- 1Lübeck Institute of Experimental Dermatology, University of Lübeck, Lübeck, Germany
- 2Azrieli Faculty of Medicine, Bar-Ilan University, Safed, Israel
- 3Unit of Dermatology and Skin Research Laboratory, Barch Padeh Medical Center, Tiberias, Israel
- 4Department of Dermatology, University Hospital Schleswig-Holstein Lübeck, Lübeck, Germany
Identification of risk factors and sequelae of any given disease is of key importance. For common diseases, primary prevention and disease management are based on this knowledge. For orphan diseases, identification of risk factors and sequelae has been challenging. With the advent of large databases, e.g., TriNetX, this can now be addressed. We used TriNetX to identify risk factors and sequelae of epidermolysis bullosa acquisita (EBA), a severe and orphan autoimmune disease. To date, there is only enigmatic information on EBA comorbidity. We recruited 1,344 EBA patients in the Global Collaborative Network of TriNetX. Using the “explore outcomes” function we identified 55 diagnoses with a different prevalence between EBA and no-EBA patients. We next performed propensity-matched, retrospective cohort studies in which we determined the risk of EBA development following any of the identified 55 diseases. Here, 31/55 diseases were identified as risk factors for subsequent EBA. Importantly, the highest risk for EBA were other chronic inflammatory diseases (CID), especially lupus erythematosus and lichen planus. Lastly, we determined the risk to develop any of the identified diseases after EBA diagnosis. Here, 38/55 diseases were identified as sequelae. Notably, EBA patients showed an increased risk for metabolic and cardiovascular disease, and thrombosis. Furthermore, the risk for CIDs, especially lupus erythematosus and lichen planus, was elevated. These insights into risk factors and sequelae of EBA are not only of clinical relevance, e.g., optimizing cardiovascular disease risk, but in addition, point to shared pathogenetic pathways between EBA and other inflammatory diseases.
Introduction
Epidermolysis bullosa acquisita (EBA) is an orphan autoimmune disease that is characterized and caused by autoantibodies targeting type VII collagen (COL7). This short statement is grounded on research that was fronted by Detlef Zillikens: In 2005 his group demonstrated the induction of experimental EBA in mice following the transfer of antibodies targeting COL7 (1). At the same time, similar findings were published by the group of David Woodley (2). With these two landmark publications, the autoimmune pathogenesis of EBA had been firmly established. In parallel to this basic research on EBA, Detlef Zillikens also pursued epidemiological research questions, mainly aimed at the determination of the incidence and prevalence of autoimmune bullous dermatoses, such as EBA. Among other findings, he demonstrated a low EBA incidence and prevalence in Germany (3, 4).
Because EBA is such a rare disease, insights into risk factors and sequelae are sparse. Based on small cohorts, reviews of case reports and case report-series, an association between EBA and inflammatory bowel diseases (IBDs), such as ulcerative colitis (UC) and Crohn’s disease (CD), as well as infectious, cardiovascular, metabolic, malignant, and neurological diseases have been reported (5–12). Whilst no association of EBA with systemic lupus erythematosus (SLE) was observed, antinuclear antibodies (ANAs), a hallmark of SLE, were described to occur at higher frequencies than expected in patients diagnosed with EBA (12). Without exception, these reports are based on case reports, case report series or a meta-analysis of these. Thus, the evidence provided is of rather limited validity. In addition, all previous publications on that topic had reported associations only, with no information of the sequence of events. Hence, insights into the sequence of events are missing.
Understanding risk factors and sequelae of EBA would, however, significantly improve patient outcomes because risk factors could be addressed to prevent disease onset, and screening for diseases that subsequently develop after EBA diagnosis may contribute to their early detection. This is, for example, exemplified in the implementation of screening for metabolic, psychiatric, and cardiovascular comorbidity in psoriasis (13). In the same line, the European League Against Rheumatism (EULAR) recently published recommendations concerning lifestyle behavior to prevent progression of rheumatic diseases. In the context of non-inflammatory diseases, such as cardiovascular diseases, preventive measures to normalize elevated blood pressure or cholesterol are common clinical practice (14, 15). These include smoking cession and weight reduction in obese patients because nicotine dependence and overweight/obesity are known risk factors for the development of rheumatic diseases, being often associated with a more severe disease and a reduced response to treatment (16).
To define the so far enigmatic risk factors and associations of EBA, we assessed the Global Collaborative Network of TriNetX that provides access to anonymized, longitudinal patient data from close to 115 million individuals. First, we determined differences in disease prevalence in patients with EBA to those without. Next, we performed several retrospective cohort studies in which we determined the risk of subsequent EBA development following the diagnosis of any of the diseases found to be different in prevalence between EBA and no-EBA patients. Lastly, we determined the risk to develop any of the identified diseases after the diagnosis of EBA.
Materials and methods
Study design and database
We performed a global population-based study with a propensity-matched retrospective cohort design. First, the Global Collaborative Network of TriNetX was used to compare differences regarding diagnoses of EBA patients (ICD10:L12.3) to those without EBA. For the latter group, individuals presenting for “Encounter for general examination without complaint, suspected or reported diagnosis” (ICD10CM:Z00) that had no diagnosis of EBA (ICD10CM:L12.3) were included. For the analysis, the “explore outcomes” function of TriNetX was used. Next, only diagnoses with a medium difference (2/3 bars) and a prevalence of 5% or more in EBA patients, and those with a high difference (3/3 bars) and a prevalence of 1% or more in EBA patients were considered further. We then performed the following retrospective cohort studies: First, the risk of EBA development following the diagnosis of any of the selected diseases was assessed. This was followed by the determination of development of any of the selected diseases following an EBA diagnosis (Figure 1).
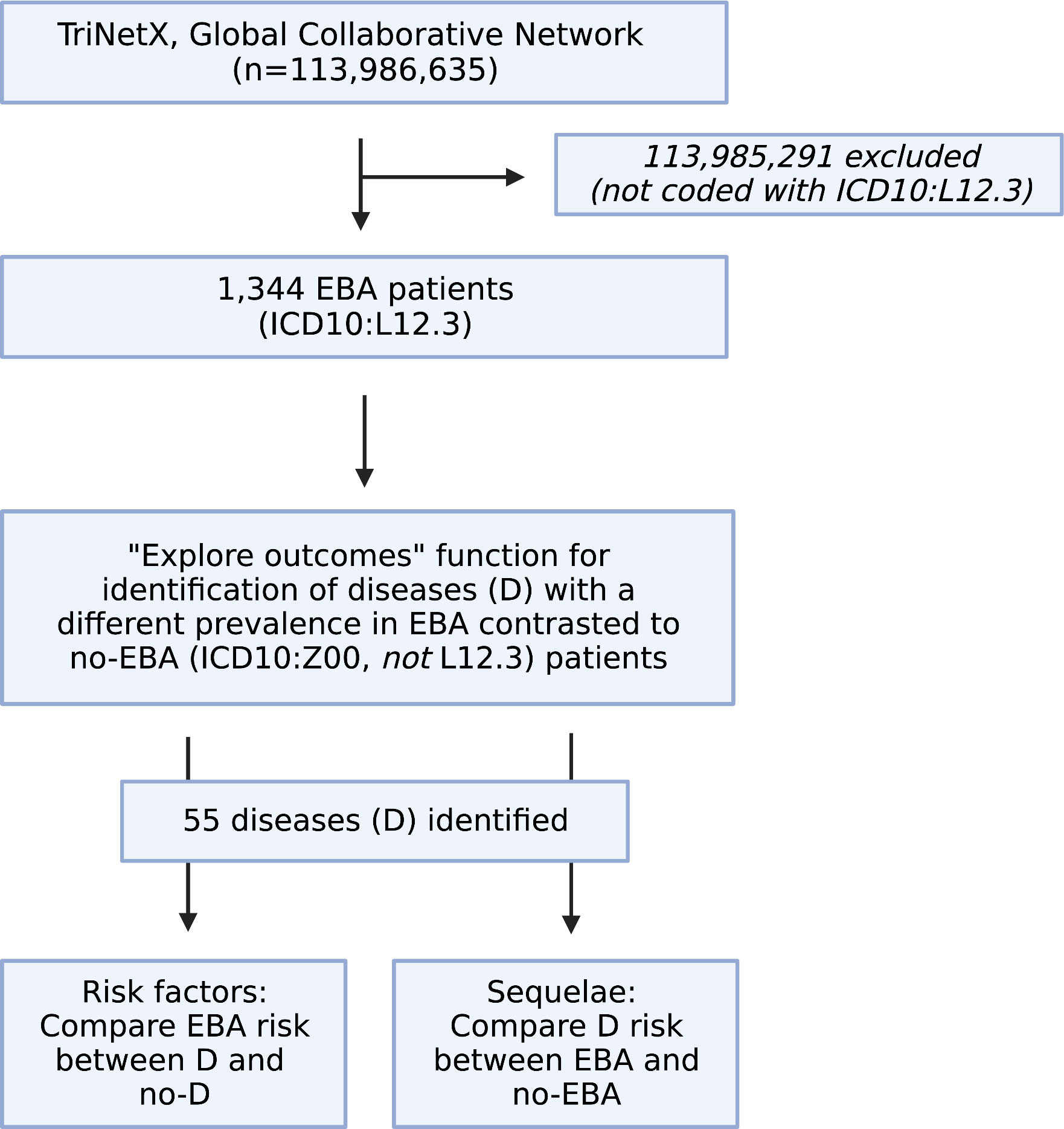
Figure 1 Study flow chart. Within the Global Collaborative Network of TriNetX that encompassed data from close to 115 million patients at the time of access (October 1st, 2022), 1,344 patients with the diagnosis of epidermolysis bullosa acquisita (EBA), defined by ICD10:L12.3 were identified. Patients without a diagnosis of EBA were excluded. Next, the “explore outcomes” function of TriNetX to identify diseases that are different in prevalence (at any given time) between EBA patients (defined by presence of ICD10:L12.3) those without EBA (defined as presence of ICD10:Z00 and absence of ICD10:L12.3). This identified 55 diseases with a different prevalence (at any given time) between EBA and no-EBA patients. Subsequently, 55 propensity-matched studies were performed to explore if any one of the identified diseases had an impact on subsequent EBA development. For this, patients with the disease, i.e., Crohn’s disease (ICD10:K50) were compared to those without the disease, i.g., no-Crohn’s disease (ICD10:Z00 and absence of ICD10:K50). Lastly, a propensity-matched study was performed to delineate the risk of EBA to develop any of the 55 identified diseases. Figure created with BioRender (https://biorender.com).
Study population and definition of eligible patients
The data used in this study was collected between October 1st to 8th, 2022, from the TriNetX Global Collaborative Network, which, at the time of analysis, provided access to electronic medical records (diagnoses, procedures, medications, laboratory values, genomic information) from 113,986,635 million patients from 90 healthcare organizations (HCO). Propensity score matching was performed for the following variables: Age, sex, ethnicity, and race. TriNetX, LLC is compliant with the Health Insurance Portability and Accountability Act (HIPAA), the US federal law which protects the privacy and security of healthcare data, and any additional data privacy regulations applicable to the contributing HCO. TriNetX is certified to the ISO 27001:2013 standard and maintains an Information Security Management System (ISMS) to ensure the protection of the healthcare data it has access to and to meet the requirements of the HIPAA Security Rule, Any data displayed on the TriNetX Platform in aggregate form, or any patient level data provided in a data set generated by the TriNetX Platform, only contains de-identified data as per the de-identification standard defined in Section §164,514(a) of the HIPAA Privacy Rule. The process by which the data is de-identified is attested to through a formal determination by a qualified expert as defined in Section §164,514(b)(1) of the HIPAA Privacy Rule. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, this study was exempted from Institutional Review Board approval.
Statistical analysis
Baseline characteristics were described by means and standard deviations (SDs) for continuous variables, and numbers and percentages for dichotomous variables. Continuous variables were compared using the student t-test, and dichotomous variables by Pearson chi-square test. Survival analyses were conducted by the Kaplan-Meier method. A log-rank test was run to determine if there were differences in the survival distribution for patients in the two investigated groups. Hazard ratios (HR)s for the study outcomes were obtained using the Cox regression model. Nelson-Aalen plots were utilized to test the proportional hazards assumption. Two-tailed P-values less than 0,05 were considered statistically significant.
Results
Cohort description and global results
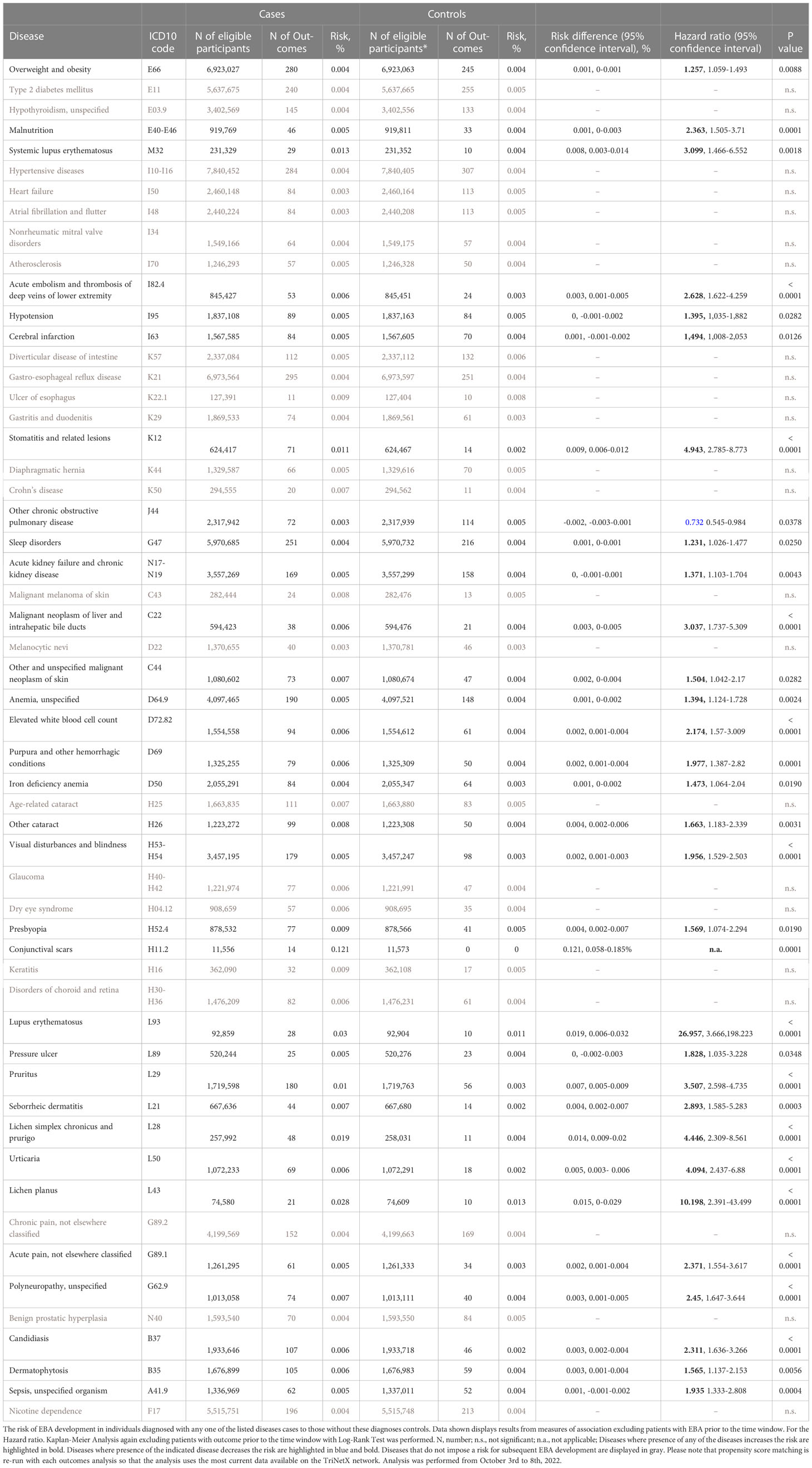
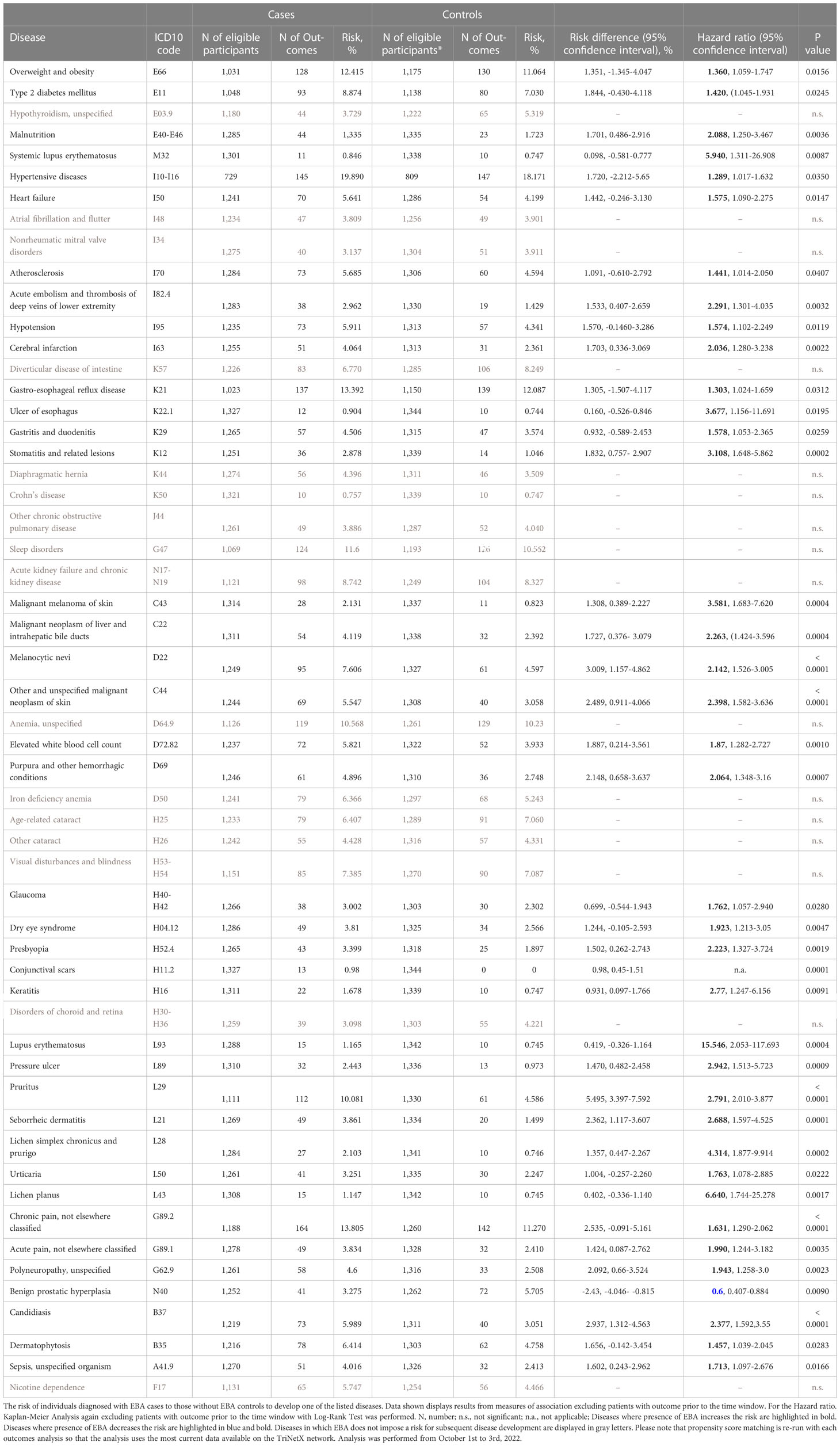
For EBA-risk factors analysis, detailed cohort descriptions and results are displayed in Supplement Tables 1–55 and Table 1. Sample sizes for the risk factor analysis ranged from 11,573 (conjunctival scars) to 7,849,544 (hypertensive diseases), with a median of 1,370,800 (845,473 to 2.337,172, 25/75-percentile). For each disease, propensity matching (age, sex, race, and ethnicity) was performed for better comparability of the groups. Globally, 31/55 diseases were associated with a higher risk of subsequent EBA, 23/55 with no impact on EBA risk, while 1/55 disease led to a reduced risk of subsequent EBA (Figure 2 and Table 1). The corresponding data for the sequalae is found in Supplement Table 56 and Table 2. Overall, we included 1,344 EBA patients (cases) and a similar number of controls. For a better comparability between cases and controls, they were again propensity matched for age, sex, race, and ethnicity. Between groups, no differences regarding these variables were observed. Overall, 37/55 diseases developed more frequently than expected following EBA diagnosis, for 17/55 diseases EBA was not a risk factor, and EBA had a lower risk for 1/55 diseases.
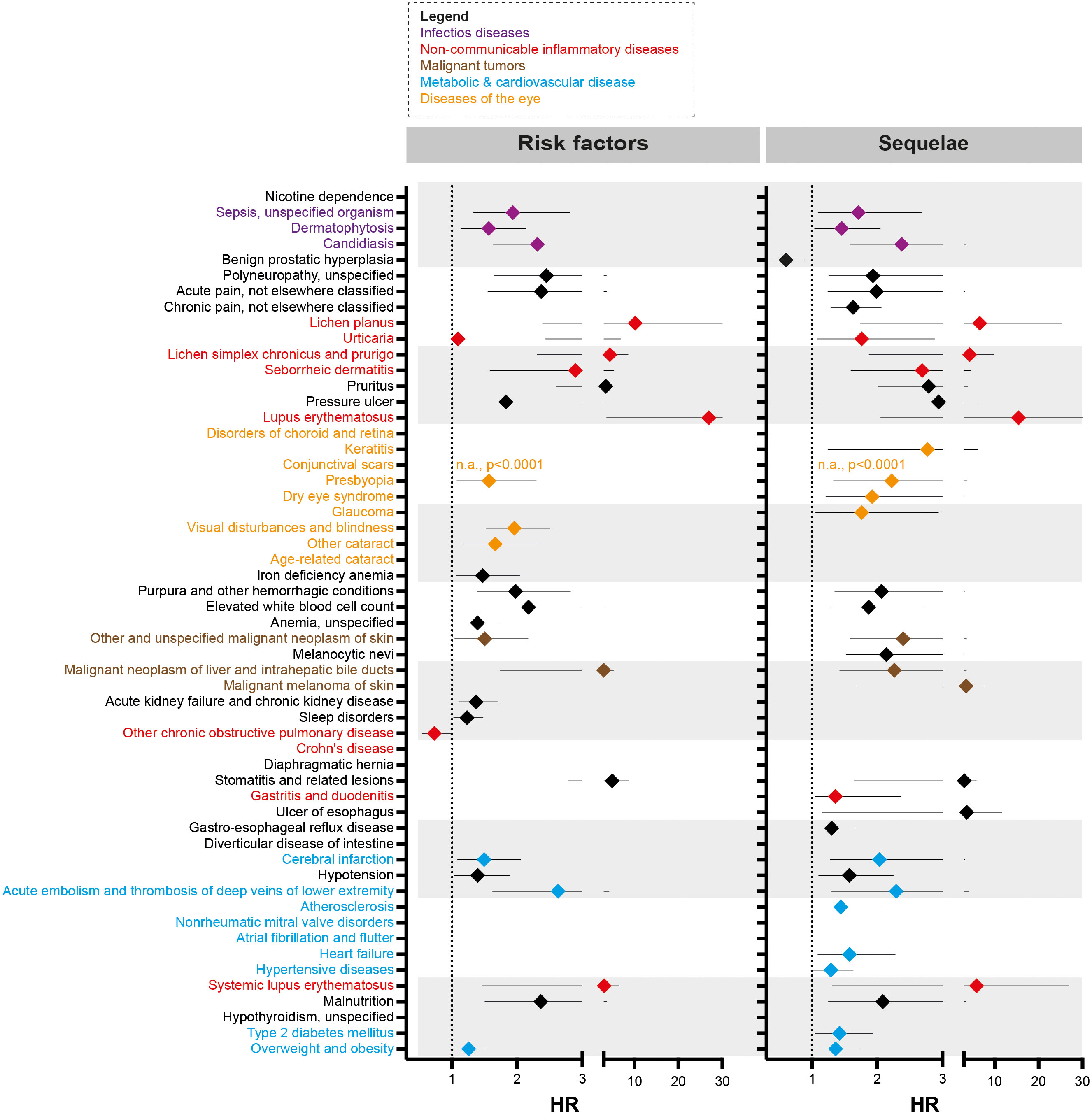
Figure 2 Risk factors and sequelae of epidermolysis bullosa acquisita. Hazard ratio (HR) event plots for the risk factors and sequelae of epidermolysis bullosa acquisita (EBA). Cohort descriptions and detailed results for risk factors are listed in Supplement Tables 1–55 and Table 1, respectively. For risk factors, these are shown in Supplement Table 56 and Table 2. Error bars indicate 5/95-percentiles. If no HR is shown, the indicated disease was not a risk factor / sequela of EBA.
Non-communicable inflammatory diseases are risk factors and sequelae of EBA
A total of 9 non-communicable inflammatory diseases (CID) were amongst the 55 diseases with a different prevalence contrasting EBA to no-EBA patients (Tables 1, 2 and Figure 2). Of these 9 CIDs, 6 were identified as risk factors for EBA. Considering that 23 diseases were identified as risk factors for EBA, CIDs are the most prevalent risk factor for subsequent EBA development. Lichen planus (HR 10.20, CI 2.39-43.50, p<0.0001), cutaneous lupus erythematosus (HR 26.96, CI 3.67-198.22, p<0.0001) and systemic lupus erythematosus (SLE, HR 3.10, CI 1.31-26.91, p=0.0087) were among the disease with the highest risk for future EBA development. Interestingly, “other chronic obstructive pulmonary disease” (COPD) was associated with a decreased risk of subsequent EBA development (HR 0.73, CI 0.55-0.98, p=0.0378).
Likewise, EBA was a risk factor for subsequent CID development. Of the 37 diseases for which EBA is a risk factor, 7 were CIDs. Again, lichen planus (HR 6.64, CI 1.74-25.28, p<0.0017), cutaneous lupus erythematosus (HR 15.55, CI 2.05-117.69, p=0.0004) and SLE (HR 5.94, CI 1.47-6.55, p=0.0018) were among the diseases manifesting following an EBA diagnosis. Albeit not a classical CID, pruritus was found to be both a risk factor (HR 3.51, CI 2.60-4.74, p<0.0001) and a sequel of EBA (HR 2.79, CI 2.01-3.88, p<0.0001).
Of note, inflammatory bowel disease, such as ulcerative colitis and Crohn’s disease, that had been previously assumed to be associated with EBA (12, 17–19) were not identified as risk factors or sequelae of EBA. Ulcerative colitis was not among the 55 diseases with a different prevalence when comparing EBA to no-EBA patients. Crohn’s disease was identified to have a different prevalence in EBA compared to no-EBA patients. However, in neither of the two, propensity matched, case-control studies, Crohn’s disease was identified as a risk factor or a sequela of EBA.
Infectious diseases are risk factors and sequelae of EBA
Sepsis, dermatophytosis and candidiasis were among the 55 diseases with a different prevalence between EBA and no-EBA patients (Tables 1, 2; Figure 2). Given that unspecific immunosuppression is the treatment of choice for EBA (12, 20–22), infectious diseases can be expected as sequelae of EBA. Indeed, sepsis, dermatophytosis and candidiasis were identified as sequelae of EBA. Interestingly, all were also risk factors for future EBA development: Sepsis (HR 1.94, CI 1.33-2.81, p=0.0004), dermatophytosis (HR 1.57, CI 1.14-2.15, p=0.0056) and candidiasis (HR 2.31, CI 1.64-3.27, p<0.0001).
Malignant melanoma, non-melanoma skin cancer and neoplasms of the liver and intrahepatic bile ducts are risk factors and sequelae of EBA
Regarding malignancy, 3/55 identified diagnoses fell into this category; namely, non-melanoma skin cancer, melanoma and malignant neoplasm of the liver and intrahepatic bile ducts. Whilst non-melanoma skin cancer and malignant neoplasm of the liver and intrahepatic bile ducts were both risk factors and sequelae of EBA, melanoma was not associated with a higher risk to develop EBA, but developed more frequently after an EBA diagnosis (Tables 1, 2 and Figure 2).
EBA has a considerable metabolic and cardiovascular disease risk
Metabolic and cardiovascular comorbidity significantly contributes to morbidity and mortality of CIDs (23–25). The potential metabolic and cardiovascular disease burden in EBA has, so far, not been addressed. Following the diagnosis of EBA, cerebral infarction (HR 2.04, CI 1.28-3.24, p=0.0004), deep vein thrombosis (HR 2.29, CI 11.30-4.04, p=0.0032), atherosclerosis (HR 1.44, CI 1.01-2.05, p=0.0407), heart failure (HR 1.58, CI 1.09-2.28, p=0.0147), hypertensive diseases (HR 1.29, CI 1.02-1.62, p=0.0350), type 2 diabetes mellitus (HR 1.42, CI 1.05-1.93, p=0.0245), and overweight & obesity (HR 1.36, CI 1.06-1.76, p=0.0156) developed more frequently than expected.
Discussion
We here determined risk factors and sequelae of EBA using a large-scale database. This led to the identification of so far unrecognized disease trajectories, as well as to the refutation of long-held beliefs, such as an association of inflammatory bowel disease with EBA.
Because EBA is such a rare disease, we believe that implementation of preventive measures based on the identification of risk factors is, for the most part, impracticable. For example, the identified EBA risk factors dermatophytosis and candidiasis are common diseases (26). The effort to screen for EBA in these patient populations would not justify the potential benefit. We, however, see some exceptions. These are pruritus, conjunctival scars, and visual disturbances. If in any of these, a definite cause cannot be determined, EBA should be excluded as a potential differential diagnosis following current diagnostic recommendations (20, 27). By contrast, the identified metabolic and cardiovascular risk profile of EBA patients warrants clinical implementation. Compared to controls, EBA patients were more prone to develop hypertensive diseases, atherosclerosis, cerebral infarction, heart failure, deep vein thrombosis, type 2 diabetes mellitus and overweight & obesity – for some, the risk was increased over 2-fold (Table 2 and Figure 2). This profile is in line with several other CIDs, for example SLE, rheumatoid arthritis and psoriasis (28–30). The finding of an increased frequency of deep vein thrombosis in EBA has also been made in bullous pemphigoid, where a prothrombotic state and an increased risk for venous thromboembolism had been noted (31, 32). Consequently, stringent control of known cardiovascular risk factors, and frequent screening for cardiovascular and metabolic disease should be implemented in the management of EBA patients.
We also found other CIDs to be risk factors and sequelae of EBA. Among these, lichen planus, SLE and cutaneous lupus were associated with the highest risk for both, future EBA development, and the probability of manifestation following an EBA diagnosis. Thus, it is tempting to speculate that EBA, lichen planus, SLE and cutaneous lupus share similar disease-driving pathomechanisms. These findings are also in line with previous clinical observations that noted a high prevalence of antinuclear autoantibodies in EBA patients (12, 33), and the co-occurrence of SLE with EBA, which coined the term bullous SLE (34–36).
Interestingly, inflammatory bowel diseases were not identified as risk factors or sequelae of EBA. An association of EBA with these had long been noted, with several subsequent publications supporting this assumption (5, 12, 17–19, 37). However, most of these observations were made before the establishment of the current diagnostic EBA criteria. Furthermore, these observations were based on single case reports, case report series and meta-analysis thereof. Considering the findings from our study, an association of EBA with inflammatory bowel disease seems rather unlikely.
Regarding malignancy, we think the increased risk of EBA patients to develop non-melanoma skin cancer and melanoma is due to regular and prolonged dermatological care. This is certainly not the case for malignant neoplasms of the liver and intrahepatic bile ducts, which were identified as a risk factor (HR 3.07, CI 1.74-5.31, p<0.0001) and a sequela of EBA (2.26, CI 1.42-3.60, p=0.0004). This necessitates regular screening for this malignancy, underscoring the need for a multidisciplinary care for EBA patients (38). The recently noted expression of COL7 in the liver (of mice) may explain this so far unnoticed association (39) – albeit this finding is not sustained by other reports (40). In the human protein atlas, COL7A1 expression has, however, been reported for the gallbladder – and to a minor extent also in the liver. Thus, autoantibody-induced inflammation in the bile ducts may promote the emergence of malignant neoplasms of the liver and intrahepatic bile ducts.
Our study has several limitations to be acknowledged. First, patient electronic health record data may suffer from misdiagnosis and/or miscoding and do not encompass all possible confounding factors. In our dataset this is underscored by the diagnosis of benign prostatic hyperplasia, where close to 0.5% of individuals with this diagnosis were coded to be female (Supplement Table 51). Second, the TriNetX database provides access to medical data from individuals who had medical encounters with healthcare systems. Thus, our analysis does not include patients with low access to healthcare facilities. Third, in the risk factor analysis, the results must be interpreted with caution if the number of EBA cases is low.
In conclusion, the use of TriNetx allowed to define risk factors and sequalae of an orphan disease, here exemplified by EBA. The identified interactions have clinical implications for the management of EBA and point towards shared pathogenic pathways among different CIDs. We envision that the here described methodology will serve as a blueprint to identify risk factors and sequelae for numerus orphan diseases.
This work has only been possible because of Detlef Zillikens. Since 2004, he continuously and methodically developed a research infrastructure for pemphigus and pemphigoid diseases at the University of Lübeck. In doing so, he spread his enthusiasm for pemphigus and pemphigoid research to improve the diagnosis and treatment of patients suffering from these diseases to those around him. All authors decided to come to Lübeck because of Detlef Zillikens. We truly miss him as an inspiring and always motivating mentor and friend, and now seek to continue his mission in pemphigus and pemphigoid.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: KK, RL; Investigation: All authors; Illustrations: AV, KB; Project Administration: RL; Resources: RL; Writing - Original Draft Preparation: RL; Writing - Review and Editing: All authors. All authors contributed to the article and approved the submitted version
Funding
This research was funded by the Cluster of Excellence “Precision Medicine in Chronic Inflammation” (EXC 2167), the Collaborative Research Centre “Pathomechanisms of Antibody-mediated Autoimmunity” (SFB 1526), the Research Training Group “Autoimmune Pre-Disease” (GRK 2633), all from the Deutsche Forschungsgemeinschaft and the Schleswig-Holstein Excellence-Chair Program from the State of Schleswig Holstein.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.1103533/full#supplementary-material
References
1. Sitaru C, Mihai S, Otto C, Chiriac MT, Hausser I, Dotterweich B, et al. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest (2005) 115:870–8. doi: 10.1172/JCI21386
2. Woodley DT, Chang C, Saadat P, Ram R, Liu Z, Chen M. Evidence that anti-type VII collagen antibodies are pathogenic and responsible for the clinical, histological, and immunological features of epidermolysis bullosa acquisita. J Invest Dermatol (2005) 124:958–64. doi: 10.1111/j.0022-202X.2005.23702.x
3. Bertram F, Brocker EB, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in lower franconia, Germany. J Dtsch Dermatol Ges (2009) 7:434–40. doi: 10.1111/j.1610-0387.2008.06976.x
4. Hübner F, Recke A, Zillikens D, Linder R, Schmidt E. Prevalence and age distribution of pemphigus and pemphigoid diseases in Germany. J Invest Dermatol (2016) 136:2495–8. doi: 10.1016/j.jid.2016.07.013
5. Livden JK, Nilsen R, Thunold S, Schjonsby H. Epidermolysis bullosa acquisita and crohn’s disease. Acta Derm Venereol (1978) 58:241–4.
6. Hughes BR, Horne J. Epidermolysis bullosa acquisita and total ulcerative colitis. J R Soc Med (1988) 81:473–5. doi: 10.1177/014107688808100821
7. Zumelzu C, Le Roux-Villet C, Loiseau P, Busson M, Heller M, Aucouturier F, et al. Black patients of African descent and HLA-DRB1*15:03 frequency overrepresented in epidermolysis bullosa acquisita. J Invest Dermatol (2011) 131:2386–93. doi: 10.1038/jid.2011.231
8. Shaw M, McKee PH, Gaminara E, Pearson TC, Evans B, McGibbon DH. Epidermolysis bullosa acquisita associated with chronic lymphatic leukaemia. Clin Exp Dermatol (1985) 10:162–8. doi: 10.1111/j.1365-2230.1985.tb00546.x
9. Etienne A, Ruffieux P, Didierjean L, Saurat JH. [Epidermolysis bullosa acquisita and metastatic cancer of the uterine cervix]. Ann Dermatol Venereol (1998) 125:321–3.
10. Endo Y, Tamura A, Ishikawa O, Miyachi Y, Hashimoto T. Psoriasis vulgaris coexistent with epidermolysis bullosa acquisita. Br J Dermatol (1997) 137:783–6. doi: 10.1111/j.1365-2133.1997.tb01119.x
11. Aractingi S, Bachmeyer C, Prost C, Caux F, Flageul B, Fermand JP. Subepidermal autoimmune bullous skin diseases associated with b-cell lymphoproliferative disorders. Med (Baltimore) (1999) 78:228–35. doi: 10.1097/00005792-199907000-00003
12. Iwata H, Vorobyev A, Koga H, Recke A, Zillikens D, Prost-Squarcioni C, et al. Meta-analysis of the clinical and immunopathological characteristics and treatment outcomes in epidermolysis bullosa acquisita patients. Orphanet J Rare Dis (2018) 13:153. doi: 10.1186/s13023-018-0896-1
13. Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol (2019) 80:1073–113. doi: 10.1016/j.jaad.2018.11.058
14. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the American college of Cardiology/American heart association task force on clinical practice guidelines. Circulation (2019) 140:e596–646. doi: 10.1161/CIR.0000000000000678
15. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet (2016) 388:2532–61. doi: 10.1016/S0140-6736(16)31357-5
16. Gwinnutt JM, Wieczorek M, Balanescu A, Bischoff-Ferrari HA, Boonen A, Cavalli G, et al. EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases. Ann Rheum (2023) 82(1):48–56. doi: 10.1136/annrheumdis-2021-222020
17. Pegum JS, Wright JT. Epidermolysis bullosa acquisita and crohn’s disease. Proc R Soc Med (1973) 66:234.
18. Raab B, Fretzin DF, Bronson DM, Scott MJ, Roenigk HHJ, Medenica M. Epidermolysis bullosa acquisita and inflammatory bowel disease. JAMA (1983) 250:1746–8. doi: 10.1001/jama.1983.03340130064034
19. Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol (2013) 38:225–30. doi: 10.1111/ced.12114
20. Koga H, Prost-Squarcioni C, Iwata H, Jonkman MF, Ludwig RJ, Bieber K. Epidermolysis bullosa acquisita: The 2019 update. Front Med (Lausanne) (2019) 5:362. doi: 10.3389/fmed.2018.00362
21. Kim JH, Kim YH, Kim SC. Epidermolysis bullosa acquisita: A retrospective clinical analysis of 30 cases. Acta Derm Venereol (2011) 91:307–12. doi: 10.2340/00015555-1065
22. Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother (2011) 12:1259–68. doi: 10.1517/14656566.2011.549127
23. Korman NJ. Management of psoriasis as a systemic disease: what is the evidence. Br J Dermatol (2020) 182:840–8. doi: 10.1111/bjd.18245
24. Amin M, Lee EB, Tsai TF, Wu JJ. Psoriasis and Co-morbidity. Acta Derm Venereol (2020) adv00033. doi: 10.2340/00015555-3387
25. Restivo V, Candiloro S, Daidone M, Norrito R, Cataldi M, Minutolo G, et al. Systematic review and meta-analysis of cardiovascular risk in rheumatological disease: Symptomatic and non-symptomatic events in rheumatoid arthritis and systemic lupus erythematosus. Autoimmun Rev (2022) 21:102925. doi: 10.1016/j.autrev.2021.102925
26. Bieber K, Harder M, Ständer S, Boch K, Kridin K, Köhler B, et al. DNA Chip-based diagnosis of onychomycosis and tinea pedis. J Dtsch Dermatol Ges (2022) 20:1112–21. doi: 10.1111/ddg.14819
27. Witte M, Zillikens D, Schmidt E. Diagnosis of autoimmune blistering diseases. Front Med (Lausanne) (2018) 5:296. doi: 10.3389/fmed.2018.00296
28. Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol (2021) 77:1670–80. doi: 10.1016/j.jacc.2021.02.009
29. Fanouriakis A, Tziolos N, Bertsias G, Boumpas DT. Update on the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis (2021) 80:14–25. doi: 10.1136/annrheumdis-2020-218272
30. Figus FA, Piga M, Azzolin I, McConnell R, Iagnocco A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun Rev (2021) 20:102776. doi: 10.1016/j.autrev.2021.102776
31. Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med (2011) 9:1. doi: 10.1186/1741-7015-9-1
32. Marzano AV, Tedeschi A, Polloni I, Crosti C, Cugno M. Prothrombotic state and impaired fibrinolysis in bullous pemphigoid, the most frequent autoimmune blistering disease. Clin Exp Immunol (2013) 171:76–81. doi: 10.1111/j.1365-2249.2012.04674.x
33. Boh E, Roberts LJ, Lieu TS, Gammon WR, Sontheimer RD. Epidermolysis bullosa acquisita preceding the development of systemic lupus erythematosus. 2319019 (1990) 22:587–93. doi: 10.1016/0190-9622(90)70077-U
34. McHenry PM, Dagg JH, Tidman MJ, Lever RS. Epidermolysis bullosa acquisita occurring in association with systemic lupus erythematosus. Clin Exp Dermatol (1993) 18:378–80. doi: 10.1111/j.1365-2230.1993.tb02224.x
35. Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol (2014) 15:517–24. doi: 10.1007/s40257-014-0098-0
36. Buijsrogge JJ, Diercks GF, Pas HH, Jonkman MF. The many faces of epidermolysis bullosa acquisita after serration pattern analysis by direct immunofluorescence microscopy. Br J Dermatol (2011) 165:92–8. doi: 10.1111/j.1365-2133.2011.10346.x
37. Licarete E, Ganz S, Recknagel M, Di Zenzo G, Hashimoto T, Hertl M, et al. Prevalence of collagen VII-specific autoantibodies in patients with autoimmune and inflammatory diseases. BMC Immunol (2012) 13:16. doi: 10.1186/1471-2172-13-16
38. Luke MC, Darling TN, Hsu R, Summers RM, Smith JA, Solomon BI, et al. Mucosal morbidity in patients with epidermolysis bullosa acquisita. Arch Dermatol (1999) 135:954–9. doi: 10.1001/archderm.135.8.954
39. Hundt JE, Iwata H, Pieper M, Pfündl R, Bieber K, Zillikens D, et al. Visualization of autoantibodies and neutrophils in vivo identifies novel checkpoints in autoantibody-induced tissue injury. Sci Rep (2020) 10:4509. doi: 10.1038/s41598-020-60233-w
Keywords: epidermolysis bullosa acquisita, TriNetX, risk factor, sequelae, systemic lupus erythematosus (SLE), inflammatory bowel disease, cardiovascular disease, lichen planus
Citation: Kridin K, Vorobyev A, Papara C, De Luca DA, Bieber K and Ludwig RJ (2023) Risk factors and sequelae of epidermolysis bullosa acquisita: A propensity-matched global study in 1,344 patients. Front. Immunol. 13:1103533. doi: 10.3389/fimmu.2022.1103533
Received: 20 November 2022; Accepted: 28 December 2022;
Published: 26 January 2023.
Edited by:
Yan Yang, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Kelly Nordyke Messingham, University of Iowa, United StatesBranka Marinovic, University Hospital Centre Zagreb, Croatia
Aikaterini Patsatsi, Aristotle University of Thessaloniki, Greece
Copyright © 2023 Kridin, Vorobyev, Papara, De Luca, Bieber and Ludwig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ralf J. Ludwig, ralf.ludwig@uksh.de
 Khalaf Kridin
Khalaf Kridin Artem Vorobyev
Artem Vorobyev Cristian Papara
Cristian Papara David A. De Luca1
David A. De Luca1 Ralf J. Ludwig
Ralf J. Ludwig