Increasing the Reinforcing Value of Exercise in Overweight Adults
- 1Department of Dietetics and Human Nutrition, University of Kentucky, Lexington, KY, United States
- 2United States Department of Agriculture-Agricultural Research Service (USDA-ARS), Grand Forks Human Nutrition Research Center, Grand Forks, ND, United States
Objectives: This study determined whether a moderate- or high-dose exercise program increases exercise reinforcement. Increasing the relative reinforcing value of exercise (RRVexercise; i.e., incentive sensitization of exercise) may increase the usual physical activity (PA) participation. Preference and/or tolerance for the intensity of exercise was also assessed.
Design: Sedentary men and women (body mass index, BMI: 25–35 kg/m2) were randomized into parallel exercise training groups expending either 300 (n = 18) or 600 (n = 18) kcal/exercise session, five sessions/week, for 12 weeks.
Methods: The RRVexercise was determined by how much work was performed for exercise relative to a sedentary alternative in a progressive ratio schedule task. Preference and tolerance for exercise intensity were determined by questionnaire.
Results: RRVexercise increased (P < 0.05) in both groups. Exercise reinforcement, defined as the amount of work completed for exercise without taking sedentary activity into account, increased (P < 0.01) in the 600 kcal group only. Preference and tolerance for exercise intensity increased (P < 0.01) in both groups, which predicted increases in RRVexercise.
Conclusion: Expending 300 or 600 kcal, 5 days/week increases RRVexercise, while 600 kcal, 5 days/week may be needed to increase exercise reinforcement.
Introduction
Successful adherence to regular exercise remains a challenge for many adults (Tucker et al., 2011; Moore et al., 2012) and there is great interest in the psychological and psychosocial mechanisms influencing exercise participation (Marcus et al., 2000; Marshall and Biddle, 2001; Speck and Harrell, 2003). Cross-sectional work has demonstrated that adults who find aerobic exercise highly reinforcing are more likely to meet physical activity (PA) guidelines for vigorous physical activity (VPA) while those who find resistance-type exercise more reinforcing are more likely to meet recommendations for muscular-strengthening activities and VPA (Flack et al., 2017a). Behavioral Choice Theory provides a framework for understanding the choices people make and how to shift choice from less healthy to healthier alternatives, such as choosing to be physically active rather than sedentary. In the case of PA or exercise, the primary determinants of the choice to exercise over a sedentary behavior include the reinforcing value of exercise relative to other available alternatives (termed relative reinforcing value or RRV) and access to either exercise or alternative sedentary behaviors (Epstein and Roemmich, 2001). If sedentary and physical activities are equally accessible, the difference in reinforcement between sedentary and physical activities determines the choice (Epstein and Roemmich, 2001). The RRV of exercise (RRVexercise) is usually low when assessed against competing sedentary activities (RRVsedendary; Epstein et al., 1999; Roemmich et al., 2008; Barkley et al., 2009), which tend to be less effortful and may be perceived as more pleasant compared to the physical discomfort of exercise. Understanding the determinants of the RRVexercise and how to increase it may offer insight into shifting choice to increase PA adherence, leading to improved health.
Increasing the reinforcing value of exercise relative to sedentary behavior (RRVexercise) should shift choice towards physically active behaviors, increase exercise participation, and result in more Americans meeting PA guidelines. Increasing RRV of a behavior can be accomplished via “incentive sensitization,” originally proposed to explain drug addiction (Robinson and Berridge, 1993). According to incentive sensitization theory, the psychological process of incentive salience transforms the perception of stimuli, increasing their salience within the environment to produce a bias of attentional processing towards the stimuli after repeated exposures (Robinson and Berridge, 1993). This produces neuroadaptations that increase the motivating value of the behavior (Epstein and Roemmich, 2001; Robinson and Berridge, 2008). The result is an increased reinforcing value of the stimulus relative to a competing alternative. Incentive sensitization theory has typically been applied to well-known, highly-reinforcing behaviors, including drug abuse, alcoholism, gambling, and eating (Robinson and Berridge, 1993; Epstein et al., 2007; Temple and Epstein, 2012; Robinson et al., 2016). These highly-reinforcing behaviors are products of the central dopamine system, initiating a dopaminergic response to modulate their reinforcing value (Arias-Carrión et al., 2014). Regulation of exercise behaviors by the central dopamine system is not fully elucidated, but evidence from animal models modifying dopamine transporter and receptor expression to influence PA behaviors points to dopamine playing a major role in voluntary PA (Rhodes and Garland, 2003; Bronikowski et al., 2004). Our group has found similar parallels in humans, where single nucleotide polymorphisms (SNPs) involved in central dopamine signaling and implicated in drug abuse reinforcement also influence RRVexercise (Flack et al., 2019). This offers an explanation as to why exercise dependency has been demonstrated in humans (Chan and Grossman, 1988; Chapman and De Castro, 1990; Belke, 1997; Holden, 2001) and that rodents will respond for exercise (Iversen, 1993; Belke, 1997, 2000; Lett et al., 2000), with some arguing that central dopamine is playing a major role in the choice to be physically active (Knab and Lightfoot, 2010). Although exercise and well-established reinforcing behaviors such as drug abuse may not share identical pathways, evidence mentioned above points to both being at least partially controlled by central dopamine signaling. Therefore, lessons learned from drug abuse or literature from other reinforcing behaviors may help inform us of exercise reinforcement and the process of incentive sensitization for exercise. This was the basis of a recent investigation by our group, demonstrating a low-dose exercise intervention to be ineffective at increasing exercise reinforcement and effective at decreasing sedentary behavior reinforcement, which resulted in less sedentary and more light-intensity PA post-intervention (Flack et al., 2019). Furthermore, this study demonstrated that tolerance to exercise discomfort was related to incentive-sensitization for exercise. It is likely that exercise session parameters such as the dose, duration, or intensity of the exercise exposures are important variables in the process of increasing RRVexercise. Greater duration of exposure is more effective at increasing drug reinforcement (Wolffgramm and Heyne, 1995; Ahmed and Koob, 1998; Heyne and Wolffgramm, 1998; Deroche-Gamonet et al., 2004; Ferrario et al., 2005). Thus, greater duration and volume of exercise completed during an exercise session may be more effective at increasing RRVexercise.
Another factor that may influence exercise behavior and RRVexercise is the preference for and/or tolerance to exercise intensity (Ekkekakis et al., 2008a; Lind et al., 2008; Flack et al., 2017a). Preference for and tolerance to the unpleasant (e.g., muscle pain, breathing hard) aspects of exercise may influence the choice to be physically active and is greater in individuals who meet PA guidelines (Flack et al., 2017a). Individuals with greater tolerance still experience these unpleasant aspects of exercise; however, they are better able to handle them, making it possible for them to find exercise more reinforcing. Preference and tolerance for exercise intensity are associated with the frequency of participation in strenuous exercise and total leisure-time exercise, independent of RRVexercise (Ekkekakis et al., 2008b). Investigations have not yet tested whether the preference or tolerance for exercise intensity can be increased with repeated exposures to exercise or whether increases in preference or tolerance mediate the effects of exercise exposures on the increase in RRVexercise. Understanding the factors that influence the incentive sensitization of exercise would yield valuable information that could be used to design exercise programs that improve aerobic fitness while concurrently increasing RRVexercise and long-term adherence to PA recommendations.
Thus, the purpose of the current study was to determine whether engaging in 12-weeks of moderate-dose (five sessions per week at 300 kcal per session, or 1,500 kcal energy expenditure/week) or high-dose (five sessions per week at 600 kcal per session, or 3,000 kcal/week) exercise training produces incentive-sensitization of RRVexercise and whether increases in preference and tolerance for exercise intensity mediate the effects of exercise dosage on increases in RRVexercise. It was hypothesized that the intervention group participating in 3,000 kcal of exercise per week would realize greater improvements in RRVexercise, which would be mediated by greater increases in preference and tolerance for exercise intensity. The analyses and results presented are secondary outcomes from a study designed to test the compensatory physiological and behavioral responses to increasing exercise energy expenditure.
Materials and Methods
Participants
A total of 36 participants (26 females) between the ages of 18 and 49 years volunteered for the study and were randomized into study groups. Of these, 29 participants completed the study (21 females) with six (five females) participants voluntarily withdrawing citing personal reasons. One participant was dismissed for non-compliance with exercise training, defined as either not adhering to the 600 or 300 kcal expenditure prescription ±100 kcal in 90% of their sessions or not completing at least 18 of the prescribed 20 exercise sessions per month (90% completion rate). All participants were inactive (i.e., exercising less than twice per week) with a body mass index (BMI) ranging from 25 to 35 kg/m2. Recruitment occurred between April and October of 2016 in the greater Grand Forks, North Dakota metropolitan area. Participants were a sample who responded to recruitment media including printed brochures and flyers and online advertisements placed on the Grand Forks Human Nutrition Research Center website. All participants were non-smokers, not dieting to lose weight, and healthy enough to participate in an exercise program assessed by the Physical Activity Readiness Questionnaire (PAR-Q; Thomas et al., 1992). All participants provided consent and the study was approved by the University of North Dakota Institutional Review Board.
Upon completion of baseline assessments, participants were randomized (1:1 allocation ratio), with allocation concealment, into parallel exercise treatment groups expending 300 or 600 kcal per exercise session, 5 days per week. The random allocation sequence was generated using the Plan procedure in SAS with a block size of four and both treatments randomly occurring twice within each block. The study statistician generated and maintained the allocation sequence and concealed the sequence until the participants were enrolled and interventions were assigned. There was no blinding of assignment to interventions. The trial is registered with ClinicalTrials.gov identifier: NCT02152501.
Exercise Intervention
Each participant received a set of personalized heart-rate based exercise sessions designed to expend the assigned energy per session (i.e., 300, 600 kcal/session or 1,500, 3,000 kcal/week) based on individual kcal expenditure rates determined from indirect calorimetry during exercise (explained below). Participants were provided two low-intensity steady-state exercise sessions and three interval-based sessions that were of greater intensity each week. To monitor heart rate and record exercise sessions, each participant received a Garmin Vivofit (Kansas City, KS, USA) for the duration of the 12-week intervention. Participants returned to the lab each week to download their workouts and to receive a new set of exercise sessions that only changed in the amount of time spent at different intensities, always resulting in the assigned energy expenditure. After repeating the incremental fitness test at 6 weeks, the average energy expenditure of each heart rate zone was recalculated so new exercise sessions administered thereafter reflected changes in aerobic fitness. To standardize the exercise environment and to prevent access to fitness facilities from presenting a barrier, participants were provided a 12-week pass to a local fitness center upon beginning the exercise intervention. Missed sessions were made-up on subsequent weeks.
Assessments
Relative Reinforcing Value of Exercise and Liking of Exercise
Participants’ reinforcing value of exercise and sedentary behaviors were assessed using their most liked exercise ergometer (elliptical, treadmill, bicycle) or running/walking on an indoor track and their most liked sedentary behavior out of the options presented (watching TV, playing video games, reading magazines, doing crossword puzzles or Sudoku). The RRVexercise was calculated by comparing their reinforcing value of exercise to their reinforcing value of sedentary behaviors (explained below). Participants rated how much they liked each exercise and sedentary activity on a 1–10 scale at baseline and post, with changes in liking calculated by subtracting the baseline score from the post score. The highest liked exercise and sedentary option were used in the reinforcement task. Reinforcing value was determined by measuring the number of responses participants made for exercise or sedentary activities on progressive variable ratio schedules of reinforcement. The task measures how much individuals want to engage in each behavior, a separate construct from liking (Bickel et al., 2000; Epstein et al., 2011; Casperson et al., 2017). The testing environment included two workstations with computers in the same room. One computer was set up for participants to earn points for their highest-liked exercises while the other for their highest-liked sedentary activity. Participants could switch between stations as much as they chose. Participants were instructed on the use of the computer-generated task they engaged in to earn points (equivalent to minutes) toward their most wanted exercise or sedentary activity, or both. The computer task presented a game that mimicked a slot machine; a point was earned each time the shapes matched. After earning five points, a schedule was completed and the participant received 5 min of exercise or 5 min of sedentary activity time depending on what was earned. The game was performed until the participant no longer wished to work for access to either behavior. The schedules of reinforcement were progressive variable ratio (±5%) schedules whereas points were delivered after every four presses initially, but then the schedule of reinforcement doubled [4, 8, 16, 32, (…) 1,024] each time five points were earned (Bickel et al., 2000; Epstein et al., 2011). Participants were awarded the time they earned for each activity after completing the game. The test was conducted in a separate lab within a large fitness center with access to exercise equipment if participants earned exercise time. Sedentary activity time was spent inside the lab, which had televisions and couches. Outcome measures included the breakpoint, or Pmax (Bickel et al., 2000), which was the last schedule of reinforcement (i.e., 4, 8, 16…) completed for the behavior (exercise or sedentary activity) and RRVexercise, which was calculated as [Pmax exercise/(Pmax sedentary + Pmax exercise)] assessed at baseline and post. Outcomes, therefore, include exercise reinforcement (Pmax exercise), sedentary behavior reinforcement (Pmax sedentary), and the RRVexercise, which takes both of these constructs into account and can be increased by reducing sedentary reinforcement or increasing exercise reinforcement.
Preference and Tolerance for Exercise Intensity
Participants’ preference and tolerance for exercise intensity were measured by a questionnaire. Participants completed the validated Preference for and Tolerance of the Intensity of Exercise Questionnaire (PRETIE-Q, Ekkekakis et al., 2005, 2008b) at baseline and post. The PRETIE-Q is an 18-item survey with nine items assessing preference for exercise intensity (i.e., “I would rather work out at low-intensity levels for a long duration than at high-intensity levels for a short duration”) and nine items assessing tolerance for exercise intensity (i.e., “Feeling tired during exercise is my signal to stop or slow down”). These items are summed for each category to produce separate scores for preference for exercise intensity and tolerance for exercise intensity. Summing scores for preference and tolerance results in the “preference and tolerance for exercise intensity” score.
Exercise Energy Expenditure
A graded exercise treadmill test was used to determine each participant’s rate of energy expenditure at four different heart-rate zones based on the heart-rate reserve (HRR) method. Resting and exercise heart rate were measured using a Garmin vivofit, which included a chest-strap heart rate monitor similar to a Polar device. Oxygen consumed and expired CO2 were analyzed by indirect calorimetry (Oxycon Mobile, CareFusion). Upon completion of a 5-min warm-up walking at 0% grade, 3.0 mph, the treadmill grade increased to 2.5% for 3 min. The treadmill grade was then increased every 3 min to produce an approximate 10 beat per minute increase in heart rate from the previous stage with the speed fixed at 3.0 mph. The test continued until a heart rate of 85% HRR was attained or the participant felt they could no longer continue. Rates of energy expenditure (kcal per minute) at different heart rate zones were calculated from the amount of oxygen consumed and CO2 expired using the Weir equation (Weir, 1949) and regressed against heart rate. Energy expenditure was then averaged across each heart rate zone for the determination of energy expenditure per minute per zone for each individual. Heart rate zones (Marcus et al., 2000; Speck and Harrell, 2003; Tucker et al., 2011; Moore et al., 2012) were calculated using HRR as (220-age)-resting HR * zone % + resting HR (Swain et al., 1998). Heart rate zone 1 ranged from 45 to 55%, zone 2 corresponded to 56–65%, zone 3 was 66–75% and zone 4 was 76–85%. Exercise sessions for each participant were prescribed by calculating the amount of time in each zone, or combinations of zones, that would achieve the appropriate energy expenditure (either 300 or 600 kcal). The treadmill test was repeated after 6 weeks to adjust the intensity and duration of the exercise training sessions to account for improvements in aerobic fitness.
Anthropometrics, Body Composition
Height was measured in triplicate to the nearest 0.1 cm using a stadiometer (Seca, Chino, CA, USA). Bodyweight was measured using a calibrated digital scale (Fairbanks Scales- Model SCB-R9000-HS; Kansas City, MO, USA) to the nearest 0.1 kg. Measures were completed with participants wearing either provided lab scrubs or light casual clothes (t-shirt, shorts) and not wearing shoes. Body composition was measured using a GE Lunar iDXA machine prior to the exercise test on the same visit. The iDXA technique allows the non-invasive assessment of soft tissue composition by region with a precision of 1–3% (Rothney et al., 2012). A total body scan was conducted with participants lying supine on the table and arms positioned to the side. Most scans were completed using the thick mode suggested by the software, as participants were overweight to obese. All scans were analyzed using GE Lunar enCORE Software (13.60.033). Automatic edge detection was used for scan analyses. The machine was calibrated before each scanning session using the GE Lunar calibration phantom.
Analytic Plan
Differences in the pre-post changes in Pmaxexercise, Pmaxsed, RRVexercise, preference, tolerance, and preference + tolerance for exercise intensity were assessed between groups (300 or 600 kcal/session) and if changes were different from zero using analysis of covariance, covarying for baseline values. Sex, age, and percent body fat were considered as additional covariates, but were not significant predictors of any of the outcomes and were not included in the final models. The models were fit with the Glimmix procedure in SAS (SAS Institute Inc., Cary, NC, USA). The Glimmix procedure can be used to fit generalized linear models using a variety of statistical distributions. The beta distribution was used to model RRV, whose values range from 0 to 1, and is therefore not normally distributed. Regression analyses were performed to determine predictors of changes in RRV and Pmax, considering changes in liking (hedonic value) of exercise or changes in preference and tolerance for exercise intensity while covarying for the corresponding baseline value of the predictor and outcome. Although there were no baseline differences between groups for any outcome, covarying for the corresponding baseline value is regarded as the best practice for clinical trials as several other factors can influence results when not covarying for baseline values (Guideline on Adjustment for Baseline Covariates in Clinical Trials, 2015).
Results
Baseline group data are presented in Table 1. As shown in Table 2 and Figure 1, RRVexercise for both the 600 kcal/session and 300 kcal/session groups increased (P < 0.05) after the 12-week intervention. Both groups saw similar decreases (P < 0.01) for Pmaxsed, while only the 600 kcal group increased (P < 0.01) their Pmaxexercise. Both groups saw similar increases (P < 0.05) in tolerance and preference + tolerance for exercise intensity after the 12-week intervention while only the 600 kcal group increased (P < 0.05) preference for exercise intensity. Changes in RRVexercise were predicted (R2 = 0.70, P < 0.01) by changes in preference and tolerance for exercise intensity (β = 0.32, semi partial R2 = 0.06, P < 0.05) when covarying for baseline RRV (β = −0.98, semi partial R2 = 0.57, P < 0.01) and baseline preference and tolerance for exercise intensity (β = 0.51, semi partial R2 = 0.08, P < 0.01). Subjective ratings of liking of exercise were not correlated with RRVexercise at baseline (r = 0.22, P = 0.22) or post (r = 0.27, P = 0.15). The change in liking of exercise also was not correlated (r = −0.01, P = 0.97) with a change in RRVexercise. Covarying for baseline liking scores did not change these results. Changes in physiological measures (body composition, resting metabolic rate) have been reported elsewhere (Flack et al., 2018, 2019) and were not significant predictors of RRVexercise, Pmax, or changes in these variables in the present analysis.
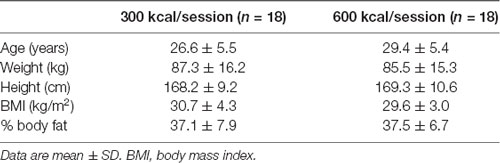
Table 1. Baseline demographic measures of study participants randomized to exercise interventions of expending 300 or 600 kcal per exercise session, 5 days per week, for 12 weeks.
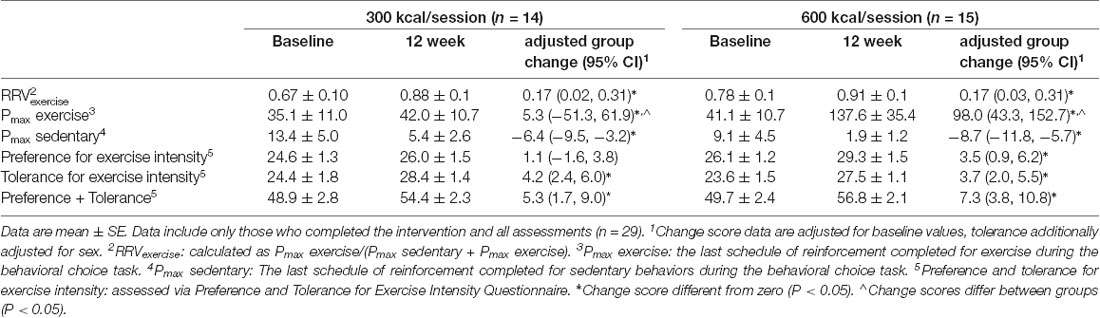
Table 2. Outcome variables at baseline and 12-weeks for participants exercising to expend either 1,500 kcal/week or 3,000 kcal/week for 12 weeks.
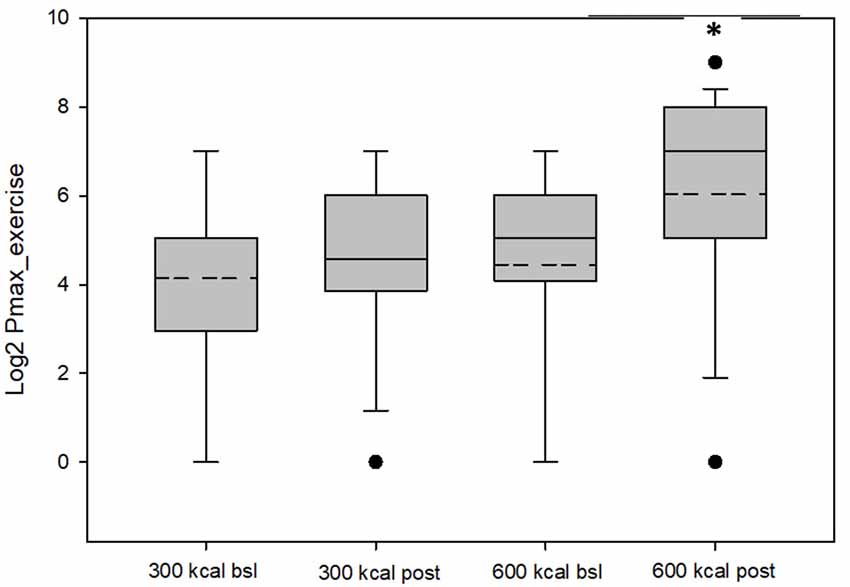
Figure 1. Values for Pmax exercise (log-transformed) for the 300 kcal per session and 600 kcal per session groups at baseline and post-intervention. The dashed lines represent the mean value, the box represents the interquartile range (25th to 75th percentile), solid line represents the median, and black circles represent outliners, which were included in the analysis and did not change overall results when removed. *Mean Pmax greater than baseline (P = 0.05).
Discussion
There is still a great deal to be learned regarding how to prescribe the number of sessions, frequency of sessions, session dose or energy expenditure, session duration, intensity, and pattern (constant load, interval) to optimally increase RRVexercise. Our previous work with a low dose exercise intervention (expending 300 or 150 kcal per session, three times per week for 6 weeks) was not successful in producing greater exercise reinforcement (Flack et al., 2019). Taking guidance from drug abuse literature, exposures to cocaine or amphetamine daily for 10 days (Mendrek et al., 1998) or every 2 days for 20 days (Covington and Miczek, 2001) have been effective at increasing drug reinforcement in rats. Given that drug reinforcement requires dopamine, it is likely that a more frequent or larger total volume of drug use is needed to instigate a dopaminergic response. Based on our and other’s findings noting a similar dopaminergic response to exercise (Rhodes and Garland, 2003; Bronikowski et al., 2004; Knab and Lightfoot, 2010; Flack et al., 2019), we are speculating the sensitization from frequent drug exposures follows a similar pattern in exercise reinforcement, in that more frequent exercise or more total exercise volume (weekly volume/energy expended through exercise) may be an important factor in producing incentive-sensitization of exercise in humans. However, little is known about the neural processes controlling incentive-sensitization for exercise, and it is possible that additional neural mechanisms are in play specific to exercise reinforcement, not following patterns of drug abuse reinforcement. The present results, coupled with our previous work, follow the evidence from drug abuse, indicating that larger doses of structured exercise (5 days per week for 12 weeks) are needed to promote increases in RRVexercise among overweight/obese individuals. RRVexercise increased and responding for sedentary activities decreased in both groups engaging in either 300 or 600 kcal per session, while only the 600 kcal experienced an increase in responding for exercise. Therefore, it appears that 300 kcal (33 min of exercise) per session, 5 days per week is a large enough amount of exercise to increase RRVexercise. This increase is primarily driven by decreasing sedentary reinforcement (Pmaxsed), which is likely to be an important step in becoming more physically active. It is possible that when sedentary people are forced to choose exercise over sedentary behaviors, as in our intervention, their sedentary pursuits become less predominate in their lives and thus lose some of their reinforcing value. However, to instill greater Pmaxexercise, sessions need to be 600 kcal or approximately 56 min per session, 5 days per week.
Exercise increased both preference and tolerance for exercise intensity discomfort in overweight to obese adults, which extends cross-sectional work demonstrating that individuals who meet activity guidelines have greater tolerance to exercise intensity discomfort (Flack et al., 2017a). This is a welcomed result as many overweight/obese individuals find exercise extremely unpleasant and therefore difficult to maintain (Ekkekakis and Lind, 2006). The increases in, tolerance and preference + tolerance for exercise intensity were not dependent on the doses of exercise tested in the present study, although increases in preference for exercise intensity was only observed in the 600 kcal group. The structure of the exercise sessions may have contributed to the improvements in preference/tolerance for exercise discomfort and RRVexercise. The intensity of exercise during the sessions were not self-selected as participants followed prescribed HR based exercise plans that resulted in them meeting their assigned energy expenditure groups. This may have resulted in the participants exercising at a greater intensity and experiencing greater discomfort than if the exercise intensity would have been self-selected (Ekkekakis and Lind, 2006).
Increases in preference and tolerance for exercise intensity predicted increases in RRVexercise when covarying for baseline RRVexercise and baseline preference and tolerance for exercise intensity. The current longitudinal results strengthen and extend previous cross-sectional work that demonstrated preference and tolerance for exercise intensity was positively associated with RRVexercise (Flack et al., 2017a).
It seems reasonable that gaining greater preference and tolerance for exercise intensity associated with exercise is necessary before exercise becomes more reinforcing. Tolerance to the unpleasant aspects of exercise may be more closely associated with the affective responses to exercise than with RRVexercise. Changes in liking of exercise were not correlated with changes in, tolerance in the present study; however, affect during exercise was not assessed. An individual that experiences exercise-induced aches and discomfort would be expected to experience negative affect during exercise if they had low tolerance for exercise discomfort. On the other hand, if the individual had greater tolerance, they may derive pleasure from such exercise even when these unpleasant sensations are present. Therefore, preference and tolerance for exercise intensity and RRVexercise could act via independent neurobiological systems to promote greater usual PA participation. Indeed, greater frontal electroencephalographic asymmetry, specifically greater left frontal activity relative to right activity, predicts positive affect following exercise (Davidson and Irwin, 1999; Davidson, 2000). In contrast, dopaminergic neurons located in the midbrain structures (substantia nigra and ventral tegmental area) control dopamine release and the reward system that mediates the reinforcing value of behaviors such as food and drugs (Arias-Carrión et al., 2014). With RRVexercise and tolerance acting independently on different neurobiological systems, their influence for PA/exercise participation may be distinct. For instance, RRVexercise may shift behavioral choice towards exercise and away from sedentary alternatives, while increasing one’s tolerance to exercise intensity discomfort could result in greater effect during and after exercise. Both would be expected to improve exercise participation, exercise as a habit, and meeting the PA guidelines (Ekkekakis et al., 2005, 2008b; Flack et al., 2017a,b). It must be stated, however, that the current study did not elucidate specific neurobiological pathways and thus it is not certain of the exact mechanism(s) at play for incentive-sensitization of exercise. It is possible that cognitive processes that work separate from central dopamine metabolism are in play (Chatzisarantis et al., 2008). Improved physical fitness may also influence RRVexercise by allowing individuals to exercise at greater intensities with reduced discomfort, or simply repeating bouts of exercise, may help people better psychologically tolerate exercise discomfort and promote greater RRVexercise. Future research may wish to examine if greater tolerance for exercise discomfort derives from other factors related to RRVexercise, such as self-efficacy or intrinsic motivation.
This study is not without limitations. As a secondary analysis of a larger study, a control group was not included. It is, therefore, possible that individuals may have increased their RRVexercise apart from the exercise intervention. Assessments of habitual PA were also not included, which would have provided information on actual behavior change and whether increasing RRVexercise, preference and tolerance for exercise intensity did indeed result in greater usual PA. However, previous research has demonstrated that these factors are associated with engaging in PA to the amount of meeting activity guidelines (Flack et al., 2017a), suggesting that the changes observed in the current study would have positively influenced participants’ PA. Since participants volunteered for this study, they may have been more motivated to start exercising than the average sedentary individual, which was indeed observed by the greater baseline Pmax for exercise than for sedentary (i.e., RRVexercise greater than 0.5). Despite this elevated RRVexercise at baseline, individuals still increased RRVexercise after 12 weeks of exercise training. It is likely that greater changes would be observed if less motivated individuals were included (Berntson et al., 1994). Additionally, of the 29 participants who completed the current study, 26 were Caucasian (one American Indian, one multi-racial, and one African American), thus limiting the generalizability to other racial/ethnic groups. Although overweight to obese, these participants were otherwise healthy young adults. It is uncertain whether those with obesity-related comorbidities or older adults can increase RRVexercise and preference/tolerance for exercise discomfort as observed in the current sample. It also may be interesting to compare the normal weight to obese individuals in their ability to increase Pmax or RRVexercise as obesity can alter reward system function (Ziauddeen et al., 2015).
In conclusion, the present study demonstrates that repeated exposures to exercise via a structured, exercise program that expends at least 300 kcal/session, performed 5 days per week, for 12 weeks increases the RRVexercise by decreasing the reinforcing value of sedentary alternatives. However, increases in RRVexercise were observed only at a greater dose of 600 kcal/session. Increases in preference and tolerance for exercise intensity predicted increases in RRVexercise, possibly pointing to a necessary antecedent for incentive-sensitization of RRVexercise to take place. These psychological and behavioral adaptations to strenuous exercise should increase PA behavior. Future studies would benefit from further investigation of exercise program parameters such as the number of sessions, frequency of sessions, session dose or energy expenditure, session duration, intensity, and pattern (constant load, interval) that most effectively improve incentive-sensitization of exercise and the tolerance and preference for exercise intensity.
Data Availability Statement
The Grand Forks Human Nutrition Research Center Data Access Committee, an entity of the United States Department of Agriculture, Agricultural Research Service, can be contacted by interested researchers inquiring about gaining access to all data presented.
Ethics Statement
The studies involving human participants were reviewed and approved by University of North Dakota Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
KF and JR designed the study. KF and KU collected the data. KF, KU and LJ analyzed the data. KF drafted the manuscript with substantial contributions from KU, LJ and JR. All authors have approved the final version.
Funding
This study was funded by the United States Department of Agriculture-Agricultural Research Service (USDA-ARS), and Project 3062-51000-051-00D. Mention of trade names, commercial products, or organizations does not imply endorsement from the U.S. government. USDA is an equal opportunity provider and employer. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Doreen Rolshoven, Jackie Nelson, and student interns for their assistance with the implementation of the protocol, data collection, and data entry.
References
Ahmed, S. H., and Koob, G. F. (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282, 298–300. doi: 10.1126/science.282.5387.298
Arias-Carrión, O., Caraza-Santiago, X., Salgado-Licona, S., Salama, M., Machado, S., Nardi, A. E., et al. (2014). Orquestic regulation of neurotransmitters on reward-seeking behavior. Int. Arch. Med. 7:29. doi: 10.1186/1755-7682-7-29
Barkley, J. E., Epstein, L. H., and Roemmich, J. N. (2009). Reinforcing value of interval and continuous physical activity in children. Physiol. Behav. 98, 31–36. doi: 10.1016/j.physbeh.2009.04.006
Belke, T. W. (1997). Running and responding reinforced by the opportunity to run:x. J. Exp. Anal. Behav. 67, 337–351. doi: 10.1901/jeab.1997.67-337
Belke, T. W. (2000). Studies of wheel-running reinforcement: parameters of Herrnstein’s (1970) response-strength equation vary with schedule order. J. Exp. Anal. Behav. 73, 319–331. doi: 10.1901/jeab.2000.73-319
Berntson, G. G., Uchino, B. N., and Cacioppo, J. T. (1994). Origins of baseline variance and the law of initial values. Psychophysiology 31, 204–210. doi: 10.1111/j.1469-8986.1994.tb01042.x
Bickel, W. K., Marsch, L. A., and Carroll, M. E. (2000). Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology 153, 44–56. doi: 10.1007/s002130000589
Bronikowski, A. M., Rhodes, J. S., Garland, T. Jr., Prolla, T. A., Awad, T. A., and Gammie, S. C. (2004). The evolution of gene expression in mouse hippocampus in response to selective breeding for increased locomotor activity. Evolution 58, 2079–2086. doi: 10.1111/j.0014-3820.2004.tb00491.x
Casperson, S. L., Johnson, L., and Roemmich, J. N. (2017). The relative reinforcing value of sweet versus savory snack foods after consumption of sugar- or non-nutritive sweetened beverages. Appetite 112, 143–149. doi: 10.1016/j.appet.2017.01.028
Chan, C. S., and Grossman, H. Y. (1988). Psychological effects of running loss on consistent runners. Percept. Mot. Skills 66, 875–883. doi: 10.2466/pms.1988.66.3.875
Chapman, C. L., and De Castro, J. M. (1990). Running addiction: measurement and associated psychological characteristics. J. Sports Med. Phys. Fitness 30, 283–290.
Chatzisarantis, N. L. D., Hagger, M. S., and Wang, J. C. K. (2008). An experimental test of cognitive dissonance theory in the domain of physical exercise. J. Appl. Sport Psychol. 20, 97–115. doi: 10.1080/10413200701601482
Covington, H. E., and Miczek, K. A. (2001). Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration "binges". Psychopharmacology 158, 388–398. doi: 10.1007/s002130100858
Davidson, R. J. (2000). Affective style, psychopathology and resilience: brain mechanisms and plasticity. Am. Psychol. 55, 1196–1214. doi: 10.1037//0003-066x.55.11.1196
Davidson, R. J., and Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 3, 11–21. doi: 10.1016/s1364-6613(98)01265-0
Deroche-Gamonet, V., Belin, D., and Piazza, P. V. (2004). Evidence for addiction-like behavior in the rat. Science 305, 1014–1017. doi: 10.1126/science.1099020
Ekkekakis, P., Hall, E. E., and Petruzzello, S. J. (2005). Some like it vigorous: measuring individual differences in the preference for and tolerance of exercise intensity. J. Sport Exerc. Psychol. 27, 350–374. doi: 10.1123/jsep.27.3.350
Ekkekakis, P., Hall, E. E., and Petruzzello, S. J. (2008a). The relationship between exercise intensity and affective responses demystified: to crack the 40-year-old nut, replace the 40-year-old nutcracker!. Ann. Behav. Med. 35, 136–149. doi: 10.1007/s12160-008-9025-z
Ekkekakis, P., Thome, J., Petruzzello, S. J., and Hall, E. E. (2008b). The preference for and tolerance of the intensity of exercise questionnaire: a psychometric evaluation among college women. J. Sports Sci. 26, 499–510. doi: 10.1080/02640410701624523
Ekkekakis, P., and Lind, E. (2006). Exercise does not feel the same when you are overweight: the impact of self-selected and imposed intensity on affect and exertion. Int. J. Obes. 30, 652–660. doi: 10.1038/sj.ijo.0803052
Epstein, L. H., Carr, K. A., Lin, H., and Fletcher, K. D. (2011). Food reinforcement, energy intake and macronutrient choice. Am. J. Clin. Nutr. 94, 12–18. doi: 10.3945/ajcn.110.010314
Epstein, L. H., Kilanowski, C. K., Consalvi, A. R., and Paluch, R. A. (1999). Reinforcing value of physical activity as a determinant of child activity level. Health Psychol. 18, 599–603. doi: 10.1037/0278-6133.18.6.599
Epstein, L. H., and Roemmich, J. N. (2001). Reducing sedentary behavior: role in modifying physical activity. Exerc. Sport Sci. Rev. 29, 103–108. doi: 10.1097/00003677-200107000-00003
Epstein, L. H., Temple, J. L., Neaderhiser, B. J., Salis, R. J., Erbe, R. W., and Leddy, J. J. (2007). Food reinforcement, the dopamine D2 receptor genotype and energy intake in obese and nonobese humans. Behav. Neurosci. 121, 877–886. doi: 10.1037/0735-7044.121.5.877
Ferrario, C. R., Gorny, G., Crombag, H. S., Li, Y., Kolb, B., and Robinson, T. E. (2005). Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol. Psychiatry 58, 751–759. doi: 10.1016/j.biopsych.2005.04.046
Flack, K. D., Johnson, L., and Roemmich, J. N. (2017a). Aerobic and resistance exercise reinforcement and discomfort tolerance predict meeting activity guidelines. Physiol. Behav. 170, 32–36. doi: 10.1016/j.physbeh.2016.11.032
Flack, K. D., Johnson, L., and Roemmich, J. N. (2017b). The reinforcing value and liking of resistance training and aerobic exercise as predictors of adult’s physical activity. Physiol. Behav. 179, 284–289. doi: 10.1016/j.physbeh.2017.06.016
Flack, K. D., Ufholz, K., Johnson, L., Fitzgerald, J. S., and Roemmich, J. N. (2018). Energy compensation in response to aerobic exercise training in overweight adults. Am. J. Physiol. Regul. Integr. Comp. Physiol. 315, R619–R626. doi: 10.1152/ajpregu.00071.2018
Flack, K. F., Ufholz, K., Johnson, L., and Roemmich, J. N. (2019). Inducing incentive sensitization of exercise reinforcement among adults who do not regularly exercise-a randomized controlled trial. PLoS One 14:e0216355. doi: 10.1371/journal.pone.0216355
Flack, K., Pankey, C., Ufholz, K., Johnson, L., and Roemmich, J. N. (2019). Genetic variations in the dopamine reward system influence exercise reinforcement and tolerance for exercise intensity. Behav. Brain Res. 375:112148. doi: 10.1016/j.bbr.2019.112148
Guideline on Adjustment for Baseline Covariates in Clinical Trials. (2015). European Medicines Agency 11. Available online at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-adjustment-baseline-covariates-clinical-trials_en.pdf
Heyne, A., and Wolffgramm, J. (1998). The development of addiction to d-amphetamine in an animal model: same principles as for alcohol and opiate. Psychopharmacology 140, 510–518. doi: 10.1007/s002130050796
Holden, C. (2001). Compulsive behaviors: "behavioral’ addictions: do they exist? Science 294, 980–982. doi: 10.1126/science.294.5544.980
Iversen, I. H. (1993). Techniques for establishing schedules with wheel running as reinforcement in rats. J. Exp. Anal. Behav. 60, 219–238. doi: 10.1901/jeab.1993.60-219
Knab, A. M., and Lightfoot, J. T. (2010). Does the difference between physically active and couch potato lie in the dopamine system? Int. J. Biol. Sci. 6, 133–150. doi: 10.7150/ijbs.6.133
Lett, B. T., Grant, V. L., Byrne, M. J., and Koh, M. T. (2000). Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite 34, 87–94. doi: 10.1006/appe.1999.0274
Lind, E., Ekkekakis, P., and Vazou, S. (2008). The affective impact of exercise intensity that slightly exceeds the preferred level: ’Pain’ for no additional ’gain’. J. Health Psychol. 13, 464–468. doi: 10.1177/1359105308088517
Marcus, B. H., Dubbert, P. M., Forsyth, L. H., McKenzie, T. L., Stone, E. J., Dunn, A. L., et al. (2000). Physical activity behavior change: issues in adoption and maintenance. Health Psychol. 19, 32–41. doi: 10.1037/0278-6133.19.suppl1.32
Marshall, S. J., and Biddle, S. J. (2001). The transtheoretical model of behavior change: a meta-analysis of applications to physical activity and exercise. Ann. Behav. Med. 23, 229–246. doi: 10.1207/S15324796ABM2304_2
Mendrek, A., Blaha, C. D., and Phillips, A. G. (1998). Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology 135, 416–422. doi: 10.1007/s002130050530
Moore, L. V., Harris, C. D., Carlson, S. A., Kruger, J., and Fulton, J. E. (2012). Trends in no leisure-time physical activity-United States, 1988–2010. Res. Q Exerc. Sport 83, 587–591. doi: 10.1080/02701367.2012.10599884
Rhodes, J. S., and Garland, T. (2003). Differential sensitivity to acute administration of Ritalin, apomorphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology 167, 242–250. doi: 10.1007/s00213-003-1399-9
Robinson, T. E., and Berridge, K. C. (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Rev. 18, 247–291. doi: 10.1016/0165-0173(93)90013-p
Robinson, T. E., and Berridge, K. C. (2008). Review. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3137–3146. doi: 10.1098/rstb.2008.0093
Robinson, M. J., Fischer, A. M., Ahuja, A., Lesser, E. N., and Maniates, H. (2016). Roles of "wanting" and "liking" in motivating behavior: gambling, food and drug addictions. Curr. Top. Behav. Neurosci. 27, 105–136. doi: 10.1007/7854_2015_387
Roemmich, J. N., Barkley, J. E., Lobarinas, C. L., Foster, J. H., White, T. M., and Epstein, L. H. (2008). Association of liking and reinforcing value with children’s physical activity. Physiol. Behav. 93, 1011–1018. doi: 10.1016/j.physbeh.2008.01.010
Rothney, M. P., Martin, F. P., Xia, Y., Beaumont, M., Davis, C., Ergun, D., et al. (2012). Precision of GE Lunar iDXA for the measurement of total and regional body composition in nonobese adults. J. Clin. Densitom. 15, 399–404. doi: 10.1016/j.jocd.2012.02.009
Speck, B. J., and Harrell, J. S. (2003). Maintaining regular physical activity in women: evidence to date. J. Cardiovasc. Nurs. 18, 282–291; quiz 292–293. doi: 10.1097/00005082-200309000-00007
Swain, D. P., Leutholtz, B. C., King, M. E., Haas, L. A., and Branch, J. D. (1998). Relationship between % heart rate reserve and %VO2 reserve in treadmill exercise. Med. Sci. Sports Exerc. 30, 318–321. doi: 10.1097/00005768-199802000-00022
Temple, J. L., and Epstein, L. H. (2012). Sensitization of food reinforcement is related to weight status and baseline food reinforcement. Int. J. Obes. 36, 1102–1107. doi: 10.1038/ijo.2011.210
Thomas, S., Reading, J., and Shephard, R. J. (1992). Revision of the physical activity readiness questionnaire (PAR-Q). Can. J. Sport Sci. 17, 338–345.
Tucker, J. M., Welk, G. J., and Beyler, N. K. (2011). Physical activity in U.S.: adults compliance with the physical activity guidelines for Americans. Am. J. Prev. Med. 40, 454–461. doi: 10.1016/j.amepre.2010.12.016
Weir, J. B. D. (1949). New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 109, 1–9. doi: 10.1113/jphysiol.1949.sp004363
Wolffgramm, J., and Heyne, A. (1995). From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav. Brain Res. 70, 77–94. doi: 10.1016/0166-4328(95)00131-c
Keywords: exercise, motivation, reward, dopamine, incentive sensitization
Citation: Flack KD, Ufholz K, Johnson L and Roemmich JN (2019) Increasing the Reinforcing Value of Exercise in Overweight Adults. Front. Behav. Neurosci. 13:265. doi: 10.3389/fnbeh.2019.00265
Received: 23 August 2019; Accepted: 20 November 2019;
Published: 03 December 2019.
Edited by:
John D. Salamone, University of Connecticut, United StatesReviewed by:
Jasper Heinsbroek, University of Colorado, United StatesVijay Mohan K. Namboodiri, University of Washington, United States
Copyright © 2019 Flack, Ufholz, Johnson and Roemmich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyle D. Flack, kyle.flack@uky.edu
 Kyle D. Flack
Kyle D. Flack Kelsey Ufholz2
Kelsey Ufholz2