- 1Department of Pharmacy, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Department of Pharmacy, Xuanwu Hospital of Capital Medical University, Beijing, China
- 3Medical Decision and Economic Group, Department of Pharmacy, Ren Ji Hospital, South Campus, School of Medicine, Shanghai Jiaotong University, Shanghai, China
- 4Department of Pharmacy, The People’s Hospital of Jiangshan, Jiangshan, China
Objective: The CAMEL clinical trial (412 patients were randomly assigned to either camrelizumab plus chemotherapy (n = 205) or chemotherapy alone (n = 207)) demonstrated that camrelizumab plus chemotherapy (CC) improved the overall survival time (OS) and progression-free survival time (PFS) of patients with metastatic nonsquamous non-small cell lung cancer (non-sq NSCLC) without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations (EGFRm and ALKm) vs. chemotherapy (C) alone. Our objective was to conduct a cost-effectiveness analysis of CC vs. C from a perspective of health - care system in China with a lifetime horizon to identify whether it will be cost-effective.
Materials and Methods: A partitioned survival model (PSM) was applied for patients with IIIB–IV non-sq NSCLC without EGFRm and ALKm. Transition parameters and proportions of three health states were derived from the CAMEL trial. The model was designed using a lifetime horizon, a 21-day cycle, and a 5% discount rate of costs and outcomes. It was deemed cost-effective in China if the incremental cost-effectiveness ratio (ICER) value is less than $32,457 per quality adjusted life-year (QALY). Deterministic and probabilistic sensitivity analyses were performed to verify the influence of parameter uncertainty on the results.
Results: In the base-case analysis, we found that the ICER of CC compared with C is $-7,382.72/QALY which meant that CC had lower costs and better outcomes. The results of the sensitivity analyses demonstrated that the result was robust for the ICERs never transcending the willingness-to-pay (WTP) threshold.
Conclusion: Camrelizumab plus chemotherapy is an obviously cost-effective therapeutic regime for patients of IIIB–IV non-sq NSCLC without EGFRm and ALKm in China at a $32,457 WTP threshold.
Introduction
Lung carcinoma is the most common malignancy all over the world. It is also the most frequent cause of cancer-related death in human beings, which is estimated to be accountable for nearly 1/5 deaths for cancer (Fitzmaurice et al., 2018). In 2015, the aggregate expenditure of lung cancer treatment reached ¥24.31 billion in China, representing 0.6% of the total health cost (Wan et al., 2020). Non-small cell lung cancer (NSCLC) bears approximately 85% of the whole lung cancer cases (Reck and Rabe, 2017), and what is worse, about more than 70% of patients have developed to the advanced stage at the time of diagnosis, leaving a 5-year survival rate of 18% (Herbst et al., 2018; Miller et al., 2019). Platinum-doublet chemotherapy (±bevacizumab) has remained the main first-line therapy for patients with metastatic NSCLC for a long time until immune checkpoint inhibitor (ICI) comes out (Sandler et al., 2006; Zhou et al., 2015; Planchard et al., 2018), such as inhibitors of programmed death 1 (PD-1) and its ligand PD-L1, which are effective therapies for metastatic NSCLC lacking sensitizing epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations (EGFRm or ALKm).
Several elegant clinical trials (Keynote-189, IMpower-130, IMpower-150, IMpower-132, and CAMEL) have shown that compared with chemotherapy alone, PD-1 or PD-L1 plus chemotherapy can significantly improve progression-free survival (PFS) and overall survival (OS) in patients with advanced non-squamous NSCLC (non-sq NSCLC), irrespective of the PD-L1 expression level (Gandhi et al., 2018; Socinski et al., 2018; West et al., 2019; Nishio et al., 2021; Zhou et al., 2021). Recently, a meta-analysis (Ferrara et al., 2020) shows that single-agent ICI in patients with NSCLC and PD-L1 ≥50% probably lead to a higher OS rate (hazard ratio (HR) 0.68, 95% confidence interval (CI) [0.60–0.76]) and may improve PFS (HR 0.68, 95% CI [0.52–0.88]) and overall response rate (ORR) (risk ratio (RR) 1.40, 95% CI [1.12–1.75]) when compared to platinum-based chemotherapy and may also lead to a lower rate of adverse events (AEs) (RR 0.41, 95% CI [0.33–0.50]) and higher health-related quality of life (HRQoL) (RR 1.51, 95% CI [1.08–2.10]). Combined ICI in patients with NSCLC and PD-L1 ≥50% also probably lead to a higher OS rate (HR 0.72, 95% CI [0.59–0.89]), but its effect on PFS, ORR, and HRQoL is unknown due to a lack of data. The rate of AEs may not differ between groups. The CAMEL study was the first phase three study for evaluating immunotherapy plus chemotherapy in Chinese patients. Camrelizumab (SHR-1210) is a humanized monoclonal antibody against PD-1, and the CAMEL trial demonstrated the remarkable clinical benefits of camrelizumab combined with chemotherapy in non-sq NSCLC as the first-line therapy (PFS 11.3 vs. 8.3 months, HR 0.60 [0.45–0.79], p = 0.0001; OS 27.9 vs. 20.5 months, HR 0.73 [0.55–0.96], p = 0.0117) (Zhou et al., 2021). Besides, in June 2020, the combination regimen of camrelizumab + standard chemotherapy (platinum and pemetrexed), as the first-line therapy for patients with metastatic non-sq NSCLC without EGFRm or ALKm, was approved by the National Medical Products Administration (NMPA) in China and was included in the Guidelines of Chinese Society of Clinical Oncology (CSCO) (Non-Small Cell Lung Cancer) (CSoCOC, 2020). Moreover, the therapeutic regime mentioned above has been included in 2020 national medical insurance catalogue with more than 85% reduction in the price of camrelizumab.
Despite these encouraging results, relative higher cost of the combination therapy (camrelizumab with chemotherapy) compared with chemotherapy alone urges us to attach importance to the pharmacoeconomic evaluation. Accordingly, our goal was to conduct a cost-effectiveness analysis of camrelizumab + chemotherapy (CC) vs. chemotherapy alone (C) in the first line treatment of patients with IIIB–IV non-sq NSCLC without EGFRm and ALKm from a perspective of health - care system in China following a lifetime horizon to confirm whether it will be cost-effective.
Materials and Methods
Model Structure
A partitioned-survival model (PSM) was established depending on the clinical data from the phase three study (CAMEL) (Zhou et al., 2021) to estimate the costs and effectiveness of the two treatments, with three health states: PFS, progressive disease (PD), and death (Figure 1). The PSM structure eliminates the need to put forward assumptions for the transition of patients between different health states and enables the direct use of the trials’ Kaplan-Meier (K-M) curves to directly divide patients into different health states. Therefore, the estimation of patients’ proportion in each health state was acquired directly from the cumulative survival probabilities in OS and PFS curves by parametric functions fitting and extrapolation. The model compared the medical cost and health outcomes for two treatments: (C group) treated with chemotherapy (carboplatin and pemetrexed) vs. (CC group) treated with camrelizumab + chemotherapy combination (camrelizumab, carboplatin, and pemetrexed). Patients could receive subsequent treatment if the disease progressed or unacceptable AEs occurred. The population was a cohort with the same characteristics as those in the CAMEL trial (patients with IIIB–IV non-sq NSCLC without EGFRm and ALKm were aged 18–70 years [Median (IQR), 59 (54–64) years and 61 (53–65) years, respectively] and male accounted for 71 and 72%, respectively). The cycle length was 21-days in keeping with the treatment schedule reported by the CAMEL trial (Zhou et al., 2021) and the time horizon was the whole life so as to ensure there were less than 1% survivors. Costs, life years (LYs), quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were calculated in each treatment group. In line with Chinese pharmacoeconomic guidelines (Liu et al., 2020), both costs and benefits are discounted at 5% (range: 0–8%) per year. The initial state is assumed to be PFS, and death is the absorbing state.
Efficacy Estimates
The model transition parameters and proportions were directly obtained from the results of CAMEL (Zhou et al., 2021). We used the GetData Graph Digitizer (version 2.26) to collect the data points from the K–M curves (PFS and OS curves) of the two arms and followed the method of Guyot et al. (2012) to reconstruct estimates of underlying individual patient data (IPD) over the clinical trial time. In terms of the IPD out of the clinical trial time, standard parametric models were used for parametric extrapolation and long-term survival estimates by using the R version 4.1.0 (https://www.r-project.org). Specifically, six parametric functions were considered including exponential, Weibull, Gompertz, log-logistic, log-normal, and generalized gamma distributions. And then, a series of methods were applied to evaluate the goodness-of-fit of each parametric survival model under appropriate circumstances, such as visual inspection and Akaike information criterion (AIC)/Bayesian information criteria (BIC) tests proposed by NICE DSU technical support document 14 (TSD14) (Latimer, 2013) (table in appendix SM1-4). Lower AIC and BIC values indicate better fit of the selected model. Superimposed graphs of the K-M curves from the trial and the predicted curves based on the best fitting parametric survival models are presented in Figure 2, Figure 3, Figure 4, and Figure 5 to intuitively inspect the survival prediction.
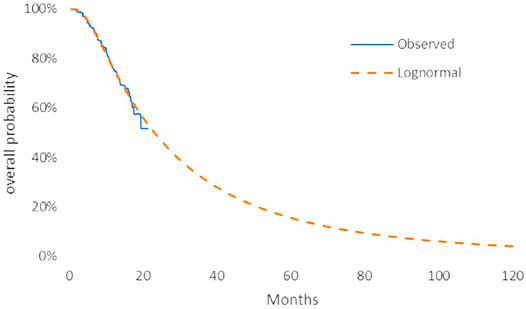
FIGURE 2. Parametric models for OS—camrelizumab plus chemotherapy. The log-normal model was chosen as the best fit model for the OS curve of the CC arm.
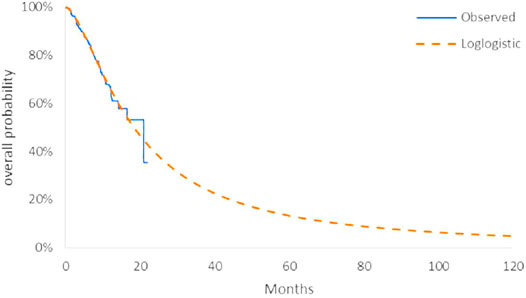
FIGURE 3. Parametric models for OS—chemotherapy. The log-logistic model was chosen as the best fit model for the OS curve of the C arm.

FIGURE 4. Parametric models for PFS—camrelizumab plus chemotherapy. The log-normal model was chosen as the best fit model for the PFS curve of the CC arm.

FIGURE 5. Parametric models for PFS—chemotherapy. The log-normal model was chosen as the best fit model for the PFS curve of the C arm.
The log-logistic model was chosen as the best fit model for the OS curve of C arm and log-normal model for CC arm and PFS curve of C arm. Considerations were as follows: (1) the lowest AIC and BIC values among all survival models; (2) the best fit with the observed curves based on visual inspection.
Utility Estimates
The health utility score reflects the level of physical, mental, and social functioning associated with a disease correlative health state that varies from 0 to 1, with 0 representing the worst health state/death and 1 representing the best. Average health utilities of the PFS and the PD state were 0.804 (Nafees et al., 2017) and 0.321 (Nafees et al., 2017), respectively, which were obtained from the previously published literature (Nafees et al., 2017). The disutility values owing to 3/4 AEs were considered in our analysis (Nafees et al., 2008; Handorf et al., 2012), but only one-time assessment was carried out during the first cycle for simplification given the trivial influence of AE disutilities. The incidence of AEs was multiplied by the corresponding disutility value to assess the QALYs loss caused by AEs. In addition, ± 20% as the boundaries of the range were used in sensitivity analyses.
Cost Inputs
In our study, only direct medical costs were considered, including cost of the drug utilization, PD-L1 test, main AEs, treatments for progression (including active treatments and supportive care), monitoring, and terminal care. Drug prices were estimated from the local bid-winning price (Drugdataexpy). Only severe AEs with great clinical impact, including anemia, neutropenia, and thrombocytopenia, were calculated because they had a relatively considerable influence on the economic evaluation by decreasing quality of life and increasing utilization of health resource. In addition, AE costs were calculated only once in the first cycle.
Costs of monitoring, AEs, terminal care, and PD-L1 tests were obtained from previously published studies (Wu et al., 2012; Zheng et al., 2018; Jiang and Wang, 2020; Wan et al., 2020). All patients were assumed to incur one-time PD-L1 test costs in the first cycle and one-time terminal care costs before death. Additionally, costs were discounted at an annual rate of 5% (Sanders et al., 2016). All costs were converted into United States dollars (USD) by exchange rate: 1 USD = 6.47 CYN. All these data are listed in Table 1. If the ICER is below $32,457 threshold (three times GDP per capita of China in 2020, ¥210,000.00), the treatment is generally considered to be cost-effective.
Clinical Inputs
According to the phase three CAMEL trial (Figure 6), the patients in the CC group (N = 205) received camrelizumab 200 mg and pemetrexed 500 mg/m2 and carboplatin at an area under the curve (AUC) 5 mg/ml per min, administered as intravenous infusion on Day 1 of each 21-day cycle for 4-6 cycles followed by optional camrelizumab 200 mg and pemetrexed 500 mg/m2 every 3 weeks (Q3W) maintenance for the remainder of the study or until recorded PD, intolerable AEs, death, or study completion; the patients in the C group (N = 207) received pemetrexed + platinum on Day 1 per 21-day cycle for 4-6 cycles followed by pemetrexed 500 mg/m2 Q3W maintenance. Patients in the C group who were confirmed disease development had an optional crossover to camrelizumab monotherapy according to investigators’ judgment, which was also taken into account in our PSM. The total exposure of camrelizumab should not be more than 2 years. After the initial treatment, 75 (81.5%, PD 92) in the CC group and 120 (94.5%, PD 127) patients in the C group received at least one subsequent anti-tumor therapy, and we assumed that the others (17 (18.5%) and 7 (5.5%), respectively) were treated with supportive care. In the C group, 79 (62.2%) patients transformed to camrelizumab monotherapy and 9 (7.1%) received other anti-PD-1 drugs, alone or combined with other therapies. Subsequent anticancer therapies included immunotherapy and chemotherapy. Referring to the other studies, we assumed that docetaxel was used as the standard second-line chemotherapy, and nivolumab and camrelizumab as the immunotherapy, which is commonly used in China. For dosage calculation, values of body surface area (BSA), weight, and creatinine clearance rate (Ccr) were 1.80 m2, 65kg, and 90 ml/min/1.73 m2, respectively (Wu et al., 2011).
(Costs and health utilities inputs were summarized in Table 1)

FIGURE 6. Flowchart of treatment regime along with inclusion and exclusion criteria. Reference: CAMEL (NCT03134872) Protocol No.: SHR-1210-III-303-NSCLC.
Sensitivity Analysis
A series of sensitivity analyses were conducted to test the robustness of the model. One-way deterministic sensitivity analyses (DSA) were used to evaluate the impact of uncertainty of a single input variable on the ICER. All parameters were adjusted within the reported 95% confidence intervals (CI) or assuming reasonable ranges of the base case values (±20%) if 95% CIs were unavailable, in accordance with established approach (Zhang et al., 2012). In the probabilistic sensitivity analysis (PSA), a Monte Carlo simulation of 1000 iterations was generated by simultaneously sampling the key model parameters from the pre-specified distributions. The variables about risk of main AEs, proportion of patients and utilities were assigned beta distributions, and the variables about costs used gamma distributions. Based on the data from 1000 iterations, a cost-effectiveness acceptability curve (CEAC) was created to represent the likelihood that the CC regime would be considered cost-effective compared with the C regime on the basis of a willingness to pay (WTP) threshold of $32,457 per QALY in China.
Results
Base Case Results
Over a lifetime horizon, the total QALYs for CC and C were estimated to be 1.55 and 1.16, respectively, and the PSM assessed the total costs to be $19,023.42 and $21,922.27, respectively, during that period, resulting in an ICER of $-7382.72/QALY (Table 2).
Sensitivity Analysis
Deterministic Sensitivity Analysis Results
Tornado diagrams of one-way sensitivity analyses are shown in Figure 7. The results indicated that the parametric models for OS curves of both chemotherapy alone and combination arms were the most sensitive parameters in the model, which had the most significant impact on the ICER. Regardless of the variation of each parameter across the wide ranges, the ICER for the CC group compared with the C group remained less than $32,457/QALY (3×GDP per capita in China 2020), even less than $10,819 per QALY (1×GDP per capita in China 2020). The DSA results indicated that model results were robust.
Probabilistic Sensitivity Analysis Results
Cost-effectiveness probabilistic acceptability curves (CEACs) (Figure 8) showed that compared with chemotherapy alone the probability of camrelizumab plus chemotherapy being cost-effective at the specified WTP threshold of $32,457 per QALY gained was 100%. The scatter plots shown in Figure 9 depict the results of the 1000 simulations of the probabilistic sensitivity analysis, in which most results generated more QALYs and less costs than chemotherapy alone.

FIGURE 8. Cost-effectiveness acceptability curves. The cost-effectiveness acceptability Frontier shows the probability of strategies being cost-effective in two strategies. Compared with chemotherapy alone, the probability of camrelizumab plus chemotherapy being cost-effective at the specified WTP threshold of $32,457 per QALY gained is nearly 100%.

FIGURE 9. Cost-effectiveness plane with scatter plot of incremental costs and incremental QALYs (WTP = $32,457). The results of Monte Carlo simulation of 1000 iterations show that in most cases camrelizumab and chemotherapy combination therapy generated more QALYs and less costs than chemotherapy alone.
Discussion
Summary and Interpretation of Results
To our knowledge, this evaluation was the first study to evaluate the cost-effectiveness of camrelizumab and chemotherapy combination therapy for the target patients with metastatic non-sq NSCLC without EGFRm or ALKm in China. Under the circumstance of the decreased price of camrelizumab from $3060.3 to $452.6, our results showed that CC strategy produced an extra 0.39 QALYs while saving $2,898.85, which resulted in an ICER of $-7,382.72/QALY. These findings suggested that the CC strategy was absolutely cost-effective in China, which was also proved by the CEACs (Figure 8). Recently, some scholars (Kazibwe et al., 2021) pointed out that using 1 to 3× GDP per capita as a cost-effectiveness threshold had resulted in a higher proportion of study interventions being found as cost-effective, which might lead to a loss of would-be health gains from deployed resources for health. However, in our study, even if the opportunity cost threshold (or 0.5× GDP per capita) is used, the CC scheme is nearly 90% likely to be cost-effective, which means that our results are robust.
Compared with other PD1/PD-L1-chemotherapy combination therapeutic regimes, including pembrolizumab, atezolizumab, and sindilimab, which were approved in China or abroad as the first line therapy for patients with metastatic non-sq NSCLC without EGFRm or ALKm, only camrelizumab-chemotherapy combination therapeutic regime showed cost-effectiveness in China according to current research. For pembrolizumab, Jiang et al. (2020), Wan et al. (2020) and Wu et al. (2020) had developed studies to assess the cost-effectiveness of combination therapy by partitional survival model, Markov model, and decision tree plus Markov model, respectively. They found that the ICERs of combination treatment vs. chemotherapy alone were $96,644/QALY, $92,533/QALY and higher than $40,000/QALY, respectively, which highly exceed the WTP threshold in China (3 times of GDP per capita). So that, compared with chemotherapy alone, the combination treatments showed no cost-effectiveness. In terms of atezolizumab, Yang et al. (2021) found that the probability of atezolizumab plus chemotherapy (ICER $325,328.71/QALY) was cost-effective was 0% at a WTP value of $30,828/QALY (Table3). Based on these findings, our study may be useful for national medical insurance negotiation and decision-making.
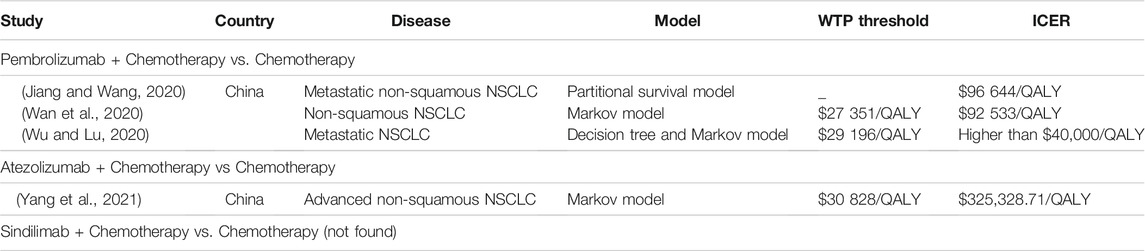
TABLE 3. Summary of cost-effectiveness analyses in China for other PD1/PD-L1-chemotherapy combination therapeutic regimes in advanced non-squamous NSCLC
Strengths and Limitations
A major strength of the basic analysis was its dependence on a direct comparison of CC and C alone, using data and information from a randomized controlled trial. The second strength was that the population included in the trial and analysis were Chinese, which eliminated the influence of different races. Besides, the PSM structure was unnecessary to produce assumptions for the transition of patients between health states but enabled researchers to partition patients to different health states directly based on the trials’ K-M curves.
Analysis limitations were as follows: First, for the longer-term extrapolation of PFS and OS, there existed an inherent uncertainty. Besides the choice of follow-up treatment would be different according to the individual situation of patients, for which additional data from real world studies could help in validating the model over the long-term. Second, the values of utilities and disutilities were from published studies, some of which were not based on Chinese populations, and the differences caused by different treatment methods were not distinguished. Nafees et al. (2017) figured out that the values of utilities for NSCLC in China were higher than other countries. Third, the published data of the phase three CAMEL trial was the interim results with a median follow-up duration of 11.9 months (IQR 9.0–14.9). Thus, it might be possible that the prediction of cost-effectiveness could be changed with additional follow-up. In addition, this study was a secondary analysis of data from the published literature which might limit the conclusions.
Conclusion
From a perspective of health - care system in China, the current model predicted that camrelizumab-chemotherapy combination therapeutic regime would offer obviously marked benefits to patients of IIIB–IV non-sq NSCLC without EGFR and ALK alteration in comparison with chemotherapy alone.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
C.Z., B.W., C.L., and H.F. contributed to conception and design of the study. G.H. and X.X. searched the database. C.Z. and G.L. performed the statistical analysis. C.Z. wrote the first draft of the manuscript. G.L. and X.X. wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.735536/full#supplementary-material
References
CSoCOC (2020). Guidelines of Chinese Society of Clinical Oncology (CSCO):Non-Small Cell Lung Cancer (Accessed May 23, 2020).
Drugdataexpy: Marketing Imformation Local Bid-Wining Price. Available From: https://datayaozhcom.
Ferrara, R., Imbimbo, M., Malouf, R., Paget-Bailly, S., Calais, F., Marchal, C., et al. (2020). Single or Combined Immune Checkpoint Inhibitors Compared to First‐line Platinum‐based Chemotherapy with or without Bevacizumab for People with Advanced Non‐small Cell Lung Cancer[J]. Cochrane Database Syst. Rev. 12, CD013257. doi:10.1002/14651858.CD013257.pub2
Fitzmaurice, C., Fitzmaurice, C., Akinyemiju, T. F., Al Lami, F. H., Alam, T., Alizadeh-Navaei, R., et al. (2018). Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 4 (11), 1553–1568. doi:10.1001/jamaoncol.2018.2706
Gandhi, L., Rodríguez-Abreu, D., Gadgeel, S., Esteban, E., Felip, E., De Angelis, F., et al. (2018). Pembrolizumab Plus Chemotherapy in Metastatic Non-small-cell Lung Cancer. N. Engl. J. Med. 378 (22), 2078–2092. doi:10.1056/NEJMoa1801005
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced Secondary Analysis of Survival Data: Reconstructing the Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Handorf, E. A., McElligott, S., Vachani, A., Langer, C. J., Bristol Demeter, M., Armstrong, K., et al. (2012). Cost Effectiveness of Personalized Therapy for First-Line Treatment of Stage IV and Recurrent Incurable Adenocarcinoma of the Lung. J. Oncol. Pract. 8 (5), 267–274. doi:10.1200/JOP.2011.000502
Herbst, R. S., Redman, M. W., Kim, E. S., Semrad, T. J., Bazhenova, L., Masters, G., et al. (2018). Cetuximab Plus Carboplatin and Paclitaxel with or without Bevacizumab versus Carboplatin and Paclitaxel with or without Bevacizumab in Advanced NSCLC (SWOG S0819): a Randomised, Phase 3 Study. Lancet Oncol. 19 (1), 101–114. doi:10.1016/S1470-2045(17)30694-0
Jiang, Y., and Wang, X. (2020). Cost-effectiveness Analysis of Pembrolizumab Plus Standard Chemotherapy versus Chemotherapy Alone for First-Line Treatment of Metastatic Non-squamous Non-small-cell Lung Cancer in China[J]. Eur. J. Hosp. Pharm., ejhpharm 2020 002208. doi:10.1136/ejhpharm-2020-002208
Kazibwe, J., Gheorghe, A., Wilson, D., Ruiz, F., Chalkidou, K., and Chi, Y-L. (2021). The Use of Cost-Effectiveness Thresholds for Evaluating Health Interventions in Low- and Middle-Income Countries from 2015 to 2020: A Review[J]. Lawrenceville, NJ: ISPOR. doi:10.1016/j.jval.2021.08.014
Latimer, N. R. (2013). Technical Support Document 14. Survival Analysis for Economic Evaluations Alongside Clinical Trials - Extrapolation with Patient-Level Data. London: National Institute for Health and Care Excellence. Available From: http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf (Accessed April 18, 2019).
Liu, G. E., Hu, S. L., Wu, J. H., Wu, J., Dong, C. H., and Li, H. C. (2020). China Guidelines for Pharmacoeconomic Evaluations.
Lu, S., Ye, M., Ding, L., Tan, F., Fu, J., and Wu, B. (2017). Cost-effectiveness of Gefitinib, Icotinib, and Pemetrexed-Based Chemotherapy as First-Line Treatments for Advanced Non-small Cell Lung Cancer in China. Oncotarget 8 (6), 9996–10006. doi:10.18632/oncotarget.14310
Miller, K. D., Nogueira, L., Mariotto, A. B., Rowland, J. H., Yabroff, K. R., Alfano, C. M., et al. (2019). Cancer Treatment and Survivorship Statistics, 2019. CA Cancer J. Clin. 69 (5), 363–385. doi:10.3322/caac.21565
Nafees, B., Lloyd, A. J., Dewilde, S., Rajan, N., and Lorenzo, M. (2017). Health State Utilities in Non-small Cell Lung Cancer: An International Study. Asia Pac. J. Clin. Oncol. 13 (5), e195–e203. doi:10.1111/ajco.12477
Nafees, B., Stafford, M., Gavriel, S., Bhalla, S., and Watkins, J. (2008). Health State Utilities for Non Small Cell Lung Cancer[J], Health Qual. Life Outcomes, 6. 84. doi:10.1186/1477-7525-6-84
Nishio, M., Saito, H., Goto, K., Watanabe, S., Sueoka-Aragane, N., Okuma, Y., et al. (2021). IMpower132: Atezolizumab Plus Platinum-Based Chemotherapy vs Chemotherapy for Advanced NSCLC in Japanese Patients. Cancer Sci. 112 (4), 1534–1544. doi:10.1111/cas.14817
Planchard, D., Popat, S., Kerr, K., Novello, S., Smit, E. F., Faivre-Finn, C., et al. (2018). Metastatic Non-small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 29 (Suppl. 4), iv192–iv237. doi:10.1093/annonc/mdy275
Reck, M., and Rabe, K. F. (2017). Precision Diagnosis and Treatment for Advanced Non-small-cell Lung Cancer. N. Engl. J. Med. 377 (9), 849–861. doi:10.1056/NEJMra1703413
Sanders, G. D., Neumann, P. J., Basu, A., Brock, D. W., Feeny, D., Krahn, M., et al. (2016). Recommendations for Conduct, Methodological Practices, and Reporting of Cost-Effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 316 (10), 1093–1103. doi:10.1001/jama.2016.12195
Sandler, A., Gray, R., Perry, M. C., Brahmer, J., Schiller, J. H., Dowlati, A., et al. (2006). Paclitaxel-carboplatin Alone or with Bevacizumab for Non-small-cell Lung Cancer. N. Engl. J. Med. 355 (24), 2542–2550. doi:10.1056/NEJMoa061884
Socinski, M. A., Jotte, R. M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., Nogami, N., et al. (2018). Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 378 (24), 2288–2301. doi:10.1056/NEJMoa1716948
Wan, N., Zhang, T. T., Hua, S. H., Lu, Z. L., Ji, B., Li, L. X., et al. (2020). Cost-effectiveness Analysis of Pembrolizumab Plus Chemotherapy with PD-L1 Test for the First-Line Treatment of NSCLC. Cancer Med. 9 (5), 1683–1693. doi:10.1002/cam4.2793
West, H., McCleod, M., Hussein, M., Morabito, A., Rittmeyer, A., Conter, H. J., et al. (2019). Atezolizumab in Combination with Carboplatin Plus Nab-Paclitaxel Chemotherapy Compared with Chemotherapy Alone as First-Line Treatment for Metastatic Non-squamous Non-small-cell Lung Cancer (IMpower130): a Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 20 (7), 924–937. doi:10.1016/S1470-2045(19)30167-6
Wu, B., Chen, H., Shen, J., and Ye, M. (2011). Cost-effectiveness of Adding Rh-Endostatin to First-Line Chemotherapy in Patients with Advanced Non-small-cell Lung Cancer in China. Clin. Ther. 33 (10), 1446–1455. doi:10.1016/j.clinthera.2011.09.016
Wu, B., Dong, B., Xu, Y., Zhang, Q., Shen, J., Chen, H., et al. (2012). Economic Evaluation of First-Line Treatments for Metastatic Renal Cell Carcinoma: a Cost-Effectiveness Analysis in a Health Resource-Limited Setting. PLoS One 7 (3), e32530. doi:10.1371/journal.pone.0032530
Wu, B., and Lu, S. (2020). The Effect of PD-L1 Categories-Directed Pembrolizumab Plus Chemotherapy for Newly Diagnosed Metastatic Non-small-cell Lung Cancer: a Cost-Effectiveness Analysis. Transl Lung Cancer Res. 9 (5), 1770–1784. doi:10.21037/tlcr-19-605
Yang, Z., Zhu, Y., Xiang, G., Hua, T., Ni, J., Zhao, J., et al. (2021). First-line Atezolizumab Plus Chemotherapy in Advanced Non-squamous Non-small Cell Lung Cancer: a Cost-Effectiveness Analysis from China[J]. Expert Rev. Pharmacoecon Outcomes Res. 21, 1061–1067. doi:10.1080/14737167.2021.1899813
Zhang, Y., Baik, S. H., Fendrick, A. M., and Baicker, K. (2012). Comparing Local and Regional Variation in Health Care Spending. N. Engl. J. Med. 367 (18), 1724–1731. doi:10.1056/NEJMsa1203980
Zheng, H., Xie, L., Zhan, M., Wen, F., Xu, T., and Li, Q. (2018). Cost-effectiveness Analysis of the Addition of Bevacizumab to Chemotherapy as Induction and Maintenance Therapy for Metastatic Non-squamous Non-small-cell Lung Cancer. Clin. Transl Oncol. 20 (3), 286–293. doi:10.1007/s12094-017-1715-1
Zhou, C., Chen, G., Huang, Y., Zhou, J., Lin, L., Feng, J., et al. (2021). Camrelizumab Plus Carboplatin and Pemetrexed versus Chemotherapy Alone in Chemotherapy-Naive Patients with Advanced Non-squamous Non-small-cell Lung Cancer (CameL): a Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Respir. Med. 9 (3), 305–314. doi:10.1016/S2213-2600(20)30365-9
Zhou, C., Wu, Y. L., Chen, G., Liu, X., Zhu, Y., Lu, S., et al. (2015). BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients with Advanced or Recurrent Nonsquamous Non-small-cell Lung Cancer. J. Clin. Oncol. 33 (19), 2197–2204. doi:10.1200/JCO.2014.59.4424
Appendix A1:
Keywords: nonsquamous non-small cell lung cancer, NSCLC, camrelizumab, CAMEL, cost-effectiveness
Citation: Zhu C, Xing X-x, Wu B, Liang G, Han G, Lin C-x and Fang H-m (2021) Cost-Effectiveness Analysis of Camrelizumab Plus Chemotherapy vs. Chemotherapy Alone as the First-Line Treatment in Patients With IIIB–IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) Without EGFR and ALK Alteration from a Perspective of Health - Care System in China. Front. Pharmacol. 12:735536. doi: 10.3389/fphar.2021.735536
Received: 03 August 2021; Accepted: 26 November 2021;
Published: 24 December 2021.
Edited by:
Filipa Alves Da Costa, University of Lisbon, PortugalReviewed by:
Dalia M. Dawoud, National Institute for Health and Care Excellence, United KingdomRavindra Deshpande, Wake Forest School of Medicine, United States
Copyright © 2021 Zhu, Xing, Wu, Liang, Han, Lin and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cai-xia Lin, 251170937@qq.com; Hong-mei Fang, fanghm@srrsh.com
†These authors have contributed equally to this work and share first authorship
 Chen Zhu
Chen Zhu Xiao-xuan Xing2†
Xiao-xuan Xing2† Bin Wu
Bin Wu






