- 1Department of Neurosurgery, University Medical Center, Johannes Gutenberg University Mainz, Mainz, Germany
- 2Department of Neurology, Klinikum Ludwigsburg, Ludwigsburg, Germany
- 3Department of Neurosurgery, University Medical Center Ulm, Günzburg, Germany
- 4Division of Epidemiology and Health Services Research, Institute of Medical Biostatistics, Epidemiology and Informatics, University Medical Center, Johannes Gutenberg University Mainz, Mainz, Germany
Objective: Supportive care needs in glioma patients often remain unrecognized, and optimization in assessment is required. First, we aimed at assessing the support needed using a simple structured questionnaire. Second, we investigated the psychosocial burden and support requested from caregivers.
Methods: Patients were assessed at three centers during their outpatient visits. They completed the Distress Thermometer (DT; score ≥ 6 indicated significant burden in brain tumor patients), the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30+BN20, and the Patients' Perspective Questionnaire (PPQ) that assessed psychosocial distress as well as support requested and received by patients for specific domains (e.g., family, doctor, and mobile care). In each subgroup, patients' caregivers were assessed simultaneously by a questionnaire developed for the study. Multivariate backward logistic regressions were performed for investigating predictors of patients' request for support.
Results: Assessments were conducted for 232 patients. Most patients (82%) had a high-grade glioma and a mean age of 52 years (range 20–87). The male to female ratio was 1.25:1. According to the PPQ results, 38% (87) of the patients felt depressed; 44% (103), anxious; and 39% (91), tense/nervous. Desired support was highest from doctors (59%) and psychologists (19%). A general request for support was associated with lower global health status (p = 0.03, odds ratio (OR) = 0.96, 95% CI: 0.92–0.99) according to EORTC QLQ-C30. Most of the assessed caregivers (n = 96) were life partners (64%; n = 61) who experienced higher distress than the corresponding patients (caregivers: 6.5 ± 2.5 vs. patients: 5.3 ± 2.4). When patients were on chemotherapy, caregivers indicated DT ≥ 6 significantly more frequently than patients themselves (p = 0.02).
Conclusion: Our data showed that glioma patients and their caregivers were both highly burdened. The PPQ allowed us to evaluate the psychosocial support requested and perceived by patients, detect supportive care needs, and provide information at a glance. Patients in poorer clinical condition are at risk of having unmet needs. The caregivers' burden and unmet needs are not congruent with the patients' need for support. In particular, caregivers of patients on chemotherapy were more highly burdened than patients themselves.
Introduction
The prognosis in high-grade glioma patients remains poor, and supportive care needs should be addressed in a timely manner. The requirement for psychosocial support and early palliative has not only become a focus of (neuro-) oncological research (1–5), but is also incorporated in guidelines for the provision of care for these patients (6, 7). Application of patient-reported outcomes (PROs) has become essential in assessing the patients' quality of life and their needs, distress, and psychosocial burden, as well as supportive care needs (8). Recently, it has been shown that monitoring symptoms via PRO measures can be very helpful for cancer patients and even influence survival (9, 10).
Palliative and end-of-life care are still partially neglected topics in current neuro-oncological outpatient care. Seibl-Leven et al. reported recently that palliative care in glioblastoma patients is either not provided at all, or not in a timely fashion, leading to a shortage of services for patients and caregivers (11). Adequate assessment of unmet needs, early integration of palliative care and timely end-of-life-care planning should be implemented as clinical routine (12).
As glioma patients suffer from neurocognitive deficits caused by both the disease itself and the treatment (13–15) they may not always be able to answer PRO questionnaires. Furthermore, as reported by our group and others, patients undergoing chemotherapy or in a poor clinical condition may be missed by PRO assessment; however, at the same time, they are those who could particularly benefit from early supportive care (5, 16–18). Therefore, we believe it is of utmost importance to adequately identify glioma patients in need of supportive care.
Caregivers face challenging situations as well, sometimes even more so than glioma patients. The neurological, psychological and cognitive symptoms of patients with gliomas represent significant challenges to their caregivers: Not only do they have to cope with the diagnosis of the family member, with the therapy and the knowledge that they will finally lose their partner or parent or child, but also accept changes in roles, relationships, social isolation, financial restriction and sooner or later, have to take care of the partner or family member day and night (19–23).
Therefore, the aims of our study were to (1) Assess support received and needed by patients and (2) Assess support needed by patients' caregivers.
Patients and Methods
Patients
During April 2015 to June 2016, patients at three German neuro-oncological centers were approached during their outpatient visits and asked to participate in the study. Inclusion criteria were diagnosis of glioma WHO grades II–IV regardless of disease stage (initial diagnosis or recurrent disease), absence of aphasia impairing communication or consent to the study, and given informed consent. Patients were asked to complete several PRO measures. Furthermore, demographic and clinical data were recorded in a database.
PRO Measures Used
Distress Thermometer (DT)
The DT is a self-reporting screening instrument developed by the National Comprehensive Cancer Network to evaluate psychological distress on a visual analog scale (0–10 points). A problem list with 40 items is included for patients to indicate the area of concern (family, financial, and physical) (24). Studies have proven its acceptance in oncological patients, and the German version for brain tumor patients was first evaluated by Goebel and Mehdorn (25). A score ≥6 indicates significant burden in brain tumor patients.
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core Module for Cancer Patients Accompanied by the Brain-Specific Module (EORTC QLQ-C30 + BN20)
The EORTC QLQ-C30 is a widely accepted questionnaire applying a 4-point Likert scale to evaluate cancer patients' quality of life. Five functional, three symptom, and six single-item scales as well as the global health status are investigated (physical, role, emotional, social, and cognitive functioning; fatigue, nausea and vomiting, pain; dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). Its validity and reliability have been proven in numerous clinical studies, and it is available in 85 languages. The additional module for brain tumor patients (BN20) consists of 20 questions specifically assessing their symptoms (3 neurological deficit scales, 1 future uncertainty scale, treatment and disease-related symptoms) (26, 27). The EORTC scores were calculated according to the user manual (28). Each scale is scored from 0 to 100, with higher scores indicating better functioning for functional scales and worse symptoms for symptom scales. In our regression and correlation analyses, we used the global health scale (GHS) as the primary endpoint.
The Patients' Perspective Questionnaire (PPQ)
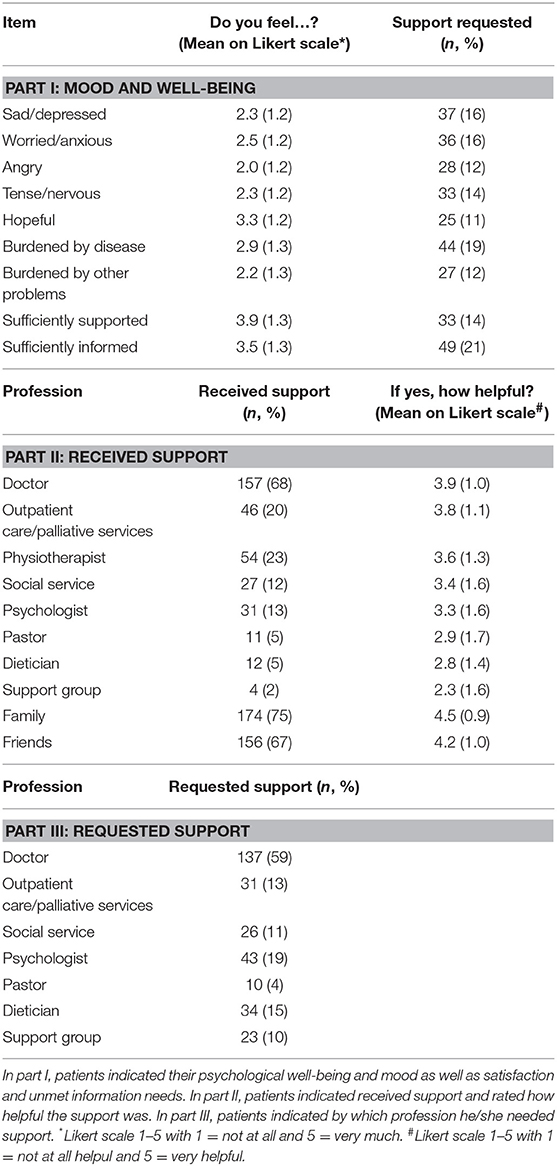
The “Patients' Perspective Questionnaire” (PPQ) is a questionnaire assessing patients' current status of support received, its subjective benefit and further needs. It was adapted for brain tumor patients based on a questionnaire used by Singer et al. (29). They applied several versions, whereas in our study we combined them into one questionnaire, added questions and items of probable interest to glioma patients according to the authors' experiences. This resulted in one questionnaire comprising three parts: Part I: The first 9 items assessed psychosocial distress (sad /worried /angry /tense /hopeful /burdened by disease/ burdened by other problems/ sufficiently supported/ sufficiently informed) by 5-step Likert-scales (scoring from 1 = not at all to 5 = very much) and if support was requested by the patient with regard to the respective item (“I need support for….”: yes/no). The latter questions were considered for the general request for support if one or more item was answered with “yes.” The next 10 items (Part II) assessed support provided and its subjective benefit on a 5-step Likert scale (scoring from 1 = support was not helpful at all to 5 = support was very helpful). The last 7 items (Part III) recorded support requested currently by the patients (from doctors, psychologist, social worker, and so on) with dichotomous answer possibilities (yes/no). Further support currently requested by any profession or next of kin was considered positive if one or more answers were “yes.” The questionnaire is provided as Supplement 1.
The Questionnaire for the Caregivers/Caregivers' Perspective Questionnaire (CPQ)
In order to provide a questionnaire for glioma patients' caregivers in line with the PPQ with regard to structure and practicability, we combined elements and items after conducting a literature search and using an expert panel in the study group. We first applied it as a pilot study in family members volunteering during an information-sharing event for brain tumor patients. According to their anonymous feedback, the questionnaire was adapted with respect to wording, font size and item specification. The final version of the questionnaire is provided as Supplement 2.
Part I assessed the psychological distress on a visual analog scale (0–10 points) according to the DT applied to patients. Further, possible problem-items were provided similarly to the DT item list with dichotomous choices with regard to practical, family or emotional problems (24). In part II, we incorporated two questions with regard to quality of life and global health with Likert scales scoring from 1 to 7 according to the items 29 and 30 of the EORTC QLQ-C30 questionnaire (26). Finally, part III provided a list of items recording the support requested by caregivers (psychologists, social care, doctor, physiotherapy, dietician, self-help, friends, family members, palliative care). The questionnaire further included a question if an explanation of any term in the questionnaire was needed (Supplement 2).
Patients' and Caregivers' Assessment
Patients completed the DT, EORTC QLQ-C30+BN20 and the PPQ by themselves. Further, patients were asked directly by the attending neuro-oncologist during the patient-doctor consultation if they would like support by a psychologist. Neuro-oncologists also indicated and recorded their own assessment with regard to patients' unmet psycho-oncological needs independent of the assessment by questionnaires after having obtained the current medical history.
In a subgroup, patients' caregivers were assessed simultaneously by the CPQ developed for the study.
Statistical Analysis
Demographic and tumor-related data, as well as Karnofsky-Index, were analyzed descriptively. Explorative Spearman's rho correlations between DT score as well as GHS and general request for support or request for support by doctors were performed.
Multivariate logistic regressions were performed with regard to “request for support in general” as well as “request for support by doctors” and “by other health care professionals.” Clinical and demographic factors probably influencing request for support were selected content driven by the authors as follows: sex (male/female), living situation (alone/in relationship), educational level (university degree/no university degree), WHO grade (low-grade/high-grade glioma), Karnofsky-Index (continuous variable, score 0–100), on chemotherapy (yes/no), GHS (continuous variable, EORTC QLQ-C30, score 0–100), DT score (continuous variable, score 0–10), surgery for recurrent disease (yes/no), and age (continuous variable, 25–85). The statistical analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY).
Ethics
The study was performed in accordance with national law, institutional ethical standards, and the Declaration of Helsinki after approval of the study protocol by the local ethics committees (Mainz, Germany and Ulm/Günzburg, Germany [No: 837.349.15 (10117)]. All patients provided written informed consent prior to data assessment.
Results
Patients
Two hundred and thirty-two patients were assessed and 84% of the patients had a high-grade glioma. Mean age was 52 years (range 25–85). Male to female ratio was 1.25:1. Most of the patients were in a relationship and 30% (n = 71) had a higher education level. Mean Karnofsky-Index was 79 and 52% of the patients were on chemotherapy during assessment. Further details are provided in Table 1.
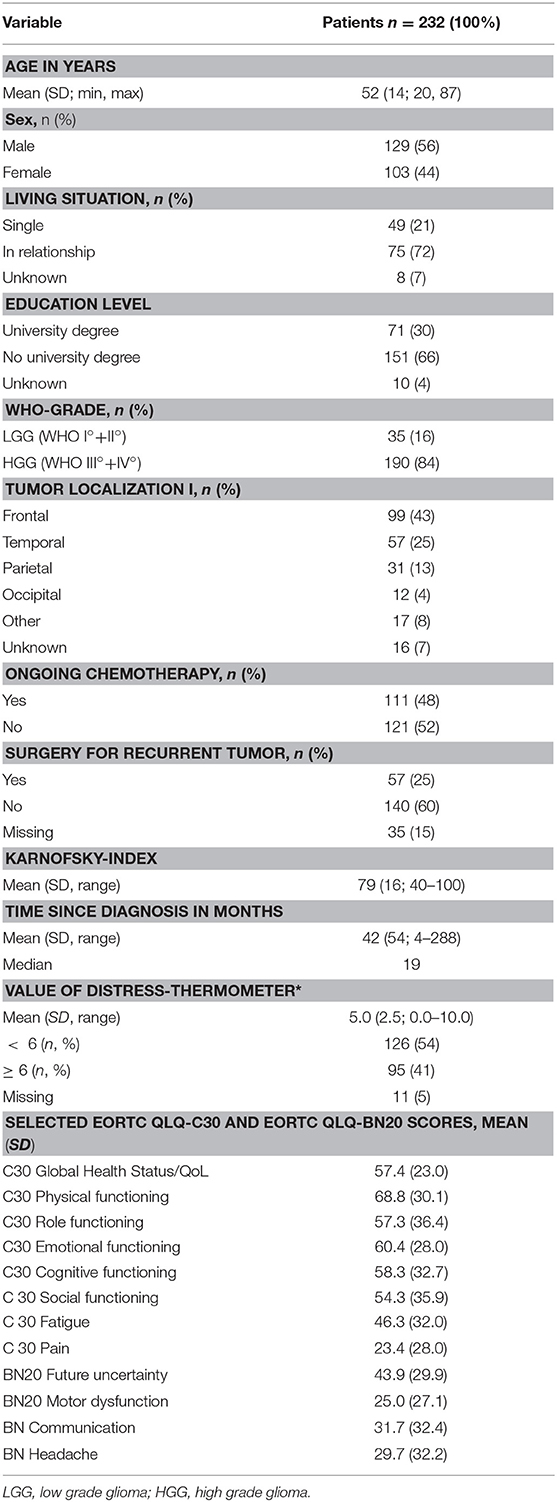
Table 1. Clinical and demographic data of the patient sample and results of the psychosocial assessment using the Distress Thermometer (DT; score ≥ 6 indicated significant burden in brain tumor patients), the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30+BN20, and the Patients' Perspective Questionnaire (PPQ).
Patients' Perceived and Requested Support According to the PPQ
We observed that 38% (87) of patients felt depressed (indicated ≥ 3 on the Likert scale), 44% (103) were anxious and 39% (91) were tense/nervous. Fifty-nine percent (138) reported to be adequately informed about the disease and therapy and 77% (180) of the patients felt sufficiently supported (Table 2).
Patients' support was reported to be highest from family (75%) and doctors (e.g., physician or attending neuro-oncologists, 68%). Only 13% were supported by psychologists. The support was mostly reported as helpful with highest mean scores being that of family and friends (mean score > 4), followed by doctors, outpatient care services and physiotherapists.
Desired support was highest from doctors (59%) and psychologists (19%) as well as from dieticians (15%, Table 2).
Patients' Requested Support in Comparison to DT, GHS, and Clinical Condition
Patients requesting support according to the PPQ generally showed higher DT scores (according to DT, p = 0.006, rs = 0.20), lower GHS (according to EORTC QLQ-C30, p < 0.001, rs = −0.34), and lower Karnofsky indices (p = 0.03, rs = −0.18). The request for support was further associated with patients' wish for psychological intervention when asked directly (p = 0.03, rs = 0.27), and the neuro-oncologist's clinical assessment of patients' unmet needs (p = 0.001, rs = 0.24).
Factors Associated With Current Requested General Support According to the PPQ
With regards to the request for support in general, we observed that patients reporting “lower GHS” were at higher risk for unmet needs (p = 0.03, odds ratio (OR) = 0.96, 95% CI: 0.92–0.99), as assessed by logistic regression analysis (step 5). Patients “living alone” indicated higher request for support; however, this was not significant. Educational level, WHO grade, Karnofsky-Index, on chemotherapy, DT score, surgery for recurrent disease and age were not significantly associated with the request for support in general.
Factors Associated With Current Requested Support by Doctors According to the PPQ
Logistic regression analyses revealed that “living situation/not in partnership” was associated with request for support by doctors according to the PPQ (living situation/in partnership p = 0.01, OR = 0.06, 95% CI: 0.01–0.53). Further, we observed a tendency of patients “on chemotherapy” to wish for greater support; however, this was not significant. Request for support from physicians was not significantly associated with educational level, GHS, sex, WHO grade, Karnofsky-Index, DT score, surgery for recurrent disease and age.
Factors Associated With Current Requested Support by Any Health Care Profession According to the PPQ
With regard to request for support by any health care profession, logistic regression revealed that “living situation/not in partnership” as well as “university degree” were associated with wish for support by any health care profession according to the PPQ; “living situation/in partnership” was protective (p = 0.01, OR = 0.076, 95% CI: 0.10–0.57), whereas having “university degree” posed a higher risk (p = 0.04, OR = 7.86, 95% CI: 1.10–56.08). There was no significant association between requested support from any health care profession and sex, GHS, WHO grade, Karnofsky-Index, on chemotherapy, DT score, surgery for recurrent disease and age.
The Caregivers' Burden
In a subgroup of 96 patients, their caregivers completed the CPQ. Patients' age in this subgroup was 56 years (SD 14.8, range 19–84). Most of the caregivers (64%, n = 61) were life partners.
Caregivers' DT mean was higher than the DT mean of the corresponding patients (caregivers: DT = 6.5, SD = 2.5 vs. patients: DT mean = 5.3, SD = 2.4). Similarly, a DT score ≥6 was reported more frequently by caregivers (55%, n = 53) than patients (47%, n = 37). Simultaneously, according to the CPQ, caregivers were highly burdened: 48% indicated to be anxious, 54% were sad and 70% reported concerns/worries. Practical problems were mostly problems with insurance or financial problems (24%), mobility and transport (38%) and working situation (25%). The changes in relationship (32%) and problems in interactions with spouse or life partner (28%) were frequently reported. Further results are presented in Table 3.
When patients were on chemotherapy, caregivers indicated DT ≥ 6 significantly more frequently than the patients themselves (patients: 33%, n = 13 vs. caregivers: 59%, n = 23, p = 0.02, Fishers' exact test).
Caregivers' Requested Support
Twenty-eight percent (n = 26) of caregivers indicated a moderate and 14% (n = 13) a poor quality of life (mean of all assessments QoL: 4.4, and global health: 4.7 on a Likert scale 1–7). Requests for support came mostly from family (26%), doctors (24%), psychologists (15%), physiotherapists (15%) as well as social service (13%). Table 3 presents results in more detail.
Discussion
In our study, we were able to apply the PPQ to glioma patients and assess support received and needed by patients as well as evaluate the accompanying caregivers by a study-specific questionnaire in a subgroup of patients. We found clinical and demographic factors (e.g., GHS, living situation, and university degree with regard to education) to be associated with higher wish for support, either global or support by a specific profession. Caregivers were even burdened more highly and needed support as well.
Patients and Required Support
In our sample of glioma patients, we observed that the male to female ratio, general conditions expressed by the Karnofsky-Index and the high rate of high-grade gliomas (HGG) represented patients seen by neuro-oncologists in outpatient settings in general. However, as we did not assess the percentage of patients refusing the assessment, we are unable to reflect on the reasons for refusal (e.g., the assessment comprising three questionnaires might not have been well-accepted and probably too demanding). Seibl-Leven et al. reported a relatively high refusal and drop-out rate in their field study (11). Further, in a previous study by our group, we observed that patients refusing an assessment or dropping out of an observational study were more often with recurrent diseases, poorer clinical condition and harbored more often a glioblastoma (30, 18). Therefore, we should take into account that a certain percentage of patients probably with unmet needs may have been missed by our assessment and assume that we observed a selection of glioma patients, leading to a lower generalizability of the results.
In general, high burden and a strong wish for support for certain mental/emotional states (e.g., worries and depression) were indicated by the patients using the PPQ, which was also reflected by the fact that higher DT scores were associated with more frequent requests for support. This is in line with other studies and emphasizes how demanding comprehensive care for glioma patients is (2, 11, 31–34).
Interestingly, 15% of the patients required support by dieticians. While rarely addressed by neuro-oncologists, this aspect should be taken into account when planning supportive care for glioma patients as they frequently suffer from dysphagia in the final phase of the disease (35, 36).
Although many patients suffered from depression, worries and sadness, only 13% were supported by psychologists and 20% by outpatient/palliative care services, and only 19% and 13% required support from these respective professions. One reason for this finding could be that patients felt stigmatized when they experienced psychological problems and hesitated to ask for and accept support. Doctors rarely refer patients proactively for psychosocial support or palliative care timeously. Ideally, the treatment team is multidisciplinary from the very beginning of the disease trajectory. Even if the patients are not healed, this team can still support the patients together (5, 7, 36). This is also strengthened by the fact that according to the findings from the PPQ in our study, many patients and caregivers required general support as well as support from doctors relatively often. As we did not define the specialist disciplines (e.g., neurologist, family doctor or oncologist) in the questionnaire, it remains difficult to interpret this high percentage. It may probably include other disciplines: Patients might hesitate to indicate the need of psychological support; however, they might prefer to indicate support by doctors. The high rate of required support by doctors compared to other disciplines could be further due to the timing of the assessment. All patients were assessed prior to the appointment. Thus, potentially relevant questions were not addressed. Patients participating in this study may have attempted to be a “compliant patient” which may have introduced a certain bias in our study. This is also a possible explanation for the finding that patients rated the support by doctors as “helpful” in part II of the PPQ.
Patients seemed to be not well informed. This is of concern and needs to be considered seriously, and as already reported by other studies on patient-doctor consultations in (neuro-) oncology, improvement in communication skills is required (37). Glioma patients suffer from neurocognitive deficits and comprehension can be impaired (38, 39). Further, due to the lack of data and effective treatment options for patients with recurrent gliomas, patients may feel that they are under-informed and hope for new therapeutic options (40).
Distress, GHS and Request for Clinical Support
We found a correlation of elevated DT, higher burden of patients and request for clinical support. Furthermore, the wish of patients when asked directly and the assessment of attending neuro-oncologists were both associated with request for general support. It is well known that doctors' views are not always congruent with the patients' views with regard to psychosocial distress and unmet needs (2). However, our data show that if there is no time for an extensive psychosocial assessment or patients are unable to complete questionnaires during the routine clinical visit, a higher level of distress directly indicated by the patient on a visual analog scale (which should be feasible for most of the patients) may signal unmet needs. Of note, the doctor in charge has to find out during the consultation the reasons for the distress and the areas of unmet needs. In our opinion, a routinely implemented question on distress during the consultation could draw attention to the patients' problems and initiate support whenever another type of screening (e.g., with questionnaires) is not possible.
Factors Associated with Required Support
We observed several clinical and demographic factors to be associated with required support. As also shown by others, patients in poor general condition (as perceived by themselves and expressed in our study by GHS) require greater support (34, 41).
In times of increasing social isolation, it seems to be an important finding of our study that patients living alone were at risk of higher unmet needs. Attending neuro-oncologists and physicians should consider the social situation of patients in order to initiate support timeously, particularly as patients with gliomas (as well as their families) are at risk for social isolation per se (42, 43).
Interestingly, patients with higher educational level longed for more support by any health care profession than did other patients. Possibly, they may have been better informed than the others or needed further support to deal with information obtained by themselves (e.g., via the internet). Presumably, all patients deal intensively with the poor prognosis, the clinical deficits, the neurocognitive impairment and psychological burden; however, those with higher education verbalize their questions better than the others. Although our analyses have to be regarded as exploratory, we observed similar results as others and the factors could serve as features signalizing patients with unmet needs to the doctors in charge (22, 34).
Caregivers' Burden
As in other studies, the caregivers of our patients reported high distress, even higher than that of the patients (1, 2, 22, 23). Our caregivers were mostly life partners accompanying their relative to the consultation. It is well known that in glioma patients, family problems occur due to role changes, or changes in relationships. Demanding financial situations and practical issues also lead to tremendous burden in caregivers along the disease trajectory—reflected in our data as well (e.g., 70% of the caregivers reported to be worried). Hence, special screening for caregivers is required using instruments such as our questionnaire which was well accepted. When patients are on chemotherapy, neuro-oncologists should take into account that mostly caregivers organize the family life, take care of the patients and are in charge when patients suffer from side effects. In order to relieve caregivers, early integration of palliative care, outpatient services, social services and interdisciplinary treatment (during ongoing tumor-specific therapies) are required (44–47).
Caregivers' Requested Support
Although caregivers reported high burden, only a minority wished for support by any profession. This may be partially due to functional coping strategies [e.g., high expectation of self-efficiency (20)] as well as feelings such as shame and fear about being unable to manage. These hinder caregivers to requesting and/or accepting “external help.” Some patients may also refuse outpatient care services as they do not like to accept the help of people they do not know and wish to stay at home in the final phase as well (36). This could lead to an enormous burden for caregivers when not supported by outpatient palliative care, which is the task of the doctor in charge (mostly physicians and neuro-oncologists) to initiate in a timely and sensitive way (45).
Strengths and Limitations of the Study
Of note, this is the first study that applied the two questionnaires in glioma patients and caregivers simultaneously in a multicenter study, with both questionnaires found to be useful for clinical and research purposes. However, the study does have several limitations.
The study required patients to be fit enough to complete several questionnaires. Therefore, patients in advanced stages of the disease and with cognitive deficits were not included, even though they might be the ones with the highest level of distress and need for supportive care. Shorter assessments are required for such patients. The study was a cross-sectional study and did not investigate the course of needs and distress during the disease trajectory. PPQ and CPQ were not validated instruments; therefore, the results should be interpreted with caution. Furthermore, the patient population was in a heterogeneous disease stage and current treatment. However, this can also represent a certain benefit as the results translate well to the general outpatient population seen at neuro-oncological centers. The content-driven selection of probable influencing clinical and demographic factors with regard to requested support for explorative correlation and logistic regression analyses reduced the statistical strength.
Conclusion
Our data show that glioma patients are highly burdened, and doctors play a crucial role in initiating these patients' psychosocial care and support. The PPQ allows us to evaluate the support requested and perceived by the patients, to detect supportive care needs and provide information at a glance. We observed clinical factors (e.g., when patients live alone in relation to support by doctors, lower GHS with regard to general requested support) and demographic factors (e.g., living alone or higher educational level with regard to support by any profession) that are possibly associated with unmet needs.
Of note, the patients' needs do not always reflect the caregivers' situations. Especially, caregivers of patients on chemotherapy are more burdened than patients themselves. Therefore, either using a questionnaire or questioning during consultation, regular assessment of relatives/caregivers accompanying the patient is required.
Author Contributions
MR contributed to the conception and design of the study and wrote the first draft of the manuscript. DM, HL, EW, and MD assessed the study data and organized the data base. MR and DM developed the questionnaire for caregivers. CW, FR, and SS contributed to the conception and design of the study and edited the manuscript. JC contributed to the conception and design of the study, performed the statistical analyses, and edited the manuscript. All authors contributed to revision of the manuscript, read and approved the submitted version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2018.00763/full#supplementary-material
Acknowledgments
We acknowledge all patients and caregivers for their study participation. Further, we acknowledge members of our study group—Minou Nadji-Ohl, Linda Stöckelmaier, Katrin Nickel, Sonja Grüninger, and Christoph Richter—for their tremendous support.
References
1. Catt S, Chalmers A, Fallowfield L. Psychosocial and supportive-care needs in high-grade glioma. Lancet Oncol. (2008) 9:884–91. doi: 10.1016/S1470-2045(08)70230-4
2. Ford E, Catt S, Chalmers A, Fallowfield L. Systematic review of supportive care needs in patients with primary malignant brain tumors. Neuro Oncol. (2012) 14:392–404. doi: 10.1093/neuonc/nor229
3. Sizoo EM, Pasman HR, Buttolo J, Heimans JJ, Klein M, Deliens L, et al. Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer (2012) 48:226–32. doi: 10.1016/j.ejca.2011.11.010
4. Sizoo EM, Pasman HR, Dirven L, Marosi C, Grisold W, Stockhammer G, et al. The end-of-life phase of high-grade glioma patients: a systematic review. Support Care Cancer (2014) 22:847–57. doi: 10.1007/s00520-013-2088-9
5. Walbert T. Integration of palliative care into the neuro-oncology practice: patterns in the United States. Neurooncol Pract. (2014) 1:3–7. doi: 10.1093/nop/npt004
6. Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of palliative care into standard oncology care: American society of clinical oncology clinical practice guideline update. J Clin Oncol. (2017) 35:96–112. doi: 10.1200/JCO.2016.70.1474
7. Pace A, Dirven L, Koekkoek JAF, Golla H, Fleming J, Ruda R, et al. European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. (2017) 18:e330–e340. doi: 10.1016/S1470-2045(17)30345-5
8. Dirven L, Taphoorn MJ, Reijneveld JC, Blazeby J, Jacobs M, Pusic A, et al. The level of patient-reported outcome reporting in randomised controlled trials of brain tumour patients: a systematic review. Eur J Cancer (2014) 50:2432–48. doi: 10.1016/j.ejca.2014.06.016
9. Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. (2016) 34:557–65. doi: 10.1200/JCO.2015.63.0830
10. Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA (2017) 318:197–8. doi: 10.1001/jama.2017.7156
11. Seibl-Leven M, von Reeken C, Goldbrunner R, Grau S, Ruge MI, Galldiks N, et al. Clinical routine assessment of palliative care symptoms and concerns and caregiver burden in glioblastoma patients: an explorative field study. J Neurooncol. (2018) 138:321–33. doi: 10.1007/s11060-018-2800-1
12. Mitchell AJ, Lord K, Slattery J, Grainger L, Symonds P. How feasible is implementation of distress screening by cancer clinicians in routine clinical care? Cancer (2012) 118:6260–9. doi: 10.1002/cncr.27648
13. Efficace F, Taphoorn M. Methodological issues in designing and reporting health-related quality of life in cancer clinical trials: the challenge of brain cancer studies. J Neurooncol. (2012) 108:221–6. doi: 10.1007/s11060-012-0819-2
14. Peterson JC, Pirraglia PA, Wells MT, Charlson ME. Attrition in longitudinal randomized controlled trials: home visits make a difference. BMC Med Res Methodol. (2012) 12:178. doi: 10.1186/1471-2288-12-178
15. Boele FW, Zant M, Heine EC, Aaronson NK, Taphoorn MJ, Reijneveld JC, et al. The association between cognitive functioning and health-related quality of life in low-grade glioma patients. Neurooncol Pract. (2014) 1:40–6. doi: 10.1093/nop/npu007
16. Renovanz M, Hickmann AK, Coburger J, Kohlmann K, Janko M, Reuter AK, et al. Assessing psychological and supportive care needs in glioma patients - feasibility study on the use of the Supportive Care Needs Survey Short Form (SCNS-SF34-G) and the Supportive Care Needs Survey Screening Tool (SCNS-ST9) in clinical practice. Eur J Cancer Care (Engl). (2016) 27:e12598. doi: 10.1111/ecc.12598
17. Walbert T, Glantz M, Schultz L, Puduvalli VK. Impact of provider level training and gender on the utilization of palliative care and hospice in neuro-oncology: a North-American survey. J Neurooncol. (2016) 126:337–45. doi: 10.1007/s11060-015-1973-0
18. Renovanz M, Hechtner M, Kohlmann K, Janko M, Nadji-Ohl M, Singer S, et al. Compliance with patient-reported outcome assessment in glioma patients: predictors for drop out. Neuro Oncol Pract. (2017) 5:129–38. doi: 10.1093/nop/npx026
19. Piil K, Juhler M, Jakobsen J, Jarden M. Daily life experiences of patients with a high-grade glioma and their caregivers: a longitudinal exploration of rehabilitation and supportive care needs. J Neurosci Nurs. (2015) 47:271–84. doi: 10.1097/JNN.0000000000000158
20. Boele FW, Given CW, Given BA, Donovan HS, Schulz R, Weimer JM, et al. Family caregivers' level of mastery predicts survival of patients with glioblastoma: a preliminary report. Cancer (2017) 123:832–40. doi: 10.1002/cncr.30428
21. Boele FW, van Uden-Kraan CF, Hilverda K, Weimer J, Donovan HS, Drappatz J, et al. Neuro-oncology family caregivers' view on keeping track of care issues using eHealth systems: it's a question of time. J Neurooncol. (2017) 134:157–67. doi: 10.1007/s11060-017-2504-y
22. Halkett GK, Lobb EA, Shaw T, Sinclair MM, Miller L, Hovey E, et al. Distress and psychological morbidity do not reduce over time in carers of patients with high-grade glioma. Support Care Cancer (2017) 25:887–93. doi: 10.1007/s00520-016-3478-6
23. Piil K, Jakobsen J, Christensen KB, Juhler M, Guetterman TC, Fetters MD, et al. Needs and preferences among patients with high-grade glioma and their caregivers-a longitudinal mixed methods study. Eur J Cancer Care (Engl). (2018) 27:e12806. doi: 10.1111/ecc.12806
24. National Comprehensive Cancer Network. Distress management. Clinical practice guidelines. J Natl Compr Canc Netw. (2003) 1:344–374.
25. Goebel S, Mehdorn HM. Measurement of psychological distress in patients with intracranial tumours: the NCCN distress thermometer. J Neurooncol. (2011) 104:357–64. doi: 10.1007/s11060-010-0501-5
26. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. (1993) 85:365–76. doi: 10.1093/jnci/85.5.365
27. Taphoorn MJ, Claassens L, Aaronson NK, Coens C, Mauer M, Osoba D, et al. An international validation study of the EORTC brain cancer module (EORTC QLQ-BN20) for assessing health-related quality of life and symptoms in brain cancer patients. Eur J Cancer (2010) 46:1033–40. doi: 10.1016/j.ejca.2010.01.012
28. Wintner LM, Sztankay M, Aaronson N, Bottomley A, Giesinger JM, Groenvold M, et al. The use of EORTC measures in daily clinical practice-A synopsis of a newly developed manual. Eur J Cancer (2016) 68:73-81. doi: 10.1016/j.ejca.2016.08.024
29. Singer S, Gotze H, Mobius C, Witzigmann H, Kortmann RD, Lehmann A, et al. Quality of care and emotional support from the inpatient cancer patient's perspective. Langenbecks Arch Surg. (2009) 394:723–31. doi: 10.1007/s00423-009-0489-5
30. Renovanz M, Hechtner M, Janko M, Kohlmann K, Coburger J, Nadji-Ohl M, et al. Factors associated with supportive care needs in glioma patients in the neuro-oncological outpatient setting. J Neurooncol. (2017) 133:653–62. doi: 10.1007/s11060-017-2484-y
31. Kvale EA, Murthy R, Taylor R, Lee JY, Nabors LB. Distress and quality of life in primary high-grade brain tumor patients. Support Care Cancer (2009) 17:793–9. doi: 10.1007/s00520-008-0551-9
32. Goebel S, von Harscher M, Mehdorn HM. Comorbid mental disorders and psychosocial distress in patients with brain tumours and their spouses in the early treatment phase. Support Care Cancer (2011) 19:1797–805. doi: 10.1007/s00520-010-1021-8
33. Boele FW, Klein M, Reijneveld JC, Verdonck-de Leeuw IM, Heimans JJ. Symptom management and quality of life in glioma patients. CNS Oncol. (2014) 3:37–47. doi: 10.2217/cns.13.65
34. Halkett GK, Lobb EA, Rogers MM, Shaw T, Long AP, Wheeler HR, et al. Predictors of distress and poorer quality of life in High Grade Glioma patients. Patient Educ Couns. (2015) 98:525–32. doi: 10.1016/j.pec.2015.01.002
35. Koekkoek JA, Dirven L, Sizoo EM, Pasman HR, Heimans JJ, Postma TJ, et al. Symptoms and medication management in the end of life phase of high-grade glioma patients. J Neurooncol. (2014) 120:589–95. doi: 10.1007/s11060-014-1591-2
36. Koekkoek JA, Chang S, Taphoorn MJ. Palliative care at the end-of-life in glioma patients. Handb Clin Neurol. (2016) 134:315–26. doi: 10.1016/B978-0-12-802997-8.00019-0
37. Sterckx W, Coolbrandt A, Clement P, Borgenon S, Decruyenaere M, De Vleeschouwer S, et al. Living with a high-grade glioma: a qualitative study of patients' experiences and care needs. Eur J Oncol Nurs. (2015) 19:383–90. doi: 10.1016/j.ejon.2015.01.003
38. Bosma I, Vos MJ, Heimans JJ, Taphoorn MJ, Aaronson NK, Postma TJ, et al. The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. (2007) 9:53–62. doi: 10.1215/15228517-2006-012
39. Klein M. Neurocognitive functioning in adult WHO grade II gliomas: impact of old and new treatment modalities. Neuro Oncol. (2012) 14(Suppl. 4):iv17–24. doi: 10.1093/neuonc/nos161
40. Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. (2017) 18:e315–29. doi: 10.1016/S1470-2045(17)30194-8
41. Aprile I, Chiesa S, Padua L, Di Blasi C, Arezzo MF, Valentini V, et al. Occurrence and predictors of the fatigue in high-grade Glioma patients. Neurol Sci. (2015) 36:1363–9. doi: 10.1007/s10072-015-2111-7
42. Cavers D, Hacking B, Erridge SE, Kendall M, Morris PG, Murray SA. Social, psychological and existential well-being in patients with glioma and their caregivers: a qualitative study. CMAJ (2012) 184:E373–82. doi: 10.1503/cmaj.111622
43. Cavers D, Hacking B, Erridge SC, Morris PG, Kendall M, Murray SA. Adjustment and support needs of glioma patients and their relatives: serial interviews. Psychooncology (2013) 22:1299–305. doi: 10.1002/pon.3136
44. Sherwood PR, Given BA, Given CW, Schiffman RF, Murman DL, Lovely M, et al. Predictors of distress in caregivers of persons with a primary malignant brain tumor. Res Nurs Health (2006) 29:105–20. doi: 10.1002/nur.20116
45. Flechl B, Ackerl M, Sax C, Oberndorfer S, Calabek B, Sizoo E, et al. The caregivers' perspective on the end-of-life phase of glioblastoma patients. J Neurooncol. (2013) 112:403–11. doi: 10.1007/s11060-013-1069-7
46. Trad W, Koh ES, Daher M, Bailey A, Kastelan M, Legge D, et al. Screening for psychological distress in adult primary brain tumor patients and caregivers: considerations for cancer care coordination. Front Oncol. (2015) 5:203. doi: 10.3389/fonc.2015.00203
47. Seekatz B, Lukasczik M, Lohr M, Ehrmann K, Schuler M, Kessler AF, et al. Screening for symptom burden and supportive needs of patients with glioblastoma and brain metastases and their caregivers in relation to their use of specialized palliative care. Support Care Cancer (2017) 25:2761–70. doi: 10.1007/s00520-017-3687-7
Keywords: supportive care needs, palliative care, glioma, brain tumor, self-assessment
Citation: Renovanz M, Maurer D, Lahr H, Weimann E, Deininger M, Wirtz CR, Ringel F, Singer S and Coburger J (2018) Supportive Care Needs in Glioma Patients and Their Caregivers in Clinical Practice: Results of a Multicenter Cross-Sectional Study. Front. Neurol. 9:763. doi: 10.3389/fneur.2018.00763
Received: 30 March 2018; Accepted: 22 August 2018;
Published: 11 September 2018.
Edited by:
Raymond Voltz, Uniklinik Köln, GermanyReviewed by:
Christoph Stretz, Yale University, United StatesMinjee Kim, Northwestern University, United States
Copyright © 2018 Renovanz, Maurer, Lahr, Weimann, Deininger, Wirtz, Ringel, Singer and Coburger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mirjam Renovanz, mirjam.renovanz@unimedizin-mainz.de
 Mirjam Renovanz
Mirjam Renovanz Dorothea Maurer1
Dorothea Maurer1 Jan Coburger
Jan Coburger