotherapy”) treatment options for depression is currently available, targeting different elements that are thought to be the underlying pathological cause in their specific theoretical framework (3). Common pharmacotherapy is predominantly based on the hypothesis that depression is caused by a deficiency of monoamine neurotransmitters, such as serotonin (5-HT), dopamine, and norepinephrine, and their receptors, which play an important role in the regulation of mood, arousal, and memory. By elevating these neurotransmitter levels to varying degrees, different types of antidepressants, such as selective serotonin reuptake inhibitors (SSRIs) or monoamine-oxidase inhibitors, are assumed to reduce depressive symptoms (44). However, while being only partially effective in severe cases of depression, antidepressants (45) may also cause severe adverse effects, such as sexual dysfunction or cardiovascular risks (46, 47). Moreover, upon discontinuation, relapse rates are high, which is why antidepressants are often taken chronically (48).
Psychotherapy includes cognitive-behavioral therapy (CBT) and interpersonal therapy, both based on different psychosocial theories, focused on modifying, respectively, behavioral and cognitive biases by means of repeated counseling sessions with a therapist (3). Despite large effects in reducing depressive symptoms (49), relapse and drop-out rates are considerably high (50, 51). For this reason, common pharmaco- and psychotherapies for MDD are frequently combined, which has been acknowledged to be more effective than either approach alone (52). Nevertheless, a substantial proportion of MDD patients that fails to achieve full recovery remains, with almost 75% after 8 weeks and approximately a quarter after 24 weeks of treatment (53).
Alternative Treatments of Depression
In response to the profound limitations of conventional treatments of depression, several alternatives have been proposed (e.g., 54–56). Among these, two approaches that originate from spiritual practice traditions of indigenous and religious communities, namely mindfulness meditation (MM) and administration of classical psychedelics, have gained scientific interest in depression research (57, 58). With regard to the latter, a limited number of clinical trials have been conducted in depressed patients who were administered ayahuasca and psilocybin (59). Although these studies give preliminary evidence of their potential in the treatment of depression, caution regarding efficacy conclusions in depression is warranted due to the currently limited number of studies and small sample sizes (60–63).
For this review we have chosen to focus on psilocybin given the known safety profile in individuals (64) and the potential future of psilocybin therapy since it has received the “breakthrough therapy for treatment-resistant depression (TRD)” designation from the FDA (October 2018). The latter means that the FDA acknowledges that “there is preliminary clinical evidence that indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development and that it will be used to treat a serious or life threatening disease or condition” (65).
MM and psilocybin are thought to exert their effects on behavior via a variety of psychological and biological mechanisms, potentially resulting in expeditious and long-lasting effects (e.g., 66–69).
Mindfulness Meditation
MM is a form of meditation derived from the Pali word “sati” that emphasizes the mental practice of present moment awareness in a non-judgemental and emotionally accepting fashion while remaining in a relaxed state (70, 71). In healthy populations, protracted MM practice (of several months) is linked to improvements in self-regulation and subjective well-being (72, 73). Of note, also shorter MM training (of e.g., four days) already has a positive impact on mood and executive functioning, while reducing fatigue and anxiety (74).
Different forms of MM may be applied, depending on the meditator’s expertise and personal goals. Focused-attention meditation (FAM) involves the direction of attention towards a focal object and gentle reinstatement of this focus when thoughts drift off or strong emotions surface (75). This variant is usually employed by novice meditators. Open-monitoring meditation (OMM) involves no focal object, but rather non-selective awareness of the present moment, and is preferably operationalized among more advanced meditators. Loving-kindness meditation (LKM), on the other hand, combines technical components of FAM and OMM, and puts strong emphasis on the fostering of compassion and positive emotions (76, 77).
MM can be used as a supplement in psychotherapy, constituting mindfulness-based interventions (MBIs), of which mindfulness-based stress reduction (MBSR) and mindfulness-based cognitive therapy (MBCT) are the most common (78). These usually entail sessions guided by a professional in addition to at-home practice over a duration of eight weeks (78). MBSR specifically targets the management of stressful situations and is recommended as a supportive means in chronic diseases, whereas MBCT teaches strategies for dealing with maladaptive thought patterns, which makes it more suitable for the prevention of depressive relapse (60, 79).
Not only have MBIs demonstrated efficacy in the treatment of depression (61), but they are also effective in reducing symptoms in a variety of other psychiatric and medical conditions, such as social anxiety, drug-resistant epilepsy, and mental fatigue following brain damage (80–82). Due to its particularly enduring effects, MM is frequently incorporated as an adjunct in maintenance treatments for the prevention of relapse of depressive symptoms (83, 84) or utilized as alternative treatment in treatment-resistant patients (61). However, effect sizes of MBIs are only moderate (85), and, in order to fully benefit from mindfulness training, a certain meditation depth is required, which depends on individual predispositions and practice frequency (86).
Psilocybin
Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine), on the other hand, is a classical tryptamine hallucinogen that can be derived from a variety of Psilocybe mushroom species (87). Upon oral administration, subjective effects become apparent after approximately 30 to 60 min, peak 90 to 180 min later and last up to 6 h in total (88). These effects are highly dose-dependent (89) and entail perceptual, cognitive, and emotional alterations, which may resemble the features of psychosis (90).
As psilocybin is metabolized into psilocin (4-hydroxy-N,N-dimethyltryptamine) upon ingestion, it is regarded as a prodrug (87, 91). Psilocin acts as a 5-HT agonist and has a particularly high affinity for the 5-HT2A receptor subtype, which is thought to be responsible for its psychotropic effects (92). An analysis by Johnson et al. (64) shows that, although psilocybin has some level of abuse potential and risk, there is no strong evidence of physical dependence, and it can in general be safely used under medical supervision. Nonetheless, adverse effects, such as anxiety or psychotic reactions, may occur when psilocybin is administered in an environment that could evoke negative emotions, as the drug tends to amplify the present affective state (93). Thus, the provision of psychological support and surroundings that are perceived as comfortable and safe are essential when applying psilocybin or other psychedelics in empirical or clinical trials (94).
If these precautions are taken, psilocybin suggests to promote long-lasting positive changes in well-being, attitude, and personality upon a single administration (88, 95). Apart from its potential therapeutic value in depression (63, 96, 97), psilocybin also holds promise for a variety of other conditions, such as anxiety in terminal illness, obsessive–compulsive disorder, and substance dependence (98–100). However, this evidence is still largely based on a limited number of small-scale controlled studies and hence preliminary, impeding its approval for clinical practice.
Aim and Outline
Due to its intrinsically mental and observant nature, MM can be seen as a psychologically focused approach; it is a specific way of paying attention, with a focus on being in the present, in a non-judgemental way. Similar to other trainings or exercises, MM has effects on neurobiological processes (101). Psilocybin is a pharmacological agent, acutely affecting neurobiological processes and inducing psychological effects. Both MM and psilocybin induce structural—longer-lasting—psychological and biological changes, and they show potential of being valuable novel alternatives in the treatment of depression.
In clinical psychedelic patient trials, psilocybin is always administered in a supportive setting, and followed by multiple integration sessions after the experience. Here, the inclusion of MM in the psychedelic-assisted psychotherapy might yield larger or longer positive effects than either treatment on its own, similar to the conjunction of conventional pharmacotherapy and psychotherapy (52).
Noteworthy here is that meditative elements, such as inward-directed attention and relaxation practices, are already being incorporated in psilocybin-assisted trials (69, 102, 103). One study in healthy volunteers concluded that determinants of the longer-lasting positive effects on prosocial attitudes and behaviors as well as psychological functioning following psilocybin administration were the psilocybin-occasioned mystical-type experience and the rate of meditative or spiritual practices (69). While very interesting and relevant in light of current developments psychedelic research, lacking here is a firm theoretical ground of such implications, as none of those studies has directly tested potential applications of a combination of both MM and psilocybin in the treatment of depression (69, 104).
Therefore, the present review aims to compare acute and long-term effects of MM and psilocybin on psychological and biological factors associated with depression in order to provide a theoretical understanding of the potential benefit when used in combination in the treatment of MDD. In each section, findings on the effects of MM and psilocybin will be presented with respect to the aforementioned six factors as depicted in Figure 1, followed by inferences about the potential beneficial effects when using them in combination.
The effects of MM and psilocybin are considered additive or complementary when their comparison suggests they both exert positive effects on the same factor, and when a theoretical combination is reasonably likely to yield superior effects over either treatment individually, with regard to effect duration (i.e., acute versus long-term effect), and/or underlying working mechanism (e.g., bottom-up versus top-down effect).
Methods
In order to gain information on the individual effects and underlying working mechanisms of MM and psilocybin on the six selected MDD-related factors (three psychological, three biological), empirical articles, textbooks, and review papers were searched between September 2018 and January 2019, using the databases PubMed and Google Scholar. Three separate search strings were employed for “depression,” “mindfulness meditation,” and “psilocybin,” which were, combined with the Boolean command “OR.”
The search string for “depression” included terms related to the six factors proposed in the introduction (i.e., depression, mood, cognitive, social, interpersonal, neuroplasticity, BDNF, network, HPA axis, cortisol, stress, inflammation). The search string for “mindfulness meditation” included MM-associated concepts and interventions (i.e., mindfulness, meditation, mindfulness-based intervention, mindfulness-based cognitive therapy, mindfulness-based stress reduction). Although the focus of the review was on psilocybin, the search string for “psilocybin” also contained other classical psychedelics, as similar mechanisms of action may allow for inferences about psilocybin’s potential effects (i.e., psilocybin, psilocin, psilocybin-assisted therapy, tryptamine, LSD, ayahuasca, DMT, 5-HT2A, psychedelic, hallucinogen).
An article was included when its focus was on psilocybin (or a classical psychedelic) and/or mindfulness, and comprised information relevant to the treatment of MDD. In addition, reference lists of included articles were searched. The literature search yielded a total of 1129 hits, of which 1047 publications were excluded since they did not match the inclusion criteria based on their title or abstract. The remaining 82 articles were individually analyzed and assigned to one or more of the six factors associated with depression; 13 articles were added after reference search lists of the included articles. In total, 95 articles were used for the present review (Figure 2). Among those, 67 were papers describing original (“experimental”) research, seven were original research articles without an experimental manipulation (correlational), three were pooled data analyses, 12 were review papers, and the remaining six included theoretical (3) or editorial (1) pieces and one book. A summary of the papers describing original research is presented in Table 1.
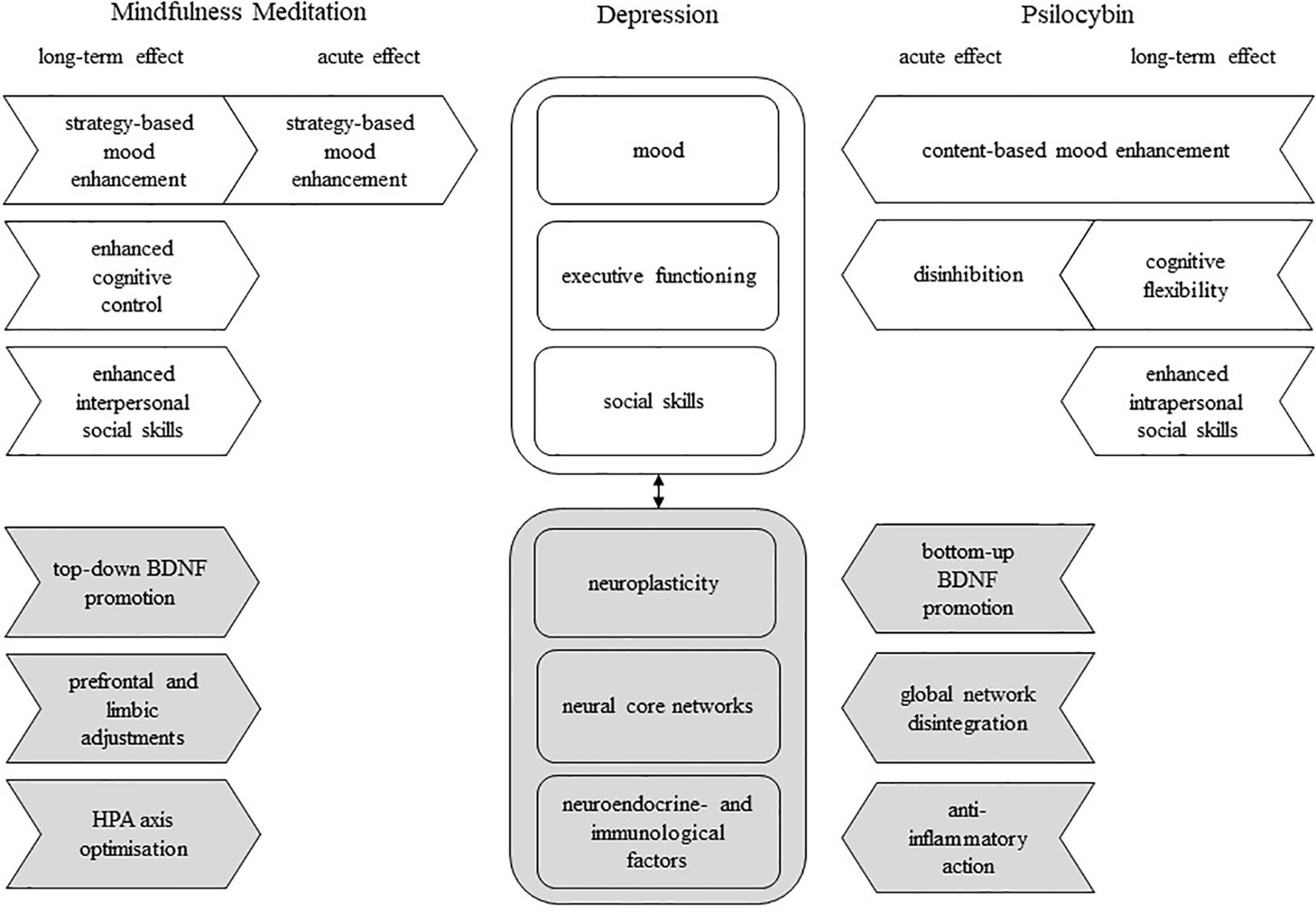
Figure 2 A model of possible complementary effects of mindfulness meditation (MM) and psilocybin on psychological and biological deficiencies associated with major depressive disorder; rounded square-shaped box, deficient factor(s) in depression; arrow-shaped box, unidirectional effect; white box, psychological factor/effect; gray box, biological factor/effect; black arrow, interdependence; BDNF, brain-derived neurotrophic factor.
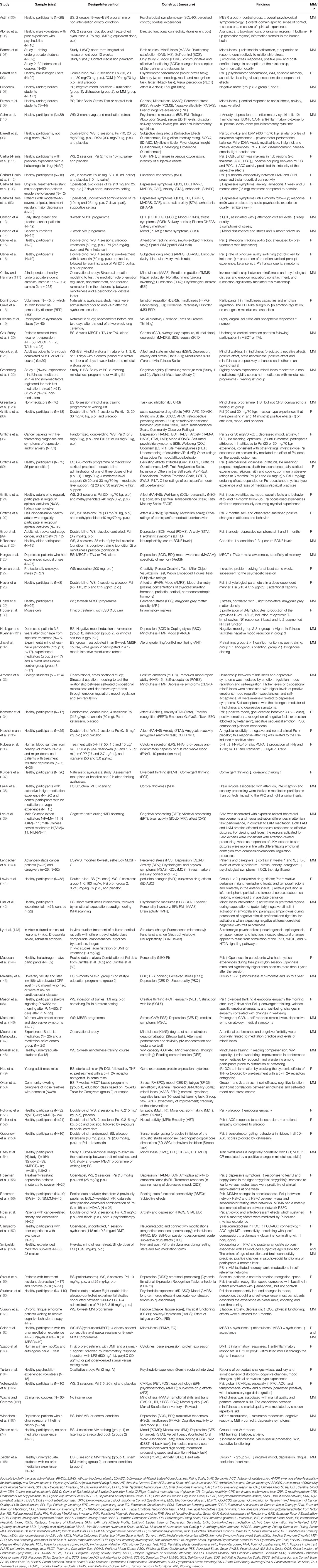
Table 1 Overview of experimental studies and other types of studies (e.g., no intervention or pooled data analysis) included in the review; only constructs and findings (increase ↑/reduction ↓) related to the model are presented; the most used abbreviations are BS, Between Subject; WS, Within Subject; MM, Mindfulness Meditation (or related); P, Psychedelic; Psi, Psilocybin; p.o., per oral; IV, intravenously; the other abbreviations are explained in the footnote of this table.
Psychological Factors
As put forward in the Introduction, the psychological deficiencies of MDD feature a rigid, negatively biased cognitive style that contributes to the recurrence of depressed mood, regulatory difficulties, and social conflict (4, 7, 16). Below, studies describing the effects of MM and psilocybin on mood, executive functioning, and social skills are summarized.
Mood
MM has been found to elevate mood in healthy participants (74, 108, 168), depressed patients (131, 167) and other conditions (114, 161). For MBSR, this effect was already apparent after training units as short as three days (168) and remained up to six months (114), while MBCT demonstrated sustained effects for three months (161). Studies of mindfulness training that was not combined with psychotherapy only demonstrated short-term mood enhancement in healthy volunteers (74, 108).
It has been proposed that such mood enhancement arises from the acquisition of mental strategies. Mindfulness-based mental strategies are thought to reduce cognitive reactivity, the tendency to engage in negative thinking in response to mildly dysphoric mood, and promotes emotion acceptance, ultimately improving affect regulation (133, 154, 167, 169). Additionally, Huffziger and Kuehner (131) showed that by encouraging non-judgemental awareness of negative thoughts in depressed patients, the association between negative thoughts and negative mood might diminish, and the perpetuation or relapse of depressive symptoms prevented (131). This is in line with the negative association between mindfulness and rumination, and the positive relationship between mindfulness and nonattachment found in healthy volunteers. The latter indicates the degree to which an individual perceives happiness as independent from external circumstances, such as financial wealth or daily-life experiences (117). It is suggested that mindful individuals ruminate less and are consequently less likely to adapt their intrinsic state to affectively salient events in their environment (117, 131).
Psilocybin has been shown to acutely enhance mood in healthy participants (69, 96, 124, 134, 135, 151) as well as depressed (63, 103, 155) and cancer patients (96, 97, 99). This occurred after one to two fixed (10 and 25 mg, p.o.) or weight-adjusted doses (range between 1-30 mg/70 kg, p.o.), and in one study, the improvement was still significant at a 14-month follow-up with 0.2 mg/kg (14 mg/70 kg, p.o.) (99). Several lines of evidence further suggest that the mood-enhancing effect of psilocybin is dose-dependent (93, 88, 89, 128).
The acute effects of psilocybin occasionally involve a “peak” experience, a blissful sense of sacredness, revelation, transcendence of time and space, or connectedness with the environment (170). This often entails psycho-spiritual insights that are reported to be of major personal value and have an enduringly positive impact on well-being, attitude and personality (69, 97, 103, 124, 144). These persisting positive (mood) effects and relative freedom from worry is also called “afterglow” and indicated as an important timeframe for psychotherapeutic interventions (171, 172).
In summary, MM and psilocybin both induce positive mood changes which might outlast the acute MM or psilocybin stage. Effects of both seem to be “dose”-related with more extensive MM practice, and higher doses of psilocybin having more pronounced effects. Nonetheless, we suggest that MM and psilocybin have a different mechanism of action to induce the same effect. For MM, repeated training promotes the use of mental strategies, altering the cognitive frame in which negative thoughts are perceived and coped with (e.g., 133). With regard to psilocybin, perceptual and thought contents are directly altered by destabilizing established belief systems resulting in a restoration of adequate mood regulation (e.g., 69, 172, 173).
The combination of the strategy-based approach of MM with psilocybin’s content-based approach (Figure 2) could possibly contribute to a potentiation or longer maintenance of induced mood enhancements. A reduction of cognitive reactivity and promotion of emotional acceptance through MM practice may prevent a relapse of negative thought patterns when the afterglow subsides. Also, Griffiths et al. (69) found that more extensive spiritual practice, including meditation, was associated with a higher frequency of psychedelic-induced peak experiences. This implies that MM practice might be able to facilitate peak experiences, which would result in an increased likelihood of personal insights and enduring positive mood effects. Likewise, positive mood state following personal insights during a psychedelic experience might, facilitate the non-judgmental observation of negative thoughts, since previous research suggests a bidirectional positive relationship between positive mood and mindfulness (121).
Whether the single or combined practice in psychiatric patients would be beneficial is another question. Two recent studies investigating the effects of ayahuasca, another psychedelic substance with similar 5-HT2A agonistic action as psilocybin was shown to increase emotion regulation and some aspects of mindfulness in healthy volunteers (162, 118). Of note, mindfulness was not increased in participants with borderline personality disorder traits (118). This may bear meaningful clinical implications, as people with certain psychopathologies, including depression, might be less receptive to a psychedelic-induced enhancement of mindfulness.
Executive Functioning
Studies have demonstrated positive effects of MM on executive functioning, including improvements in cognitive flexibility (122). Repeated training of mindfulness has been shown to improve WM as well as attentional and inhibitory capacities (74, 123, 132, 147, 148).
A possible explanation for MM-induced executive function enhancement builds on an incremental reduction of mind-wandering together with an enhancement of meta-awareness. The former describing the tendency to drift off with one’s thoughts, while the latter can be conceptualized as the acknowledgement of ongoing mental processes, which shares a neural signature with that of executive functions. Accordingly, this hypothesis was supported empirically (74, 126, 149). A positive “side” effect of MM’s cognition-enhancing effects is a more pronounced subjective sense of control, as shown in a study investigating the effects of MBSR (105).
In contrast to MM’s homogenously positive effects across cognitive domains, psilocybin tends to acutely impair some aspects of executive functioning like inhibition, attention, and WM (93, 115, 116, 153), while improving others by, for example, inducing a greater bias towards positive stimuli (134), or leaving some processes unaffected, like spatial WM (107, 115, 116, 153).
The feeling of loss of control over thoughts or perceptions is frequently reported in psilocybin trials, which is linked to adverse reactions, and may reflect the induced decreases in executive control (128, 160, 164). On the other hand, psilocybin’s dys-executive effects have also been proposed to offer therapeutic implications as to surface suppressed emotions and thoughts in order to confront them and, hence, restore emotional responsiveness in MDD (155).
Further, consistent with the notion that psilocybin impairs cognitive focus and control, 5-HT2A agonism has been implicated in an acute decline in convergent thinking, which critically relies on adequate executive functioning (137). In line with this, it was shown that LSD-induced impairment of working memory, executive functions, and cognitive flexibility was mediated by the 5-HT2A receptor (174). Conversely, 5-HT2A agonism is also associated with increased cognitive flexibility and divergent thinking (106, 119, 127, 137, 144, 175). The latter findings might be suggested to underlie decreased executive control and a loosening of associations via neuroplastic changes in core neural networks, although this hypothesis has to be tested. Additionally, it is suggested that these psychedelic-induced increases in cognitive flexibility are potentially long-lasting (119, 127, 144).
Taken together, MM demonstrates relatively global cognition-enhancing effects upon repeated training, whereas psilocybin’s effects on executive functioning build on increased acute disinhibition and enduring cognitive flexibility (Figure 2). Supporting psilocybin-assisted therapy with MM practice may have the potential to buffer feelings of loss of control associated with the acute psychedelic effects by boosting both subjective and objective executive functioning and, consequently, reducing the risk of adverse reactions. Alternatively, an interference with the individual effects of either treatment is also possible. For instance, by improving cognitive control, MM may reduce psilocybin’s cathartic effects or, conversely, psilocybin might exacerbate the practice of MM during acute effects, as certain aspects of mindfulness, especially in FAM, build on attentional capacities (139).
Social Skills
With regard to social skills, MM has been linked to greater relationship satisfaction, as it supposedly fosters a more adequate expression and recognition of feelings and reduces the degree to which an individual is emotionally affected by distressing social events (107, 166, 169). This led to the emergence of variants of MBIs that specifically focus on the interpersonal aspects of MM, such as mindful relating (166) or mindfulness-based relationship enhancement (MBRE) (176). These trainings are encompassed by the frame term “relational mindfulness” and lay emphasis on fostering compassion and attentive communication to others.
Psilocybin has positive acute and subacute effects on some aspects of empathy (95, 151, 177). A recent study (159) further showed that psilocybin improved emotional face recognition (cognitive empathy) in TRD patients, while another study demonstrated reduced feelings of social exclusion and in healthy volunteers following psilocybin administration compared to placebo (153).
Pahnke (171) suggested that, in the afterglow, the willingness “to enter into close interpersonal relationships” may be heightened, which is in agreement with self- and other reports of positive changes in social attitudes and behavior following psychedelic peak experiences (69, 88, 96, 102). One aspect of peak experiences in particular, namely the phenomenon of ego dissolution, could be meaningful in this context. Ego dissolution can be described as the loss of sense of identity that is separate from its surroundings and is, therefore, accompanied by an intense feeling of connectedness with the environment. Such an experience may contribute to the destabilization of self-centered belief systems and open the individual up to his or her social surroundings (172). This theoretical implication is supported by the finding of enduring increases in the personality trait “openness” following a psilocybin session (69, 144).
Both MM and psilocybin appear to induce long-term enhancements of social skills. Based on studies conducted up until now it is suggested that MM does so by influencing the way an individual deals emotionally with social encounters, which, as a result, promotes adequate social behavior, or interpersonal social skills. Psilocybin seems to predominantly act on an intrapersonal level of social skills by means of enhanced empathic abilities and a changed personality (Figure 2). Combining MM and psilocybin could potentially enhance social relationships more efficiently, as changes in social cognition effectuated by psilocybin would be expected to complement changes in social behavior induced by MM.
Biological Factors
Studies have shown a range of biological deficiencies to be implicated in MDD among which impaired neuroplasticity, an imbalance in core neural networks, and disturbances in stress responses which are visible as disruptions in neuroendocrine and neuroimmune parameters (21, 22, 29). In the next section MM and psilocybin effects on these processes are summarized.
Neuroplasticity
For MM, BDNF-promoting effects appear to be linked to prolonged, repeated practice (110) rather than a single, brief training session (125). The exact mechanisms underlying these effects are unclear, though. While MM’s relation to serotonin signaling has not been investigated yet (101), the expression of BDNF may be enhanced by either frontal activation following active engagement of attention, vagal stimulation or a reduction in stress response (110).
Psilocybin and related classical psychedelics, such as lysergic acid diethylamide (LSD) or N,N-dimethyltryptamine (DMT), are hypothesized to promote neuroplasticity through mechanisms involving 5-HT2A agonism (67). Serotonin 2A receptors, to which psilocin binds, are especially prominent on large glutamatergic pyramidal neurons in deep cortical layers projecting to layer V pyramidal neurons of the PFC, and on layer V itself. These receptors are suggested to rapidly increase in activity as psilocybin is ingested, hypothetically resulting in an elevated expression of BDNF (67, 143). The resulting temporarily state of heightened neuroplasticity may already occur after a single, psychotropic dose and could allow for major synaptic changes, which was suggested to offer an important opportunity for psychotherapeutic interventions (67).
This indicates that the effects of psilocybin and MM on neuroplasticity differ in aetiology and magnitude. Psilocybin could induce a transient, but powerful neuroplastic boost, which is driven by bottom-up glutaminergic processes. In contrast, MM supposedly relies on top-down regulatory efforts and encourages plasticity incrementally throughout the progress of training (Figure 2). These approaches may support one another, as MM could possibly serve to prolong the potential neuroplastic state induced by psilocybin and psilocybin might boost the rate at which BDNF rises throughout MM training.
Neural Core Networks
MM has differential effects on the SN and CEN with SN regions, the insula and anterior cingulate cortex (ACC), being engaged during mediation, whereas the activity of CEN regions, the lateral PFC and parietal cortex, decreases (75). As the insula and ACC are involved in interoceptive processes (178) and the lateral PFC and parietal cortex in external awareness (179), this is thought to reflect inward-focused attention during the practice of mindfulness (75, 77). Moreover, MM promotes the activation of the dorsolateral PFC, a key region of the CEN, which is important for cognitive control (142, 180). Long-term meditators show increased cortical thickness in the insula, sensory cortices, and PFC as well as reduced volume of the amygdala, a region involved in fear responses (129, 138). This supposedly represents decreased emotional over-reactivity and increased regulatory control that has been manifested through repeated practice, which is in line with the effects on mood and executive functioning, as discussed above.
Psilocybin was proposed to globally decrease functional neural integrity within, while increasing connectivity between networks, which may be responsible for the experience of hallucinations, loosening of strong associations, and increases in cognitive flexibility following its administration (112, 156, 175). Most notably, the cortical disintegration of the DMN has been implicated in the occurrence of social skill-related ego dissolution and increases in some aspects of mindfulness (77, 157, 181). In addition, an increased functional connectivity between SN and CEN contrasts the aforementioned effects of MM on these networks (156), which might relate to the treatments’ opposing cognitive effects. The glutaminergic action of 5-HT2A receptors discussed in the previous section would suggest that psilocybin induces widespread cortical activations, particularly in association cortices, where 5-HT2A receptors are most abundant (182). Consistent with this expectation, some studies appear to endorse acute psilocybin-induced hyper-activation in frontal regions, as opposed to more posterior regions, and this pattern of activity correlated positively with the measures of psychotic symptoms, especially ego dissolution (106, 141, 165). An fMRI study of psilocybin’s acute effects showed deactivations in cortical hub regions, such as the posterior cingulate cortex and thalamus. This could be explained by an involvement of GABAergic interneurons within psilocybin’s pathway of action, which, when excited, inhibits subsequent neurons (111). The apparent paradox between the frontal hyper-frontality shown in one study (165) and the decreased perfusion in frontal regions by another study (111) was suggested not to be in contrast, but rather dependent on the method of analysis (141). It was suggested to interpret the relative changes in perfusion in relation to absolute signal variations, and to report two analyses, with and without this “correction” for global activity as a solution to enhance transparency, reduce inconsistencies, and help in the interpretation of findings (141). Nonetheless, as cortical hubs play a crucial role in coordinating the flow of information across functionally discerned brain areas, their inhibition might result in sub-optimal communication between brain areas involved in executive control, reflecting the disinhibition effects of psilocybin (183).
While jointly working to resolve DMN dominance associated with excessive rumination (22, 23, 77), MM and psilocybin seem to alter circuits differentially. MM additionally targets areas related to interoception and executive control, while psilocybin has a more wide-spread effect on functional integrity, potentially promoting flexible cognition (Figure 2). This appears to reflect the MM- and psilocybin-induced psychological changes described earlier. By reorganizing the connectivity between the DMN, CEN and SN, MM, and psilocybin may restore normal functional integration in patients, which could contribute to a reduction of negative and rigid thinking patterns. Relevant in this light is the recent study by Smigielski et al. (158) who administered a single dose of psilocybin (0.315 mg/kg, p.o.) to healthy, experienced meditators, during a five-day mindfulness retreat. The pre-post brain resting state analysis revealed a decoupling of medial prefrontal and posterior cingulate cortices, which was associated with the psilocybin-induced subjective ego dissolution. Of note, the extent of ego dissolution and brain connectivity predicted positive changes in psycho-social functioning of participants 4 months later.
Neuroendocrine and Neuroimmunological Factors
The attenuation of stress responses has been suggested to be a central mechanism through which MM exerts its beneficial effects on mental and physical health. MM may do so by, reducing stress-reactivity, in addition to promoting regulatory prefrontal pathways, involving a reduction in amygdalar projections and HPA axis activity (68). However, although MM training generally reduces subjective psychological stress (140, 150), its effect on cortisol secretion varies across populations. In healthy volunteers, eight weeks of MBSR training had no effect on cortisol levels (145, 150), whereas, in cancer patients, cortisol levels decreased significantly under comparable intervention settings (113, 140).
As diseases represent sources of profound stress, this may imply that the association between MM and cortisol only holds for highly stressful situations, which was supported empirically by Brown et al. (109). Accordingly, it is conceivable that MM also reduces cortisol levels in depressed patients. Instead, what has been observed by Matousek et al. (146) was that MBSR increased the CAR in cancer patients who demonstrated depressive symptoms. This conforms an alternative hypothesis, namely that MM not merely reduces cortisol, but rather optimizes HPA responsivity (78, 184). However, another study investigating the effect of MBCT on the CAR in patients remitted from recurrent depression did not support the findings by Matousek et al. (146) (120), which may be due to the use of MBCT rather than MBSR (78). MBSR, as opposed to MBCT, is implicated in being a particularly suitable means for diminishing overall stress symptomatology, as it promotes specific stress coping strategies (79, 113). Although MM might additionally reduce pro-inflammatory cytokines, including IL-6, these findings are inconsistent (140, 145), but may originate from vagal stimulation, which is thought to induce a cholinergic anti-inflammatory reflex (185).
Psilocybin, on the other hand, is associated with an acute increase in cortisol levels (128). In accordance with the involvement of cortisol in attention and memory, this transient elevation could possibly facilitate extinction learning of negative associations by prioritizing the formation of new memories over the retrieval of older memories (186, 187).
Moreover, psilocybin reduced subjective stress in terminally ill cancer patients during the first three months following administration (99). An incremental down-regulation of 5-HT2A receptors is suggested to play a role in this as prefrontal 5-HT2A receptors were found to be involved in stress response pathways (188). Furthermore, by activating prefrontal areas, psilocybin might encourage top-down control of stress responses in limbic structures, such as the amygdala (67).
5-HT2A agonism has also been linked to major anti-inflammatory action, as 5-HT2A receptors are integrated in an abundance of cells throughout the immune system (149, 189, 190). Psilocybin and related psychedelics are hypothesized to distort cell signaling within the immune system by selectively stimulating anti-inflammatory pathways (191, 192). Although this is yet to be tested with psilocybin, LSD, DMT, and 2,5-Dimethoxy-4-iodoamphetamine (DOI) were found to have anti-inflammatory action, inhibiting the production of IL-6 (130, 149, 163) which might account for enduring antidepressant psychedelics effects (193). Nonetheless, there is evidence that 5-HT2A receptors are also involved in pro-inflammatory responses. The extent to which 5-HT is immunosuppressive or immune-activating may depend on its blood concentration (136).
The neuroendocrine and neuroimmune system are interdependent networks that communicate by means of hormone and cytokine signaling (194). MM and psilocybin act differentially and possibly complementarily on these systems. Through the progressive strengthening of regulatory control and reduction of stress-responsiveness, MM optimizes HPA axis functioning and may, eliminate immune system disruptions. Psilocybin, one the other hand, could transiently reduce inflammatory responses by means of 5-HT2A agonism and consequently reduce the stimulation of the HPA axis through anti-inflammatory cytokines (Figure 2).
Discussion
Depression is a major public health problem, to which conventional treatments represent an insufficient solution (1, 45, 51). MM and psilocybin appear to be promising novel treatments, and combined their resulting therapeutic effect might even be greater. However, the current literature is limited to theoretical and empirical underpinnings of their singular use in treatment (e.g., 61, 63). The present review therefore aimed to identify possible additive or complementary effects of MM and psilocybin on six factors (mood, executive functioning, social skills, neuroplasticity, neural core networks, neuroendocrine, and neuroimmunological factors) associated with MDD in order to offer theoretical implications for future clinical research of depression. Findings showed that MM and psilocybin exerted similar effects on mood, social skills, and neuroplasticity; different effects were found on executive functioning, neural core networks, and neuroendocrine and neuroimmune system markers. The effects on mood were “dose”-dependent, with more MM practice or higher psilocybin doses leading to more pronounced mood effects; effects on neuroplasticity were already visible after a single dose of psilocybin, while more MM practice sessions were needed before effects were visible. While for most factors the combination of MM and psilocybin is potentially beneficial, this was not clear for executive functions.
From a psychological perspective, MM employs mental strategies that augment emotional and cognitive self-regulation in the long term (73, 133, 154), whereas psilocybin has neuromodulatory effects that induce a state of apparent “flexible” cognition, and may lead to personal insights that diminish negative biases (102, 111). A combination of MM and psilocybin could possibly shift both the cognitive frame and content of thoughts towards a more positive, open-minded outlook, promote the feeling of control over strong emotions that might occur under the acute effects of psilocybin, or improve communication skills. This may ultimately enhance psychological factors, such as mood, cognitive control, and relationship satisfaction. Recent research suggests that the extent of psilocybin-induced ego dissolution during a mindfulness session might play a very important role in the endurance of positive changes in psycho-social functioning (158).
From a biological perspective, MM serves to adjust prefrontal and limbic activity and HPA reactivity through repeated top-down control (129, 138, 184). Psilocybin, on the other hand, promotes global network disintegration and anti-inflammatory effects involving transient bottom-up processes (175, 191). Pairing these effects may result in a two-way reorganization of neural networks, especially those involved in rumination, and downregulation of neuroendocrine and neuroinflammatory responses. Part of this suggestion was investigated and supported by a recent study that showed decoupling in self-referential networks and the psilocybin-induced change in self-experience, during a mediation retreat, to be predictive of enduring positive changes in psycho-social functioning (158). Together these findings offer several implications for future clinical research into MDD.
Implications for Future Research
The present findings suggest that the combination of MM and psilocybin could possibly exert larger or longer-lasting effects in the treatment of MDD than either treatment alone. These effects may particularly relate to enhancements in mood, social skills, neuroplasticity, and a reduction of stress-related neuroendocrine and neuroinflammatory markers. Testing this hypothesis requires comparisons of changes in these variables in depressed patient groups in a—preferably—randomized, double-blind, placebo-controlled trial with repeated measurements to test acute and persistent effects, weeks to months after treatment.
Ideally, psilocybin-assisted MBI is compared to psilocybin and MM alone, and to a conventional antidepressant (SSRI). To test the effects of MBI on the variables of interest a “psychological” placebo, e.g., minimal psychological support based on CBT principles, complementing the pharmacological manipulation, is needed. This is also warranted since the administration of psilocybin without psychological support is not recommended (94). Primary endpoints would focus on depressive symptomatology assessed with daily diaries and weekly assessments with the Hamilton Depression Inventory or the Beck Depression Inventory (BDI) (195–197). Secondary endpoints would be social skills and executive functioning, assessed with cognitive tests, self-reports, and structural and functional brain imaging; neuroplasticity (BDNF), neuroendocrine (cortisol, oxytocin) and neuroinflammatory (cytokines) factors assessed in blood samples. To add, self-reports from patients and observational reports from significant others could be used to test whether depressed patients indicate less conflict and higher relationship satisfaction following a psilocybin-assisted MBI than their respective control groups as both treatments are known to alter the perception of social relations (107, 172). Cognitive tests at different time points in the treatment will be useful to dissociate short- and long-term effects of the combination of MM and psilocybin on executive functioning and clarify potential opposing effects on such processes as suggested by the inconclusive findings in the present review.
To date, no norms regarding the exact procedure, type of psychological support, dose(s) of the psychedelic, or duration of the psychedelic therapy have been agreed upon (198). With regard to the psychological component of psilocybin-assisted MBI therapy, the typical treatment duration of eight weeks MBI may be appropriate (78). To add, it has not yet been determined whether MM should precede or follow the administration of psilocybin. The present findings would endorse MM practice prior to a psilocybin session, as it may have the potential to reduce the risk of adverse effects in depressed patients due to its positive effects on mood and cognition (e.g., 74). Moreover, the findings imply that MM could facilitate the occurrence of peak experiences upon psilocybin administration (69), something that has been shown to be important in the treatment response (97, 155).
Considering the potential benefits of these implications, future studies could test if (eight weeks of) MM practice prior to a psilocybin session can decrease potential adverse reactions such as anxiety, and increase the chance of having a peak experience, or increase the intensity of the experience during a psilocybin session, compared to appropriate control conditions (69, 102, 124, 144).
Lastly, a combination of MM and psilocybin may also bear benefits for MDD patients in a more indirect way, as findings indicate. Mindfulness could represent a useful asset to the training of psychedelic therapists (199, 200). Future studies may test if patients of psychedelic therapists trained in mindfulness demonstrate better outcomes on psychological measures of depression in comparison to patients of therapists that were not trained in mindfulness.
Limitations
Upon discussing scientific implications that the findings offer, it is important to mention that the present review features a number of limitations. First, due to different methodologies, findings of the included studies are difficult to compare. For example, studies examining the effects of MM have used diverse assessment methods to measure similar variables in different populations, which could explain the inconsistent findings across studies. For instance, while Carlson et al. (114) demonstrated positive effects of MBSR on mood states (Profile of Mood States) of cancer patients compared to pre-MM scores, Astin (105) did not demonstrate significant effects of MBSR on mood (Symptom Check List-90-R (SCL-90-R)) in undergraduate students. While the (physical and mental) difference in groups are apparent, the construct differences between questionnaires might not be that obvious. Whereas the POMS is specifically designed to assess mood states, the SCL-90-R screens for a broad range of clinical symptoms (201–203).
Another example is the significant decrease shown in immune markers (salivary IL-6 level) in healthy participants (cancer patient caregivers) after six weeks of MBSR, and the absence of this finding in university staff and students following an 8-week-long low-dose MBI (145). Despite both groups being regarded as healthy, it is apparent that they were exposed to dissimilar kinds of stressors, which precludes inferences about general effects of MM on immune system markers.
Another methodological issue noted in the reviewed MM studies is the general lack of active control groups, which impedes the differentiation of effects that are specific to MM from those that apply to any other psychological treatment. Hence, points of attention when conducting a study investigating the effects of MM are to use gold standard tests to assess certain constructs and the inclusion of active control groups (203).
As for studies investigating the effects of psilocybin there are a number of methodological issues that at this moment withhold from making firm statements about potential implications. Examples are the small number of patients samples (96, 99, 103, 159), the use of an open-label design, and no control group (103). These methodological choices make the generalization of findings to larger populations not possible at this stage, and due to the use of open-label or uncontrolled designs, pharmacological effects cannot be separated from expectancy or placebo effects. Additionally, psilocybin is routinely combined with psychological support, making it difficult to dissociate the psychotropic from general care effects (62, 63, 69).
Moreover there are conceptual issues regarding the definition of MM, as it comprises various forms, such as FAM, MBCT or MBRE. These techniques emphasize different aspects of mindfulness and consequently yield diverse psychological and biological effects (60, 75, 176). For example, the effect of MM on cortisol (CAR) differs between MBCT and MBSR (78, 120). Hence, findings in one study may not necessarily apply to all forms of MM and introduce methodological noise.
With regard to psilocybin, and its mechanism of action, the discussion largely pertained to 5-HT2A agonism (67, 136, 143, 149, 175, 188–191, 204) while psilocybin is also known to act on other neurotransmitter systems (87) which might be relevant for the comparison with MM.
Further, in some of the included papers, hypotheses were proposed that have not been subjected to sufficient empirical testing, such as proposed mechanisms regarding the immunosuppressive action of psilocybin and BDNF-promoting effects of MM (67, 68, 110, 191). To draw definite conclusions, premises based on concrete empirical evidence are needed, and therefore, the inferential power of the present review with regard to the aforementioned is limited. These hypotheses were nevertheless incorporated with other reviewed literature in order to speculate on potential interaction points between psilocybin and MM that may be of value in the treatment of depression upon investigation.
Conclusion
The present review provides an extensive overview of the current scientific knowledge on the effects of MM and psilocybin on specific pathological depressive features, and on how both interventions might be complementary or even synergistic when combined, in the treatment of depression. With this a valuable theoretical ground for future research is presented. Future studies investigating these effects in both healthy and depressed populations, using rigorous control conditions and representative samples, will provide more knowledge on possible implementation of psilocybin-assisted MBI in clinical practice.
Author Contributions
KH and KK conceptualized the review question. KH conducted the literature search. KH conceptualized the first version and figures. KH and KK wrote the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. World Health Organization. Depression and other common mental disorders: global health estimates. Vol. 99. Geneva. (2017) p. 124–30. Retrieved from https://apps.who.int/.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DMS V. In: Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. American Psychiatric Publishing (2013). doi: 10.1176/appi.books.9780890425596.744053
3. Nolen-Hoeksema S. Mood Disorders and Suicide. In: Abnormal Psychology. Boston: McGraw-Hill (2017). p. 172–213.
4. Beck AT. Depression: Clinical experimental and theoretical aspects. New York: Harper & Row (1967).
5. Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking Rumination. Perspect psychol Sci (2008) 3(5):400–24. doi: 10.1111/j.1745-6924.2008.00088.x
6. Davis RN, Nolen-Hoeksema S. Cognitive inflexibility among ruminators and nonruminators. Cogn Ther Res (2000) 24(6):699–711. doi: 10.1023/A:1005591412406
7. Meiran N, Diamond GM, Toder D, Nemets B. Cognitive rigidity in unipolar depression and obsessive compulsive disorder: Examination of task switching, Stroop, working memory updating and post-conflict adaptation. Psychiatry Res (2011) 185(1-2):149–56. doi: 10.1016/j.psychres.2010.04.044
8. Rose EJ, Ebmeier KP. Pattern of impaired working memory during major depression. J Affect Disord (2006) 90(2-3):149–61. doi: 10.1016/j.jad.2005.11.003
9. Dalgleish T, Watts FN. Biases of attention and memory in disorders of anxiety and depression. Clin Psychol Rev (1990) 10(5):589–604. doi: 10.1016/0272-7358(90)90098-U
10. Stordal KI, Lundervold AJ, Egeland J, Mykletun A, Asbjørnsen A, Landrø NI, et al. Impairment across executive functions in recurrent major depression. Nord J Psychiatry (2004) 58(1):41–7. doi: 10.1080/08039480310000789
11. Segrin C. Social skills deficits associated with depression. Clin Psychol Rev (2000) 20(3):379–403. doi: 10.1016/S0272-7358(98)00104-4
12. Hames JL, Hagan CR, Joiner T. Interpersonal Processes in Depression. SSRN (2013) 9:355–77. doi: 10.1146/annurev-clinpsy-050212-185553
13. Cusi AM, MacQueen GM, Spreng RN, McKinnon MC. Altered empathic responding in major depressive disorder: Relation to symptom severity, illness burden, and psychosocial outcome. Psychiatry Res (2011) 188(2):231–6. doi: 10.1016/j.psychres.2011.04.013
14. Thoma P, Zalewski I, von Reventlow HG, Norra C, Juckel G, Daum I. Cognitive and affective empathy in depression linked to executive control. Psychiatry Res (2011) 189:373–8. doi: 10.1016/j.psychres.2011.07.030
15. Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry (2009) 166(10):1178–84. doi: 10.1176/appi.ajp.2009.09020149
16. Hammen C. Stress and depression. Annu Rev Clin Psychol (2005) 1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938
17. Konarski JZ, Mcintyre RS, Kennedy SH, Rafi-tari S, Soczynska JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: Bipolar disorder versus major depressive disorder. Bipolar Disord (2008) 10(1):1–37. doi: 10.1111/j.1399-5618.2008.00435.x
18. Saveanu RV, Nemeroff CB. Etiology of Depression: Genetic and Environmental Factors. Psychiatr Clinics North America (2012) 35(1):51–71. doi: 10.1016/j.psc.2011.12.001
19. Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus (2006) 16(3):239–49. doi: 10.1002/hipo.20156
20. D’Addario C, Dell’Osso B, Galimberti D, Palazzo MC, Benatti B, Di Francesco A, et al. Epigenetic modulation of BDNF gene in patients with major depressive disorder. Biol Psychiatry (2013) 73(2):e6–7. doi: 10.1016/j.biopsych.2012.07.009
21. Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol (2008) 11(8):1169–80. doi: 10.1017/S1461145708009309
22. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry (2015) 72(6):603–11. doi: 10.1001/jamapsychiatry.2015.0071.Large-scale
23. Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist (2012) 18(3):251–70. doi: 10.1177/1073858411403316
24. Miller EK, Cohen JD. An Integrative Theory of Prefrontal Cortex Function. Annu Rev Neurosci (2001) 24:167–. doi: 10.1146/annurev.neuro.24.1.167
25. Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williamsa LM, et al. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U States America (2013) 110(49):19944–9. doi: 10.1073/pnas.1311772110
26. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct (2010) 214(5-6):655–67. doi: 10.1007/s00429-010-0262-0
27. Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiol Dis (2013) 52:4–11. doi: 10.1016/j.nbd.2012.01.015
28. Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, et al. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci (2014) 7:930. doi: 10.3389/fnhum.2013.00930
29. Southwick SM, Vythilingam M, Charney DS. The Psychobiology of Depression and Resilience to Stress: Implications for Prevention and Treatment. Annu Rev Clin Psychol (2005) 1:255–91. doi: 10.1146/annurev.clinpsy.1.102803.143948
30. Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, Von Auer K, Jobst S, et al. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sci (1997) 61(26):2539–49. doi: 10.1016/S0024-3205(97)01008-4
31. Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol Psychol (2009) 80(3):265–78. doi: 10.1016/j.biopsycho.2008.10.004
32. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A Meta-Analysis of Cytokines in Major Depression. Biol Psychiatry (2010) 67(5):446–57. doi: 10.1016/j.biopsych.2009.09.033
33. Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep (2011) 13(6):467–75. doi: 10.1007/s11920-011-0232-0
34. Mastorakos G, Chrousos GP, Weber JS. Recombinant interleukin-6 activates the hypothalamic-pituitary-adrenal axis in humans. J Clin Endocrinol Metab (1993) 77(6):1609–94. doi: 10.1210/jcem.77.6.8263159
35. Sharma RP, Tun N, Grayson DR. Depolarization induces downregulation of DNMT1 and DNMT3a in primary cortical cultures. Epigenetics (2008) 3(2):74–80. doi: 10.4161/epi.3.2.6103
36. Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry (2008) 13:131–46. doi: 10.1038/sj.mp.4002067
37. Everaert J, Koster EHW, Derakshan N. The combined cognitive bias hypothesis in depression. Clin Psychol Rev (2012) 32:413–24. doi: 10.1016/j.cpr.2012.04.003
38. Lin CH, Yen YC, Chen MC, Chen CC. Depression and pain impair daily functioning and quality of life in patients with major depressive disorder. J Affect Disord (2014) 166:173–8. doi: 10.1016/j.jad.2014.03.039
39. Pyne JM, Patterson TL, Kaplan RM, Gillin JC, Koch WL, Grant I. Assessment of the quality of life of patients with major depression. Psychiatr Serv (1997) 48(2):224–30. doi: 10.1176/ps.48.2.224
40. Angst J, Angst F, Stassen HH. Suicide risk in patients with major depressive disorder. J Clin Psychiatry (1999) 60:57–62.
41. Lerner D, Adler DA, Chang H, Lapitsky L, Hood MY, Perissinotto C, et al. Unemployment, job retention, and productivity loss among employees with depression. Psychiatr Serv (2004) 55(12):1371–8. doi: 10.1176/appi.ps.55.12.1371
42. Hysenbegasi A, Hass SL, Rowland CR. The impact of depression on the academic productivity of university students. J Ment Health Policy Econ (2005) 8:145–51.
43. Kessler RC, Heeringa S, Lakoma MD, Petukhova M, Rupp AE, Schoenbaum M, et al. Individual and societal effect of mental disorders on earnings in the United States: Results from the National Comorbidity Survey Replication. Am J Psychiatry (2008) 165:703–11. doi: 10.1176/appi.ajp.2008.08010126
44. Stahl SM. Stahl′s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Mens Sana Monogr (2013) 8(1):146–50. doi: 10.4103/0973-1229.58825
45. Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: A patient-level meta-analysis. JAMA—J Am Med Assoc (2010) 303(1):47–53. doi: 10.1001/jama.2009.1943
46. Stimmel GL, Gutierrez MA. Sexual Dysfunction and Psychotropic Medications. CNS Spectr (2006) 8(9):24–30. doi: 10.1017/S1092852900026730
47. Julien R, Advocat C, Comaty J. A primer of drug action. In: A series of books in psychology, 12th ed. New York: Freeman (2011).
48. Thase ME, Denko T. Pharmacotherapy of mood disorders. Annu Rev Clin Psychol (2008) 4:53–92. doi: 10.1146/annurev.clinpsy.2.022305.095301
49. Robinson LA, Berman JS, Neimeyer RA. Psychotherapy for the treatment of depression: A comprehensive review of controlled outcome research. psychol Bull (1990) 108(1):30–49. doi: 10.1037/0033-2909.108.1.30
50. Swift JK, Greenberg RP. Premature discontinuation in adult psychotherapy: A meta-analysis. J Consult Clin Psychol (2012) 80(4):547–59. doi: 10.1037/a0028226
51. Steinert C, Hofmann M, Kruse J, Leichsenring F. Relapse rates after psychotherapy for depression - Stable long-term effects? A meta-analysis. J Affect Disord (2014) 168:107–18. doi: 10.1016/j.jad.2014.06.043
52. Cuijpers P, Dekker J, Hollon SD, Andersson G. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: A meta-analysis. J Clin Psychiatry (2009) 70(9):1219–29. doi: 10.4088/JCP.09r05021
53. Novick D, Montgomery W, Vorstenbosch E, Moneta MV, Dueñas H, Haro JM. Recovery in patients with major depressive disorder (MDD): Results of a 6-month, multinational, observational study. Patient Prefer Adherence (2017) 11:1859–68. doi: 10.2147/PPA.S138750
54. George MS, Nahas Z, Molloy M, Speer AM, Oliver NC, Li XB, et al. A controlled trial of daily left prefrontal cortex TMS for treating depression. Biol Psychiatry (2000) 48(10):962–70. doi: 10.1016/S0006-3223(00)01048-9
55. Hammond DC. Neurofeedback treatment of depression and anxiety. J Adult Dev (2005) 12(2-3):131–7. doi: 10.1007/s10804-005-7029-5
56. Pilkington K, Kirkwood G, Rampes H, Richardson J. Yoga for depression: The research evidence. J Affect Disord (2005) 89(1-3):13–24. doi: 10.1016/j.jad.2005.08.013
57. Van Gordon W, Shonin E, Griffiths MD, Singh NN. There is Only One Mindfulness: Why Science and Buddhism Need to Work Together. Mindfulness (2014) 6(1):49–56. doi: 10.1007/s12671-014-0379-y
58. McKenna D, Riba J. New world tryptamine hallucinogens and the neuroscience of ayahuasca. Curr Top Behav Neurosci (2018) 36:283–311. doi: 10.1007/7854_2016_472
59. Muttoni S, Ardissino M, John C. Classical psychedelics for the treatment of depression and anxiety: A systematic review. J Affect Disord (2019) 258:11–24. doi: 10.1016/j.jad.2019.07.076
60. Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clin Psychol: Sci Pract (2003) 10(2):125–43. doi: 10.1093/clipsy/bpg015
61. Kenny MA, Williams JMG. Treatment-resistant depressed patients show a good response to Mindfulness-based Cognitive Therapy. Behav Res Ther (2007) 45(3):617–25. doi: 10.1016/j.brat.2006.04.008
62. Barnby JM, Mehta MA. Psilocybin and Mental Health–Don’t Lose Control. Front Psychiatry (2018) 9(293):1–3. doi: 10.3389/fpsyt.2018.00293
63. Carhart-Harris RL, Bolstridge M, Day CMJ, Rucker J, Watts R, Erritzoe DE, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology (2018) 235(2):399–408. doi: 10.1007/s00213-017-4771-x
64. Johnson MW, Griffiths RR, Hendricks PS, Henningfield JE. The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology (2018) 142:143–66. doi: 10.1016/j.neuropharm.2018.05.012
65. FDA. (2018). Fact Sheet: Breakthrough Therapies. U.S. Food and Drug Administration. Available at: https://www.fda.gov/regulatory-information/food-and-drug-administration-safety-and-innovation-act-fdasia/fact-sheet-breakthrough-therapies.
66. Dunn BR, Hartigan JA, Mikulas WL. Concentration and mindfulness meditations: Unique forms of consciousness? Appl Psychophysiol Biofeedback (1999) 24(3):147–65. doi: 10.1023/A:1023498629385
67. Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat Rev Neurosci (2010) 11:642–51. doi: 10.1038/nrn2884
68. Creswell JD, Lindsay EK. How Does Mindfulness Training Affect Health? A Mindfulness Stress Buffering Account. Curr Dir psychol Sci (2014) 13(6):401–7. doi: 10.1177/0963721414547415
69. Griffiths RR, Johnson MW, Richards WA, Richards BD, Jesse R, MacLean KA, et al. Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol (2018) 31(1):49–69. doi: 10.1177/0269881117731279
70. Kabat-Zinn J. Mindfulness-based interventions in context: Past, present, and future. Clin Psychol: Sci Pract (2003) 10(2):144–56. doi: 10.1093/clipsy/bpg016
71. Awasthi B. Issues and perspectives in meditation research: In search for a definition. Front Psychol (2013) 3613:1–9. doi: 10.3389/fpsyg.2012.00613
72. Lykins ELB, Baer RA. Psychological Functioning in a Sample of Long-Term Practitioners of Mindfulness Meditation. J Cogn Psychother (2009) 23(3):226–41. doi: 10.1891/0889-8391.23.3.226
73. Keng SL, Smoski MJ, Robins CJ. Effects of mindfulness on psychological health: A review of empirical studies. Clin Psychol Rev (2011) 31(6):1041–56. doi: 10.1016/j.cpr.2011.04.006
74. Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: Evidence of brief mental training. Conscious Cogn (2010a) 19(2):597–605. doi: 10.1016/j.concog.2010.03.014
75. Manuello J, Vercelli U, Nani A, Costa T, Cauda F. Mindfulness meditation and consciousness: An integrative neuroscientific perspective. Conscious Cogn (2016) 40:67–87. doi: 10.1016/j.concog.2015.12.005
76. Vago DR, Silbersweig DA. Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. Front Hum Neurosci (2012) 6:296. doi: 10.3389/fnhum.2012.00296
77. Millière R, Carhart-Harris RL, Roseman L, Trautwein FM, Berkovich-Ohana A. Psychedelics, meditation, and self-consciousness. Front Psychol (2018) 9(1475):1–29. doi: 10.3389/fpsyg.2018.01475
78. O’Leary K, O’Neill S, Dockray S. A systematic review of the effects of mindfulness interventions on cortisol. J Health Psychol (2016) 21(9):2108–21. doi: 10.1177/1359105315569095
79. Fjorback LO, Arendt M, Ornbol E, Fink P, Walach H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy - a systematic review of randomized controlled trials. Acta Psychiatr Scand (2011) 124(2):102–19. doi: 10.1111/j.1600-0447.2011.01704.x
80. Tang V, Poon WS, Kwan P. Mindfulness-based therapy for drug-resistant epilepsy: An assessor-blinded randomized trial. Neurology (2015) 85(13):1094. doi: 10.1212/WNL.0000000000001967
81. Goldin PR, Gross JJ. Effects of Mindfulness-Based Stress Reduction (MBSR) on Emotion Regulation in Social Anxiety Disorder. Emotion (2010) 10(1):83–91. doi: 10.1037/a0018441
82. Johansson B, Bjuhr H, Rönnbäck L. Mindfulness-based stress reduction (MBSR) improves long-term mental fatigue after stroke or traumatic brain injury. Brain Inj (2012) 26(13-14):1621–8. doi: 10.3109/02699052.2012.700082
83. Ma SH, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: Replication and Exploration of Differential Relapse Prevention Effects. J Consult Clin Psychol (2004) 72(1):31–40. doi: 10.1037/0022-006X.72.1.31
84. Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol (2000) 68(4):615–25. doi: 10.1037//0022-006X.68.4.615
85. Hofmann SG, Sawyer AT, Witt AA, Oh D. The Effect of Mindfulness-Based Therapy on Anxiety and Depression: A Meta-Analytic Review. J Consult Clin Psychol (2010) 78(2):169–83. doi: 10.1037/a0018555
86. Hölzel BK, Ott U. Relationships between meditation depth, absorption, meditation practice and mindfulness: a latent variable approach. J Transpersonal Psychol (2006) 38(2):179–99. doi: 10.1002/pola.20555
87. Passie T, Seifert J, Schneider U, Emrich HM. The pharmacology of psilocybin. Addict Biol (2002) 7:357–64. doi: 10.1080/1355621021000005937
88. Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. Psilocybin occasioned mystical-type experiences: Immediate and persisting dose-related effects. Psychopharmacology (2011) 218(4):649–65. doi: 10.1007/s00213-011-2358-5
89. Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J Psychopharmacol (2011) 25(11):1434–52. doi: 10.1177/0269881110382466
90. Vollenweider FX, Vollenweider-Scherpenhuyzen MFI, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. NeuroReport (1998) 9(17):3897–902. doi: 10.1097/00001756-199812010-00024
91. Brown RT, Nicholas CR, Cozzi NV, Gassman MC, Cooper KM, Muller D, et al. Pharmacokinetics of Escalating Doses of Oral Psilocybin in Healthy Adults. Clin Pharmacokinet (2017) 56(12):1543–54. doi: 10.1007/s40262-017-05406
93. Barrett FS, Carbonaro TM, Hurwitz E, Johnson MW, Griffiths RR. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: effects on cognition. Psychopharmacology (2018) 235(10):2915–27. doi: 10.1007/s00213-018-4981-x
94. Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: Guidelines for safety. J Psychopharmacol (2008) 22(6):603–20. doi: 10.1177/0269881108093587
95. Mason NL, Mischler E, Uthaug MV, Kuypers KPC. Sub-Acute Effects of Psilocybin on Empathy, Creative Thinking, and Subjective Well-Being. J Psychoact Drugs (2019) 51(12):123–34. doi: 10.1080/02791072.2019.1580804
96. Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol (2016) 30(12):1181–97. doi: 10.1177/0269881116675513
97. Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J Psychopharmacol (2016) 30(12):1165–80. doi: 10.1177/0269881116675512
98. Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry (2006) 67(11):1735–40. doi: 10.4088/JCP.v67n1110
99. Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstad AL, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry (2011) 68(1):71–8. doi: 10.1001/archgenpsychiatry.2010.116
100. de Veen BTH, Schellekens AFA, Verheij MMM, Homberg JR. Psilocybin for treating substance use disorders? Expert Rev Neurother (2017) 17(2):203–12. doi: 10.1080/14737175.2016.1220834
101. Young SN. Biologic effects of mindfulness meditation: Growing insights into neurobiologic aspects of the prevention of depression. J Psychiatry Neurosci (2011) 36(2):75–7. doi: 10.1503/jpn.110010
102. Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (2006) 187(3):268–83. doi: 10.1007/s00213-006-0457-5
103. Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry (2016) 3(7):619–27. doi: 10.1016/S2215-0366(16)30065-7
104. Soler J, Elices M, Franquesa A, Barker S, Friedlander P, Feilding ,A, et al. Exploring the therapeutic potential of Ayahuasca: Acute intake increases mindfulness-related capacities. Psychopharmacology (2016) 233(5):823–9. doi: 10.1007/s00213-015-4162-0
105. Astin JA. Stress reduction through mindfulness meditation. Psychother Psychosom (1997) 66:97–106. doi: 10.1159/000289116
106. Alonso FJ, Romero S, Mañanas MA, Riba J. Serotonergic psychedelics temporarily modify information transfer in humans. Int J Neuropsychopharmacol (2015) 18(8):1–9. doi: 10.1093/ijnp/pyv039
107. Barnes S, Brown KW, Krusemark E, Campbell WK, Rogge RD. The role of mindfulness in romantic relationship satisfaction and responses to relationship stress. J Marital Family Ther (2007) 33(4):482–500. doi: 10.1111/j.1752-0606.2007.00033.x
108. Broderick PC. Mindfulness and coping with dysphoric mood: Contrasts with rumination and distraction. Cogn Ther Res (2005) 29(5):501–10. doi: 10.1007/s10608-005-3888-0
109. Brown KW, Weinstein N, Creswell JD. Trait mindfulness modulates neuroendocrine and affective responses to social evaluative threat. Psychoneuroendocrinology (2012) 37(12):2037–41. doi: 10.1016/j.psyneuen.2012.04.003
110. Cahn BR, Goodman MS, Peterson CT, Maturi R, Mills PJ. Yoga, Meditation and Mind-Body Health: Increased BDNF, Cortisol Awakening Response, and Altered Inflammatory Marker Expression after a 3-Month Yoga and Meditation Retreat. Front Hum Neurosci (2017) 11(315):1–13. doi: 10.3389/fnhum.2017.00315
111. Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci (2012) 109(6):2138–43. doi: 10.1073/pnas.1119598109
112. Carhart-Harris RL, Leech R, Erritzoe D, Williams TM, Stone JM, Evans J, et al. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull (2013) 39(6):1343–51. doi: 10.1093/schbul/sbs117
113. Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med (2003) 65(4):571–81. doi: 10.1097/01.PSY.0000074003.35911.41
114. Carlson LE, Ursuliak Z, Goodey E, Angen M, Speca M. The effects of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients: 6-month follow-up. Support Care Cancer (2001) 9(2):112–3. doi: 10.1007/s005200000206
115. Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cogn Neurosci (2005) 17:1497–508. doi: 10.1162/089892905774597191
116. Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (2007) 195(3):415–24. doi: 10.1007/s00213-007-0930-9
117. Coffey KA, Hartman M. Mechanisms of action in the inverse relationship between mindfulness and psychological distress. Complement Health Pract Rev (2008) 13(2):79–91. doi: 10.1177/1533210108316307
118. Domínguez-Clavé E, Soler J, Pascual JC, Elices M, Franquesa A, Valle M, et al. Ayahuasca improves emotion dysregulation in a community sample and in individuals with borderline-like traits. Psychopharmacology (2019) 236(2):572–80. doi: 10.1007/s00213-018-5085-3
119. Frecska E, Móré CE, Vargha A, Luna LE. Enhancement of creative expression and entoptic phenomena as after-effects of repeated ayahuasca ceremonies. J Psychoact Drugs (2012) 44:191–9. doi: 10.1080/02791072.2012.703099
120. Gex-Fabry M, Jermann F, Kosel M, Rossier MF, Van der Linden M, Bertschy G, et al. Salivary cortisol profiles in patients remitted from recurrent depression: One-year follow-up of a mindfulness-based cognitive therapy trial. J Psychiatr Res (2012) 46(1):80–6. doi: 10.1016/j.jpsychires.2011.09.011
121. Gotink RA, Hermans KSFM, Geschwind N, De Nooij R, De Groot WT, Speckens AEM. Mindfulness and mood stimulate each other in an upward spiral: a mindful walking intervention using experience sampling. Mindfulness (2016) 7(5):1114–22. doi: 10.1007/s12671-016-0550-8
122. Greenberg J, Reiner K, Meiran N. “Mind the Trap”: Mindfulness Practice Reduces Cognitive Rigidity. PloS One (2012) 7(5):1–8. doi: 10.1371/journal.pone.0036206
123. Greenberg J, Reiner K, Meiran N. “Off with the old”: Mindfulness practice improves backward inhibition. Frontiers in Psychology. 3 (2013) 618. doi: 10.3389/fpsyg.2012.00618
124. Griffiths RR, Richards WA, Johnson MW, McCann UD, Jesse R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol (2008) 22(6):621–32. doi: 10.1177/0269881108094300
125. Håkansson K, Ledreux A, Daffner K, Terjestam Y, Bergman P, Carlsson R, et al. BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J Alzheimer’s Dis (2017) 55(2):645–57. doi: 10.3233/JAD-160593
126. Hargus E, Crane C, Barnhofer T, Williams JMG. Effects of Mindfulness on Meta-Awareness and Specificity of Describing Prodromal Symptoms in Suicidal Depression. Emotion (2010) 10(1):34–42. doi: 10.1037/a0016825
127. Harman WW, McKim RH, Mogar RE, Fadiman J, Stolaroff MJ. Psychedelic agents in creative problem-solving: a pilot study. psychol Rep (1966) 19:211–27. doi: 10.2466/pr0.1966.19.1.211
128. Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological affects of psilocybin in healthy humans: A double-blind, placebo-controlled dose-effect study. Psychopharmacology (2004) 172(2):145–56. doi: 10.1007/s00213-003-1640-6
129. Hölzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci (2009) 5(1):11–7. doi: 10.1093/scan/nsp034
130. House RV, Thomas PT, Bhargava HN. Immunological consequences of in vitro exposure to lysergic acid diethylamide (LSD). Immunopharmacol Immunotoxicol (1994) 16(1):23–40. doi: 10.3109/08923979409029898
131. Huffziger S, Kuehner C. Rumination, distraction, and mindful self-focus in depressed patients. Behav Res Ther (2009) 47(3):224–30. doi: 10.1016/j.brat.2008.12.005
132. Jha AP, Krompinger J, Baime MJ. Mindfulness meditation modifies subsystems of attention. Cogn Affect Behav Neurosci (2007) 7(2):109–19. doi: 10.3758/CABN.7.2.109
133. Jimenez SS, Niles BL, Park CL. A mindfulness model of affect regulation and depressive symptoms: Positive emotions, mood regulation expectancies, and self-acceptance as regulatory mechanisms. Pers Individ Dif (2010) 49(6):645–50. doi: 10.1016/j.paid.2010.05.041
134. Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry (2012) 72(11):898–906. doi: 10.1016/j.biopsych.2012.04.005
135. Kraehenmann R, Preller KH, Scheidegger M, Pokorny T, Bosch OG, Seifritz E, et al. Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol Psychiatry (2015) 78(8):572–81. doi: 10.1016/j.biopsych.2014.04.010
136. Kubera M, Kenis G, Bosmans E, Scharpé S, Maes M. Effects of serotonin and serotonergic agonists and antagonists on the production of interferon-γ and interleukin-10. Neuropsychopharmacology (2000) 23(1):89–98. doi: 10.1016/S0893-133X(99)00150-5
137. Kuypers KPC, Riba J, de la Fuente Revenga M, Barker S, Theunissen EL, Ramaekers JG. Ayahuasca enhances creative divergent thinking while decreasing conventional convergent thinking. Psychopharmacology (2016) 233(18):3395–403. doi: 10.1007/s00213-016-4377-8
138. Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, et al. Meditation experience is associated with increased cortical thickness. NeuroReport (2005) 16(17):1893–7. doi: 10.1097/01.wnr.0000186598.66243.19
139. Lee TMC, Leung MK, Hou WK, Tang JCY, Yin J, So KF, et al. Distinct neural activity associated with focused-attention meditation and loving-kindness meditation. PloS One (2012) 7(8):1–11. doi: 10.1371/journal.pone.0040054
140. Lengacher CA, Kip KE, Barta M, Post-White J, Jacobsen PB, Groer M, et al. A Pilot Study Evaluating the Effect of Mindfulness-Based Stress Reduction on Psychological Status, Physical Status, Salivary Cortisol, and Interleukin-6 Among Advanced-Stage Cancer Patients and Their Caregivers. J Holist Nurs (2012) 30(3):170–85. doi: 10.1177/0898010111435949
141. Lewis CR, Preller KH, Kraehenmann R, Michels L, Staempfli P, Vollenweider FX. Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. NeuroImage (2017) 159:70–8. doi: 10.1016/j.neuroimage.2017.07.020
142. Lutz J, Herwig U, Opialla S, Hittmeyer A, Jäncke L, Rufer M, et al. Mindfulness and emotion regulation-an fMRI study. Soc Cogn Affect Neurosci (2013) 9(6):776–85. doi: 10.1093/scan/nst043
143. Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep (2018) 23(11):3170–82. doi: 10.1016/j.celrep.2018.05.022
144. MacLean KA, Johnson MW, Griffiths RR. Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. J Psychopharmacol (2011) 25(11):1453–61. doi: 10.1177/0269881111420188
145. Malarkey WB, Jarjoura D, Klatt M. Workplace based mindfulness practice and inflammation: A randomized trial. Brain Behav Immun (2013) 27:145–54. doi: 10.1016/j.bbi.2012.10.009
146. Matousek RH, Pruessner JC, Dobkin PL. Changes in the cortisol awakening response (CAR) following participation in Mindfulness-Based Stress Reduction in women who completed treatment for breast cancer. Complement Ther Clin Pract (2011) 17:65–70. doi: 10.1016/j.ctcp.2010.10.005
147. Moore A, Malinowski P. Meditation, mindfulness and cognitive flexibility. Conscious Cogn (2009) 18(1):176–86. doi: 10.1016/j.concog.2008.12.008
148. Mrazek MD, Franklin MS, Phillips DT, Baird B, Schooler JW. Mindfulness Training Improves Working Memory Capacity and GRE Performance While Reducing Mind Wandering. psychol Sci (2013) 24(5):776–81. doi: 10.1177/0956797612459659
149. Nau F, Yu B, Martin D, Nichols CD. Serotonin 5-HT2AReceptor Activation Blocks TNF-α Mediated Inflammation In Vivo. PloS One (2013) 8(10):1–8. doi: 10.1371/journal.pone.0075426
150. Oken BS, Fonareva I, Haas M, Wahbeh H, Lane JB, Zajdel D, et al. Pilot Controlled Trial of Mindfulness Meditation and Education for Dementia Caregivers. J Altern Complement Med (2010) 16(19):1031–8. doi: 10.1089/acm.2009.0733
151. Pokorny T, Preller KH, Kometer M, Dziobek I, Vollenweider FX. Effect of psilocybin on empathy and moral decision-making. Int J Neuropsychopharmacol (2017) 20(9):747–57. doi: 10.1093/ijnp/pyx047
152. Preller KH, Pokorny T, Hock A, Kraehenmann R, Stämpfli P, Seifritz E, et al. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A (2016) 113(18):5119–24. doi: 10.1073/pnas.1524187113
153. Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology (2012) 37:630–40. doi: 10.1038/npp.2011.228
154. Raes F, Dewulf D, Van Heeringen C, Williams JMG. Mindfulness and reduced cognitive reactivity to sad mood: Evidence from a correlational study and a non-randomized waiting list controlled study. Behav Res Ther (2009) 47(7):623–7. doi: 10.1016/j.brat.2009.03.007
155. Roseman L, Demetriou L, Wall MB, Nutt DJ, Carhart-Harris RL. Increased amygdala responses to emotional faces after psilocybin for treatment-resistant depression. Neuropharmacology (2018) 142:263–169. doi: 10.1016/j.neuropharm.2017.12.041
156. Roseman L, Leech R, Feilding A, Nutt DJ, Carhart-Harris RL. The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci (2014) 8(204):1–11. doi: 10.3389/fnhum.2014.00204
157. Sampedro F, Revenga MDLF, Valle M, Roberto N, Domínguez-Clavé E, Elices M, et al. Assessing the psychedelic “after-glow” in ayahuasca users: Post-acute neurometabolic and functional connectivity changes are associated with enhanced mindfulness capacities. Int J Neuropsychopharmacol (2017) 20(9):698–711. doi: 10.1093/ijnp/pyx036
158. Smigielski L, Scheidegger M, Kometer M, Vollenweider FX. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. NeuroImage (2019) 196:207–15. doi: 10.1016/j.neuroimage.2019.04.009
159. Stroud JB, Freeman TP, Leech R, Hindocha C, Lawn W, Nutt DJ, et al. Psilocybin with psychological support improves emotional face recognition in treatment-resistant depression. Psychopharmacology (2018) 235(2):459–66. doi: 10.1007/s00213-017-4754-y
160. Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of psilocybin response in healthy volunteers. PloS One (2012) 7(2):1–12. doi: 10.1371/journal.pone.0030800
161. Surawy C, Roberts J, Silver A. The effect of mindfulness training on mood and measures of fatigue, activity, and quality of life in patients with chronic fatigue syndrome on a hospital waiting list: A series of exploratory studies. Behav Cogn Psychother (2005) 33(1):103–9. doi: 10.1017/S135246580400181X
162. Soler J, Elices M, Dominguez-Clavé E, Pascual JC, Feilding A, Navarro-Gil M, et al. Four weekly ayahuasca sessions lead to increases in “acceptance” capacities: A comparison study with a standard 8-week mindfulness training program. Front Pharmacol (2018) 9(224):1–8. doi: 10.3389/fphar.2018.00224
163. Szabo A, Kovacs A, Frecska E, Rajnavolgyi E. Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PloS One (2014) 9(8):1–12. doi: 10.1371/journal.pone.0106533
164. Turton S, Nutt DJ, Carhart-Harris RL. A qualitative report on the subjective experience of intravenous psilocybin administered in an FMRI environment. Curr Drug Abuse Rev (2014) 7(2):117–27. doi: 10.2174/1874473708666150107120930
165. Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology (1997) 16:357–72. doi: 10.1016/S0893-133X(96)00246-1
166. Wachs K, Cordova JV. Mindful relating: Exploring mindfulness and emotion repertoires in intimate relationships. J Marital Family Ther (2007) 33(4):464–81. doi: 10.1111/j.1752-0606.2007.00032.xhttp://citeseerx.ist.psu.edu/
167. Winnebeck E, Fissler M, Gärtner M, Chadwick P, Barnhofer T. Brief training in mindfulness meditation reduces symptoms in patients with a chronic or recurrent lifetime history of depression: A randomized controlled study. Behav Res Ther (2017) 99:124–30. doi: 10.1016/j.brat.2017.10.005
168. Zeidan F, Johnson SK, Gordon NS, Goolkasian P. Effects of Brief and Sham Mindfulness Meditation on Mood and Cardiovascular Variables. J Altern Complement Med (2010b) 16(8):867–73. doi: 10.1089/acm.2009.0321
169. Dekeyser M, Raes F, Leijssen M, Leysen S, Dewulf D. Mindfulness skills and interpersonal behaviour. Pers Individ Differ (2008) 44(5):1235–45. doi: 10.1016/j.paid.2007.11.018
170. Pahnke W. Drugs and mysticism: An analysis of the relationship between psychedelic drugs and the mystical consciousness. Ph.D. dissertation. Harvard University. (1963).
172. Majić T, Schmidt TT, Gallinat J. Peak experiences and the afterglow phenomenon: When and how do therapeutic effects of hallucinogens depend on psychedelic experiences? J Psychopharmacol (2015) 29(3):241–53. doi: 10.1177/0269881114568040
173. Rocha JM, Osório FL, Crippa JAS, Bouso JC, Rossi GN, Hallak JEC, et al. Serotonergic hallucinogens and recognition of facial emotion expressions: a systematic review of the literature. Ther Adv Psychopharmacol (2019) 9:1–11. doi: 10.1177/2045125319845774
174. Pokorny T, Duerler P, Seifritz E, Vollenweider F, Preller K. LSD acutely impairs working memory, executive functions, and cognitive flexibility, but not risk-based decision-making. psychol Med (2019), 1–10. doi: 10.1017/S0033291719002393
175. Carhart-Harris RL, Nutt DJ. Serotonin and brain function: A tale of two receptors. J Psychopharmacol (2017) 31(9):1191–20. doi: 10.1177/0269881117725915
176. Carson JW, Carson KM, Gil KM, Baucom DH. Mindfulness-Based Relationship Enhancement (MBRE) in Couples. In: . Mindfulness-Based Treatment Approaches. Behavior Therapy (2006). p. 309–31. doi: 10.1016/B978-012088519-0/50015-0
177. Preller KH, Pokorny T, Kraehenmann R, Dziobek I, Staempfli P, Vollenweider FX. The Effect of 5-HT2A/1a Agonist Treatment On Social Cognition, Empathy, and Social Decision-making. Eur Psychiatry (2015) 30(1):28–31. doi: 10.1016/s0924-9338(15)30017-1
178. Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Frontiers in Psychology. 2 (2012) 930:1–16. doi: 10.3389/fpsyg.2011.00395
179. Demertzi A, Soddu A, Laureys S. Consciousness supporting networks. Curr Opin Neurobiol (2013) 23(2):239–44. doi: 10.1016/j.conb.2012.12.003
180. Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science (1999) 183(5408):1657–61. doi: 10.1126/science.283.5408.1657
181. Carhart-Harris RL, Kaelen M, Nutt D. How do hallucinogens work on the brain? Psychol (2014a) 27(9):662–5.
182. Beliveau V, Ganz M, Feng L, Fisher PM, Svarer C, Greve D, et al. (2016). A high-resolution molecular imaging atlas of the human brain 5-HT. J Neurosci (2017) 37(1):120–28 .
183. van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci (2013) 17(12):683–96. doi: 10.1016/j.tics.2013.09.012
184. Mikolajczak M, Quoidbach J, Vanootighem V, Lambert F, Lahaye M, Fillée C, et al. Cortisol awakening response (CAR)’s flexibility leads to larger and more consistent associations with psychological factors than CAR magnitude. Psychoneuroendocrinology (2010) 35(5):752–7. doi: 10.1016/j.psyneuen.2009.11.003
186. De Quervain DJF, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci (2000) 3(4):313–4. doi: 10.1038/73873
187. Lupien SJ, Wilkinson CW, Brière S, Ménard C, Ng Ying Kin NMK, Nair NPV. The modulatory effects of corticosteroids on cognition: Studies in young human populations. Psychoneuroendocrinology (2002) 27(3):401–16. doi: 10.1016/S0306-4530(01)00061-0
188. Vázquez-Borsetti P, Cortés R, Artigas F. Pyramidal neurons in rat prefrontal cortex projecting to ventral tegmental area and dorsal raphe nucleus express 5-HT2A-receptors. Cereb Cortex (2009) 19(7):1678–86. doi: 10.1093/cercor/bhn204
189. dos Santos RG, Osório FL, Crippa JAS, Riba J, Zuardi AW, Hallak JEC. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): a systematic review of clinical trials published in the last 25 years. Ther Adv Psychopharmacol (2016) 6:193–213. doi: 10.1177/2045125316638008
190. Stefulj J, Jernej B, Cicin-Sain L, Rinner I, Schauenstein K. mRNA expression of serotonin receptors in cells of the immune tissues of the rat. Brain Behav Immun (2000) 14(3):219–24. doi: 10.1006/brbi.1999.0579
191. Flanagan TW, Nichols CD. Psychedelics as anti-inflammatory agents. Int Rev Psychiatry (2018) 30(4):363–75. doi: 10.1080/09540261.2018.1481827
192. Szabo A. Psychedelics and immunomodulation: Novel approaches and therapeutic opportunities. Front Immunol (2015) 6(358):1–11. doi: 10.3389/fimmu.2015.00358
193. Kyzar EJ, Nichols CD, Gainetdinov RR, Nichols DE, Kalueff AV. Psychedelic Drugs in Biomedicine. Trends Pharmacol Sci (2017) 38(11):992–1005. doi: 10.1016/j.tips.2017.08.003
194. Chryssikopoulos A. The relationship between the immune and endocrine systems. Ann New Y Acad Sci (1997) 816(1):83–93. doi: 10.1111/j.1749-6632.1997.tb52132.x
195. Lenderking WR, Hu M, Tennen H, Cappelleri JC, Petrie CD, Rush AJ. Daily process methodology for measuring earlier antidepressant response. Contemp Clin Trials (2008) 29(6):867–77. doi: 10.1016/j.cct.2008.05.012
196. McDowell I. Measuring Health: A guide to rating scales and questionnaires. In: . Measuring Health: A Guide to Rating Scales and Questionnaires. New York: Oxford Scholarship Online (2009). p. 238–86. doi: 10.1093/acprof:oso/9780195165678.001.0001
197. Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Rev Bras Psiquiatr (2013) 35(4):416–31. doi: 10.1590/1516-4446-2012-1048
198. Danforth A. Focusing-oriented Psychotherapy as a Supplement to Preparation for Psychedelic Therapy. J Transpersonal Psychol (2009) 41(2):151–60.
199. Phelps J. Developing Guidelines and Competencies for the Training of Psychedelic Therapists. J Humanistic Psychol (2017) 57(5):450–87. doi: 10.1177/0022167817711304
201. Derogatis LR, Unger R. Symptom Checklist-90-Revised. In: The Corsini Encyclopedia of Psychology. (John Wiley & Sons, Inc.) (2010). doi: 10.1002/9780470479216.corpsy0970
202. McNair DM, Lorr M, Droppleman OF. EITS Manual for the Profile of Mood States. (San Diego, CA) (1971).
203. Toneatto T, Nguyen L. Does mindfulness meditation improve anxiety and mood symptoms? A review of the controlled research. Can J Psychiatry (2007) 52(4):260–6. doi: 10.1177/070674370705200409
Keywords: depression, mindfulness meditation, psilocybin, psychedelics, review
Citation: Heuschkel K and Kuypers KPC (2020) Depression, Mindfulness, and Psilocybin: Possible Complementary Effects of Mindfulness Meditation and Psilocybin in the Treatment of Depression. A Review. Front. Psychiatry 11:224. doi: 10.3389/fpsyt.2020.00224
Received: 22 June 2019; Accepted: 06 March 2020;
Published: 31 March 2020.
Edited by:
Stefan Borgwardt, University of Basel, SwitzerlandReviewed by:
Kai G. Kahl, Hannover Medical School, GermanyBartosz Zurowski, University Medical Center Schleswig-Holstein, Germany
Copyright © 2020 Heuschkel and Kuypers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kim P.C. Kuypers, k.kuypers@maastrichtuniversity.nl
 Kristin Heuschkel
Kristin Heuschkel Kim P.C. Kuypers
Kim P.C. Kuypers