- 1Basic Medical Sciences Neuroscience and Sense Organs Department, University of Bari Aldo Moro, Bari, Italy
- 2Unit for Severe Disabilities in Developmental Age and Young Adults, Developmental Neurology and Neurorehabilitation, Scientific Institute IRCCS E. Medea, Brindisi, Italy
Over the last few years, new studies focused their attention on the gender-related features in high-functioning autism spectrum disorder (HFA), often leading to controversial results. Another interesting aspect of these subtype of patients is linked to the complexity of clinical presentation, where besides core symptoms, other co-occurrence disorders may complicate the diagnostic evaluation. Therefore, we retrospectively studied 159 HFA patients, male and female, investigating their comorbidities and to find any gender difference. For each patient, were evaluated the presence/absence, type and gender distribution of psychopathological comorbidities, according to DSM-5 diagnostic criteria. The total sample was divided in 100 male and 59 female patients, age and intelligence quotient matched. In our sample, the psychiatric comorbidities observed were Attention Deficit Hyperactivity Disorder, Anxiety Disorders, Depressive Disorders, Bipolar Disorder, Obsessive-Compulsive Disorder, and Anorexia Nervosa. No statistical significant differences were found between male and female HFA patients comorbidities except for Anorexia Nervosa. In both male and female patients, attention deficit and hyperactivity disorder and anxiety disorders were found in high percentage. In conclusion, our investigation showed that a statistical significant difference of comorbidity between male and female HFA patients was found only for AN diagnosis. However, the question about the distinction between female and male HFA patients remains quite interesting and an open area of research for future studies.
Introduction
It is well-known that in Autism Spectrum Disorder (ASD), males are over-represented than females, with an average gender ratio of 4.3 males to 1 female (1). Many theories have emerged in order to explain this difference in gender distribution but no one explanation appears to be conclusive (2–5). Females are different from males both in core symptoms and in comorbidity; this probably causes the difficulty in their detection. Data show that the gender ratio M:F is intelligence quotient (IQ)-related, varying from 5.75:1 in cognitively high-functioning children (HF; full-scale IQ higher than 70) to 1.9:1 in low-functioning children (LF; full-scale IQ lower than 70) (5–9).
The definition of High Functioning Autism Spectrum Disorder (HFA) refers to the category of ASD “without cognitive impairment” specified by the Diagnostic and Statistical Manual of Mental Disorders—Fifth Edition (10).
This variance shows that HF females have been the most difficult to detect, probably because of milder symptom presentation (11–13) or methodological bias (ex. lack of specific diagnostic tools) (14–16). With increased agreement on the definition of ASD and improvement in case detection, recent studies are identifying more HF females (17). Beyond the sex differences in phenotype, males and females could differ in coexisting psychopathology (5) so there has been an emergence of research focusing on gender differences in ASD regarding comorbidity. The most common practice to date for identifying comorbid psychopathologies had been the use of the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria. Literature on comorbid psychopathologies, based on the new edition of DSM criteria (DSM-5), is beginning to develop, even if with mixed findings, probably caused by different methodologies (related to stratification for IQ and age) and by small samples.
A report described that boys with ASD are more likely to experience externalizing disorders such as ADHD and oppositional defiant disorder (18), above all in childhood, while it is reported that girls with ASD may be at especially high risk for internalizing psychopathology (8).
Moreover, studies have shown more hyperactive behaviors in HF boys than girls with ASD (19), while no gender differences in LF children (20–23) were found.
Nevertheless, differences also emerged with respect to internalizing disorders as HF girls with ASD evidenced significant anxiety symptoms compared to boys, mainly in adolescence (8), suggesting that the typical gender developmental trajectory of anxiety may be present. However, it might be quite difficult to distinguish if anxiety symptoms or repetitive behaviors would belong to the core ASD symptoms or might be signs of a comorbid condition with an anxiety and/or an obsessive-compulsive disorder (24). In the same way as for anxiety symptoms, other affective disorders, such as depressive disorders, are reported in childhood and at levels dramatically higher in adolescent girls (16, 19, 25). Lastly, in ASD patients are largely reported abnormal eating behaviors and/or conducts (26).
However, sex differences in HFA comorbidities is still poorly understood. Therefore, the aim of this retrospective study was to investigate whether male and female HFA patients might develop specific comorbidities phenotype, using well-defined samples regarding factors like age and IQ.
Materials and Methods
This retrospective study included children and adolescents admitted to the Child and Adolescent Neuropsychiatry Unit between April 2016 and May 2018 and diagnosed with HF ASD, according to DSM-5 diagnostic criteria. For each patient, were evaluated the presence/absence, type and gender distribution of psychopathological comorbidities. All participants were drug-naïve.
All demographic and clinical variables were subjected to statistical analysis. Descriptive analysis was conducted for sociodemographic and clinical features. Quantitative variables (IQ and age) were presented as mean ± standard deviation (SD); qualitative variables (psychopathological comorbidities and ASD severity level) were expressed as percentages. The chi-square test (χ2) was used to compare qualitative variables between male and female with ASD. The χ2 enabled us to compare observed and expected frequencies of dichotomous variables objectively. Statistical significance, in this case, is due to the difference between observed and expected frequencies. Both groups were compared with the independent sample t test for comparison of age and IQ mean score. All the statistical analyses were considered significant with a p-value equal or lower than 0.05. For statistical processing, we used the Statistical Package for Social Science version 20.0.
Results
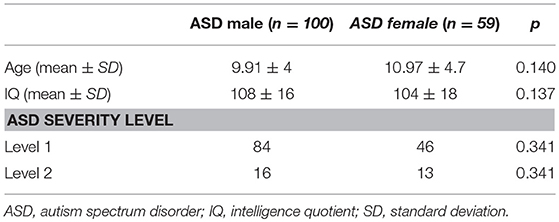
The sample included 159 patients with HF ASD diagnosis. Table 1 summarizes patients demographic and clinical data. The total sample included 100 males (mean age: 9.91 ± 4 DS) and 59 females (mean age: 10.97 ± 4.7 DS). No significant statistical differences for age and Intelligent Quotient were found between male and female patients. In 44% of male patients at least one comorbidity was diagnosed, while, in female patients, 72.8% presented at least one comorbidity.
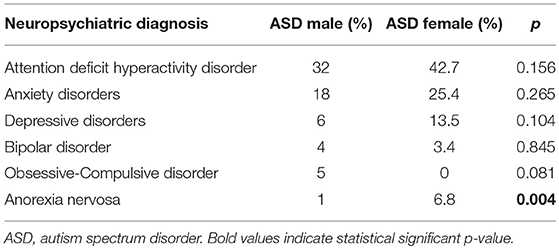
In both male and female patients, ADHD and Anxiety Disorders were the most frequent diagnoses. Anorexia Nervosa diagnosis resulted more frequent among female patients, with a statistical significant difference (p = 0.04) (Table 2).
Discussion
It is well-established that the most difficult challenge in ASD diagnostic process is based on the recognition and discrimination of the frequent co-occurrence of this disorder with other conditions, such as other neurodevelopment and/or psychiatric disorders. In fact, core symptoms of ASD often mask psychiatric comorbid symptoms and viceversa (27–29). Furthermore, there are potential similarities of how the symptoms of these disorders appear (24).
The purpose of this study was to investigate HF ASD psychopathological comorbidities between male and female patients. In our sample, high rates of comorbidities were found. Moreover, 55% of the total sample presented at least one comorbidity, with a higher rate in females (72.8%) than males (44%). This finding about the high prevalence of comorbidities in ASD is in accordance with literature data (28). The difference between the comorbidities rates between male and female patients in our sample may be a statistical bias due to the disproportion of the sample sizes. Eventually larger sample studies would contribute to clarify if this percentage difference of comorbidity exists or not. Moreover, in female HFA patients, it is more difficult to recognize psychiatric comorbidities for several reasons. First, diagnostic criteria and/or tools are mainly male-targeted complicating their detection; moreover, females less refer to clinical attention since their impairment is even less clear than in male patients.
In our sample, the psychiatric comorbidities observed (Table 2) were ADHD, Anxiety Disorders (AD), Depressive Disorders (DD), Bipolar Disorder (BD), Obsessive-Compulsive Disorder (OCD), and Anorexia Nervosa (AN). No statistical significant differences were found between male and female HFA patients comorbidities except for AN.
The most frequent comorbidity was ADHD with no statistical significant difference between male and female subjects. Over the years, and lastly after the publication of DSM-5, the overlap between ASD and ADHD has been supported by an increasing number of studies (28, 30–35). A recent study estimated that the prevalence of the co-occurring of ASD and ADHD is about 37–85% of children with ASD (35), supporting the hypothesis of a common neurobiological pathogenesis (35, 36). Moreover, comparing male with female, we found that ADHD with “Predominantly inattention presentation” prevails on female subgroup; while in male subgroup “Combined presentation” is the most frequent clinical presentation. Potentially, associated difficulties commonly occurring in HFA, like externalizing/disruptive behaviors, could differ between boys and girls prompting gender differences in the clinical referral. Girls without externalizing behaviors/hyperactivity could be overlooked for assessment and educational support despite largely similar cognitive, academic and behavioral profiles to boys (37, 38).
In our sample, Anxiety Disorders was found in high percentage of both male and female patients, without any statistical significant difference. In a recent review, Tarazi et al. (39) found that people with HFA experience more anxiety symptoms (such as tension, apprehension, panic, attention deficits) compared to other ASD patients. It is suggested that, in this subtype of patients, anxiety is more likely related to their inability to face social interactions, changing in daily routines or modulation of their emotional experiences.
In our sample, Depressive and Bipolar Disorders appeared to be less frequent than other comorbidities. This finding is probably due to the typical adolescent onset of these mood disorders in ASD, and the mean age of our sample was about 10 years old (8, 16).
In our total sample, OCD diagnosis was found in 3%; this finding probably is understandable considering that OCD could appear particularly difficult to identify in the context of an ASD because of their potential similarities only in five male patients. Moreover, OCD was diagnosed only in five male patients; this may be probably explained by the fact that restrictive interests and ritualistic behaviors in female HFA patients appear to be more socially acceptable (40).
Anorexia Nervosa was more frequent among female subjects with a statistical significant difference. This result reflects the epidemiology of AN, considering that this disorder predominantly affects female patients in early adolescence (41). It is suggested that both genetic and psychosocial risk factors may contribute to this gender difference of AN prevalence. Firstly, during puberty, ovarian hormones are directly involved in genetic effects as transcriptional mediators of neural expression of transmission systems disrupted in eating disorder (e.g., serotoninergic system) (42, 43). Secondly, young girls are more exposed than males to sociocultural factors that may contribute to increase the risk of AN (e.g., pressure about weight and body image, thinness and cultural model) (43, 44). Moreover, it is well-known how early testosterone exposure has a huge impact on brain plasticity and organization, and this effect may contribute to protect male subjects from developing an eating disorder (45–48). The association between AN and ASD was firstly described in 1980s when Gillberg observed that three male autistic patients had a familiar history for AN (49, 50). The author hypothesized that common factors may contribute to develop autism in young boys and AN in female relatives. On the other hand, dysfunctional eating behaviors (such as restrictive and/or limited food intake or repertoire) represents one of the main clinical aspects of ASD (51). Moreover, it is reported that abnormal eating features may be precursors of AN in patients with ASD (52). Nevertheless, patients affected by AN share clinical similarity with ASD (e.g., set-shifting deficits, reduced cognitive flexibility, deficits in emotion recognition, and social cognition) and some of these features might be potential risk factors to develop AN (41). It is suggested that these aspects may be explained by a possible neuropsychological overlap between the two disorders (deficit in central coherence, theory of mind and executive functions theories) (53). A recent study investigated gray matter volumetric aspects in female patients affected by AN and autistic traits. The results showed that higher autistic traits correlate with volumetric alterations of brain regions involved in the social cognition, supporting a valuable link of the association between the two disorders (54). In addition, the overlap between ASD and AN may be supported by other common neurobiological basis, including the involvement of the dopaminergic system. Variations in dopamine transporters and dopamine receptors gene (e.g., DAT1 and DRD4) are strongly linked to ASD (55–58). Nevertheless, it is known that dopamine is one the crucial neuromediator involved in feeding behavior, distortion of body image perception (59, 60). In fact, mesolimbic dopamine pathways play a crucial role in food reward, anticipation of food and social recognition mechanisms (61, 62). Moreover, specific genotype of DRD4 (DRD4 7R/R), leading to a reduced expression of the receptor, appears to be strongly linked to the risk of AN (59, 63). However, the complete neurobiological basis of the overlap between AN and ASD are quite uncertain and still not defined.
In conclusion, our investigation showed that a statistical significant difference of comorbidity between male and female HFA patients was found only for AN diagnosis. However, the question about the distinction between female and male HFA patients remains quite interesting and open. Furthermore, female HFA patients are often overlooked, misdiagnosed and underestimated; therefore, this retrospective study might be preliminary for future and larger-sampled research on this category of patients.
Ethics Statement
For this study, an ethical review process by the Local Ethics Committee of Azienda Ospedaliero-Universitaria Policlinico di Bari (Italy) was not required, since all the procedures within the study assessment are included in the diagnostic protocol of our Child and Adolescence Neuropsychiatry Unit. All the participants were recruited after obtaining a written informed consent by their parents.
Author Contributions
LM, PV, and FM designed and supervised the study. RP, AP, and CdG contributed to the recruitment and the revision of the medical records. FC performed the statistical analyses. All authors equally contributed to the draft of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. (2009) 65:591–8. doi: 10.1203/PDR.0b013e31819e7203
2. Kopp S, Gillberg C. Girls with social deficits and learning problems: Autism, atypical Asperger syndrome or a variant of these conditions. Eur Child Adolesc Psychiatry. (1992) 1:89–99. doi: 10.1007/BF02091791
4. Baron-Cohen S. About 1% of children in the South Thames region have an autistic spectrum disorder. Evid Based Ment Health. (2007) 10:28. doi: 10.1136/ebmh.10.1.28
5. Holtmann M, Bölte S, Poustka F. Autism spectrum disorders: sex differences in autistic behaviour domains and coexisting psychopathology. Dev Med Child Neurol. (2007) 49:361–6. doi: 10.1111/j.1469-8749.2007.00361.x
6. Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord. (2003) 33:365–82.
7. Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. (2003) 289:49–55.
8. Solomon M, Miller M, Taylor SL, Hinshaw SP, Carter CS. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J Autism Dev Disord. (2012) 42:48–59. doi: 10.1007/s10803–011-1215-z
9. Frazier TW, Georgiades S, Bishop SL, Hardan AY. Behavioral and cognitive characteristics of females and males with autism in the simons simplex collection. J Am Acad Child Adolesc Psychiatry. (2014) 53:329–40.e1–3. doi: 10.1016/j.jaac.2013.12.004
10. American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Publishing (2013).
11. Constantino JN1, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. (2003) 60:524–30. doi: 10.1001/archpsyc.60.5.524
12. Russell G, Steer C, Golding J. Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Soc Psychiatry Psychiatr Epidemiol. (2011) 46:1283–93. doi: 10.1007/s00127–010-0294-z
13. Dworzynski K, Ronald A, Bolton P, Happé F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry. (2012) 51:788–97. doi: 10.1016/j.jaac.2012.05.018
14. Van Wijngaarden-Cremers PJ, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, Van der Gaag RJ. Gender and age differences in the core triad of impairments in autism spectrum disorders: a systematic review and meta-analysis. J Autism Dev Disord. (2014) 44:627–35. doi: 10.1007/s10803–013-1913–9
15. Beggiato A, Peyre H, Maruani A, Scheid I, Rastam M, Amsellem F, et al. Gender differences in autism spectrum disorders: Divergence among specific core symptoms. Autism Res. (2017) 10:680–689. doi: 10.1002/aur.1715
16. Hull L, Mandy W, Petrides KV. Behavioural and cognitive sex/gender differences in autism spectrum condition and typically developing males and females. Autism. (2017) 21:706–27. doi: 10.1177/1362361316669087
17. Zwaigenbaum L, Bryson SE, Szatmari P, Brian J, Smith IM, Roberts W, et al. Sex differences in children with autism spectrum disorder identified within a high-risk infant cohort. J Autism Dev Disord. (2012) 42:2585–96. doi: 10.1007/s10803–012-1515-y
18. American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Washington DC: American Psychiatric Association.
19. Oswald TM, Winter-Messiers MA, Gibson B, Schmidt AM, Herr CM, Solomon M. Sex Differences in internalizing problems during adolescence in autism spectrum disorder. J Autism Dev Disord. (2016) 46:624–36. doi: 10.1007/s10803–015-2608–1
20. Gadow KD, Devincent CJ, Pomeroy J, Azizian A. Comparison of DSM-IV symptoms in elementary school-age children with PDD versus clinic and community samples. Autism. (2005) 9:392–415. doi: 10.1177/1362361305056079
21. Brereton AV, Tonge BJ, Einfeld SL. Psychopathology in children and adolescents with autism compared to young people with intellectual disability. J Autism Dev Disord. (2006) 36:863–70. doi: 10.1007/s10803-006-0125-y
22. Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. (2008) 47:921–9. doi: 10.1097/CHI.0b013e318179964f
23. Mandy W, Charman T, Gilmour J, Skuse D. Toward specifying pervasive developmental disorder-not otherwise specified. Autism Res. (2011) 4:121–31. doi: 10.1002/aur.178
24. Matson JL, Cervantes PE. Commonly studied comorbid psychopathologies among persons with autism spectrum disorder. Res Dev Disabil. (2014) 35:952–62. doi: 10.1016/j.ridd.2014.02.012
25. Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil. (2011) 32:1910–7. doi: 10.1016/j.ridd.2011.03.025
26. Karjalainen L, Råstam M, Paulson-Karlsson G, Wentz E. Do autism spectrum disorder and anorexia nervosa have some eating disturbances in common? Eur Child Adolesc Psychiatry. (2018) 28:69–78. doi: 10.1007/s00787–018-1188-y
27. Mazzone L, Ruta L, Reale L. Psychiatric comorbidities in asperger syndrome and high functioning autism: diagnostic challenges. Ann Gen Psychiatry. (2012) 11:16. doi: 10.1186/1744–859X-11–16
28. Salazar F, Baird G, Chandler S, Tseng E, O'sullivan T, Howlin P, et al. Co-occurring psychiatric disorders in preschool and elementary school-aged children with autism spectrum disorder. J Autism Dev Disord. (2015) 45:2283–94. doi: 10.1007/s10803–015-2361–5
29. Postorino V, Kerns CM, Vivanti G, Bradshaw J, Siracusano M, Mazzone L. Anxiety disorders and obsessive-compulsive disorder in individuals with autism spectrum disorder. Curr Psychiatry Rep. (2017) 19:92. doi: 10.1007/s11920–017-0846-y
30. Taylor MJ, Charman T, Robinson EB, Plomin R, Happé F, Asherson P, et al. Developmental associations between traits of autism spectrum disorder and attention deficit hyperactivity disorder: a genetically informative, longitudinal twin study. Psychol Med. (2013) 43:1735–46. doi: 10.1017/S003329171200253X
31. Cooper M, Martin J, Langley K, Hamshere M, Thapar A. Autistic traits in children with ADHD index clinical and cognitive problems. Eur Child Adolesc Psychiatry. (2014) 23:23–34. doi: 10.1007/s00787–013-0398–6
32. Taylor MJ, Charman T, Ronald A. Where are the strongest associations between autistic traits and traits of ADHD? Evidence from a community-based twin study. Eur Child Adolesc Psychiatry. (2015) 24:1129–38. doi: 10.1007/s00787–014-0666–0
33. Antshel KM, Zhang-James Y, Wagner KE, Ledesma A, Faraone SV. An update on the comorbidity of ADHD and ASD: a focus on clinical management. Exp Rev Neurother. (2016) 16:279–93. doi: 10.1586/14737175.2016.1146591
34. Craig F, Margari F, Legrottaglie AR, Palumbi R, de Giambattista C, Margari L. A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr Dis Treat. (2016) 12:1191–202. doi: 10.2147/NDT.S104620
35. Leitner Y. The co-occurrence of autism and attention deficit hyperactivity disorder in children—what do we know? Front Hum Neurosci. (2014) 8:268. doi: 10.3389/fnhum.2014.00268
36. Lamanna AL, Craig F, Matera E, Simone M, Buttiglione M, Margari L. Risk factors for the existence of attention deficit hyperactivity disorder symptoms in children with autism spectrum disorders. Neuropsychiatr Dis Treat. (2017) 15:1559–67. doi: 10.2147/NDT.S132214
37. May T, Cornish K, Rinehart N. Does gender matter? A one year follow-up of autistic, attention and anxiety symptoms in high-functioning children with autism spectrum disorder. J Autism Dev Disord. (2014) 44:1077–86. doi: 10.1007/s10803–013-1964-y
38. Mowlem FD, Rosenqvist MA, Martin J, Lichtenstein P, Asherson P, Larsson H. Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. Eur Child Adolesc Psychiatry. (2018). doi: 10.1007/s00787–018-1211–3. [Epub ahead of print].
39. Tarazi FI, Sahli ZT, Pleskow J, Mousa SA. Asperger's syndrome: diagnosis, comorbidity and therapy. Exp Rev Neurother. (2015) 15:281–93. doi: 10.1586/14737175.2015.1009898
40. Hiller RM, Young RL, Weber N. Sex differences in autism spectrum disorder based on DSM-5 criteria: evidence from clinician and teacher reporting. J Abnorm Child Psychol. (2014) 42:1381–93. doi: 10.1007/s10802–014-9881-x
41. Treasure J, Zipfel S, Micali N, Wade T, Stice E, Claudino A, et al. Anorexia nervosa. Nat Rev Dis Primers. (2015) 26:15074. doi: 10.1038/nrdp.2015.74
42. Klump KL, Keel PK, Sisk C, Burt SA. Preliminary evidence that estradiol moderates genetic influences on disordered eating attitudes and behaviors during puberty. Psychol Med. (2010) 40:1745–53. doi: 10.1017/S0033291709992236
43. Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, Nigg JT. The effects of puberty on genetic risk for disordered eating: evidence for a sex difference. Psychol Med. (2012) 42:627–37. doi: 10.1017/S0033291711001541
44. Cafri G, Yamamiya Y, Brannick M, Thompson JK. The influence of sociocultural factors on body image: a meta-analysis. Clin Psychol. (2005) 12:421–33. doi: 10.1093/clipsy/bpi053
45. Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: A comparison of opposite- and same-sex twins. Arch Gene Psychiatry. (2008) 65:329–336. doi: 10.1001/archgenpsychiatry.2007.47
46. Culbert KM, Burt SA, Sisk CL, Nigg JT, Klump KL. The effects of circulating testosterone and pubertal maturation on risk for disordered eating symptoms in adolescent males. Psychol Med. (2014) 44:2271–86. doi: 10.1017/S0033291713003073
47. Smith AR, Hawkeswood SE, Joiner TE. The measure of a man: Associations between digit ration and disordered eating in males. Int J Eating Disord. (2010) 43:543–8. doi: 10.1002/eat.20736
48. Gagnidze K, Pfaff DW, Mong JA. Gene expression in neuroendocrine cells during the critical period for sexual differentiation of the brain. Progress Brain Res. (2010) 186:97–111. doi: 10.1016/B978-0-444-53630-3.00007-5
49. Gillberg C. Are autism and anorexia nervosa related? Br J Psychiatry. (1983) 142:428b. doi: 10.1192/bjp.142.4.428b
50. Gillberg C. Autism and anorexia nervosa: related conditions? Nordic J Psychiatry. (1985) 39:307–12. doi: 10.3109/08039488509101911
51. Matson JL, Fodstad JC. The treatment of food selectivity and other feeding problems in children with autism spectrum disorders. Res Autism Spectrum Disord. (2009) 3:455–61. doi: 10.1016/j.rasd.2008.09.005
52. Mandy W, Tchanturia K. Do women with eating disorders who have social and flexibility difficulties really have autism? A case series. Mol Autism. (2015) 6:6. doi: 10.1186/2040–2392-6–6
53. Zhou ZC, McAdam DB, Donnelly DR. Endophenotypes: a conceptual link between anorexia nervosa and autism spectrum disorder. Res Dev Disabil. (2018) 82:153–65. doi: 10.1016/j.ridd.2017.11.008
54. Björnsdotter M, Davidovic M, Karjalainen L, Starck G, Olausson H, Wentz E. Grey matter correlates of autistic traits in women with anorexia nervosa. J Psychiatry Neurosci. (2018) 43:79–86. doi: 10.1503/jpn.170072
55. Gadow KD, Roohi J, DeVincent CJ, Hatchwell E. Association of ADHD, tics, and anxiety with dopaminetransporter (DAT1) genotype in autism spectrumdisorder. J Child Psychol Psychiatry. (2008) 49:1331–8. doi: 10.1111/j.1469-7610.2008.01952.x
56. Anderson BM, Schnetz-Boutaud N, Bartlett J, Wright HH, Abramson RK, Cuccaro ML, et al. Examination of association to autism of common geneticvariationin genes related to dopamine. Autism Res. (2008) 1:364–9. doi: 10.1002/aur.55
57. Sun X, Yue J, Zheng C. Study of dopamine transporterimaging on the brain of children with autism. J Biomed Eng. (2008) 25:327–330
58. Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. (2010) 16:e92–123. doi: 10.1111/j.1755–5949.2010.00154.x
59. Gervasini G, Gordillo I, García-Herráiz A, Flores I, Jiménez M, Monge M, Carrillo JA. Influence of dopamine polymorphisms on the risk for anorexia nervosa and associated psychopathological features. J Clin Psychopharmacol. (2013) 33:551–5. doi: 10.1097/JCP.0b013e3182970469
60. Södersten P, Bergh C, Leon M, Zandian M. Dopamine and anorexia nervosa. Neurosci Biobehav Rev. (2016) 60:26–30. doi: 10.1016/j.neubiorev.2015.11.003
61. Caine SB, Koob GF. Effects of mesolimbic dopaminedepletion on responding maintained by cocaine andfood. J Exp Anal Behav. (1994) 61:213–21.
62. Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolichormones, dopamine circuits, and feeding. Front Neuroendocrinol. (2010) 31:104–12. doi: 10.1016/j.yfrne.2009.10.004
Keywords: high-functioning, autism spectrum disorder, psychopathological comorbidities, gender distribution, anorexia nervosa, ADHD, anxiety disorders, mood disorders
Citation: Margari L, Palumbi R, Peschechera A, Craig F, de Giambattista C, Ventura P and Margari F (2019) Sex-Gender Comparisons in Comorbidities of Children and Adolescents With High-Functioning Autism Spectrum Disorder. Front. Psychiatry 10:159. doi: 10.3389/fpsyt.2019.00159
Received: 22 November 2018; Accepted: 04 March 2019;
Published: 26 March 2019.
Edited by:
Manuel Fernando Casanova, University of South Carolina, United StatesCopyright © 2019 Margari, Palumbi, Peschechera, Craig, de Giambattista, Ventura and Margari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Margari, lucia.margari@uniba.it
 Lucia Margari
Lucia Margari Roberto Palumbi
Roberto Palumbi Antonia Peschechera
Antonia Peschechera Francesco Craig2
Francesco Craig2 Francesco Margari
Francesco Margari