Paratonia in Dementia: A Systematic Review
Abstract
Background:
Paratonia is a dementia-induced motor abnormality. Although paratonia affects virtually all people with dementia, it is not well known among clinicians and researchers.
Objective:
The aim of this study was to perform a systematic review of the literature on the definition, pathogenesis, diagnosis, and intervention of paratonia as well as to propose a research agenda for paratonia.
Methods:
In this systematic review, the Embase, PubMed, CINAHL, and Cochrane CENTRAL databases were searched for articles published prior to December 2019. Two independent reviewers performed data extraction and assessed the risk of bias of the studies. The following data were extracted: first author, year of publication, study design, study population, diagnosis, assessment, pathogenesis, therapy and interventions.
Results:
Thirty-five studies met the inclusion criteria and were included. Most studies included in the review mention clinical criteria for paratonia. Additionally, pathogenesis, method of assessment, diagnosis, and paratonia severity as are interventions to address paratonia are also discussed.
Conclusion:
This systematic review outlines what is currently known about paratonia, as well as discusses the preliminary research on the underlying mechanisms of paratonia. Although paratonia has obvious devastating impacts on health and quality of life, the amount of research to date has been limited. In the last decade, there appears to have been increased research on paratonia, which hopefully will increase the momentum to further advance the field.
INTRODUCTION
According to the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM V), dementia is a major neurocognitive disorder that affects 6 distinctive cognitive domains [1]. The effects of dementia on the perceptual-motor domain in particular highlight the fact that motor impairments are correlated with cognitive impairments in dementia. Motor decline in dementia has been demonstrated over decades [2] but still lacks widespread recognition as a major cause of disability and dependency in dementia. Often, movement disorders are present prior to detectable cognitive changes. Studies have shown that gait speed starts declining up to 12 years prior to the development of mild cognitive impairments (MCI) and up to 7 years prior to the clinical onset of dementia [3, 4]. The extent of motor impairment varies depending on the type of dementia [5, 6].
One manifestation of motor abnormalities obser-ved in dementia is paratonia (Fig. 1). Paratonia was first described and observed by Friedlander [7] in 1828 and later observed by Dupré [8] in 1910; they characterized paratonia as “an inability to relax muscles in the setting of cognitive impairment”. In 1927, Kleist [9] observed a hypertonic response to passive movement in late-stage dementia and called it “Gegenhalten” (i.e., counter pull). In 1949, Kral [10] described facilitory paratonia as a form of pathologic assistance (i.e., actively assisting passive movement or involuntary cooperation). Both types of paratonia have been shown to be associated with cognitive impairment or mental disorders [11, 12]. Involuntary resistance to passive movement, also called “oppositional paratonia”, has been estimated to be present in 5% of patients with MCI and in up to 100% of patients with advanced dementia [13, 14]. In advanced dementia, paratonia may lead to fixed postures (contractures). Fixed postures may lead to skin breakdown, infection, and pain upon movement, thereby reducing comfort and quality of life. Some of the consequences of paratonia include difficulties with washing, dressing, feeding, and providing general care to the patient as well as increased caregiver burden and stress [15].
Fig. 1
Typical (fetal) posture of patient with advanced paratonia with flexion and adduction of the extremities.
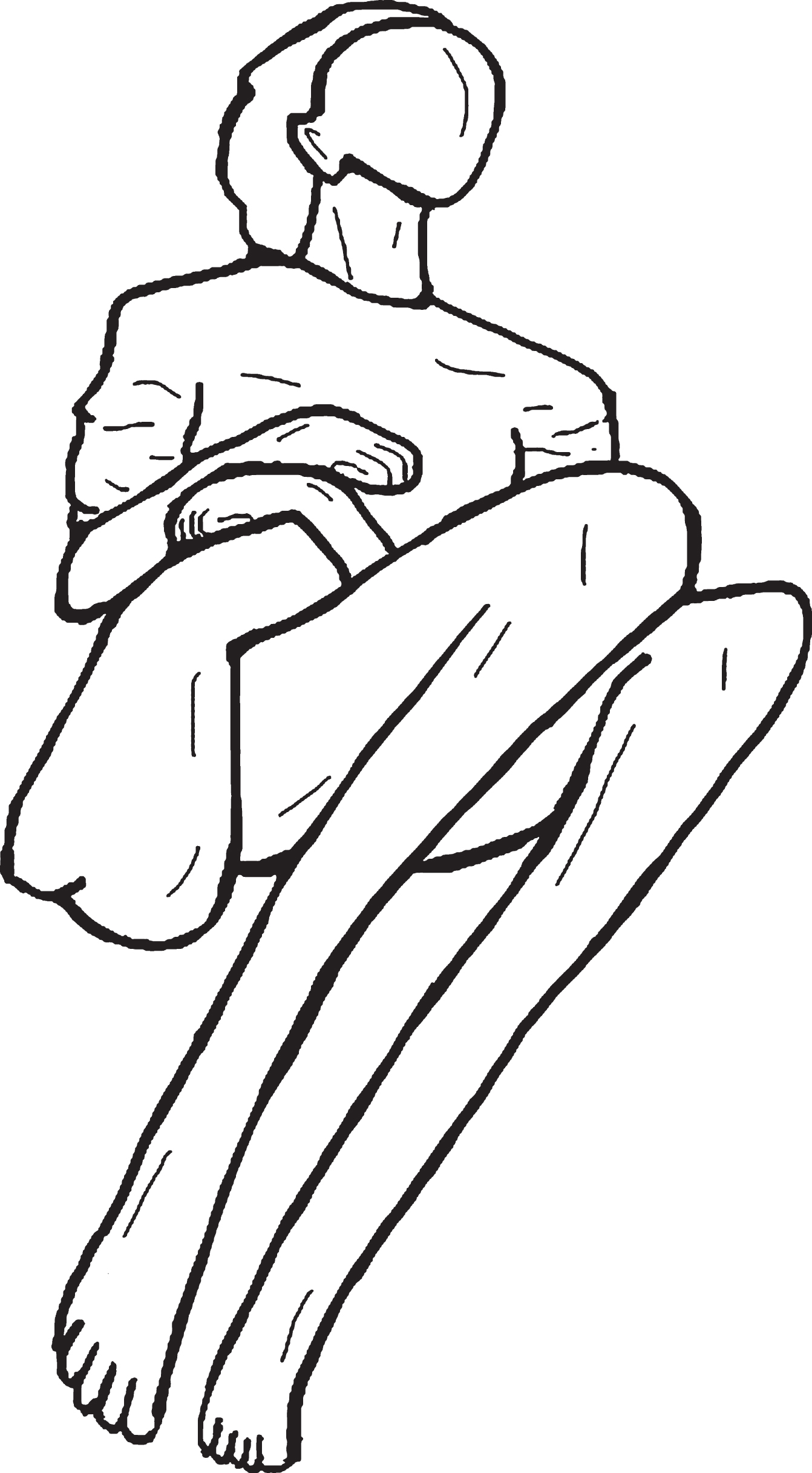
Despite the fact that paratonia eventually affects virtually all people with dementia and leads to devastating health consequences and poor quality of life [16], this condition is not well known among clinicians and researchers. Additionally, research-based data are scarce, and clinical guidelines are lacking.
Therefore, the aim of this study is to perform a systematic review of the literature on the definition, pathogenesis, diagnosis, and intervention of paratonia. With this overview of the existing scientific evidence, we aim to increase the awareness of paratonia in the clinical setting as well as to propose a research agenda on paratonia for future work.
METHODS
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were adopted [17]. A checklist is provided as Supplementary Table 1.
Search strategy
The Embase, PubMed, CINAHL, and Cochrane CENTRAL databases were searched for articles published prior to December 2019. Search terms were both employed as free text words and mapped to subject headings terms with explosion when feasible. We utilized all possible relevant terminology regarding dementia, paratonia/hypertonia and older people using the PICO process [18]. For detailed information on the search terms, see Table 1. Bibliographies from identified articles were reviewed for additional references.
Table 1
Key words regarding dementia, paratonia/hypertonia and older people used in the search string
| Entry | Keywords |
| Dementia | “Dementia” OR “Alzheimer Disease” OR “Alzheimer” OR “Lewy Body Disease” OR “Lewy Bod” OR “Parkinson Disease” OR “Parkinson” OR “Dementia, Vascular” |
| AND | |
| Paratonia/hypertonia | “Muscle Rigidity” OR “Muscle Hypertonia” OR “Paratonia” OR “Gegenhalten” OR “Facilitory Paratonia” OR “Mitgehen” OR “Muscle Rigidit” OR “Counterpull” OR “Motor negativism” OR “Hypertonia” OR “Hypertonie d’opposition” OR “Muscle resistance” OR “Motor resistance” OR Muscle ton* OR “Muscle stiffness” OR “Motor Abnormalities” |
| AND | |
| Older people | “Adult” OR “Middle Aged” OR “Aged” OR “Aged, 80 and over” OR “Frail Elderly” OR “Adult” OR “Elder” OR “Senior” OR “Geriatric” OR “Nursing home” |
Study selection
Initially, abstracts and titles were screened independently by two reviewers using the online program Rayyan [19]. Any disagreements were solved by discussion until a consensus was reached.
Inclusion criteria
All types of study designs and all articles written in English, Dutch, French, or German were eligible for inclusion when paratonia or its synonyms (i.e., Gegenhalten, mitgehen, oppositional paratonia, facilitory paratonia, counterpull, motor negativism, hypertonie d’opposition) were described. Studies were also included if they described a direct relationship between dementia, muscle stiffness, or range of motion (i.e., extrapyramidal signs, muscle rigidity, stiffness, hypertonia, contractures, muscle resistance, motor resistance, variable muscle tone, muscle tone disorder, muscle stiffness, or movement disorder).
Exclusion criteria
Studies related to stroke or Parkinson’s disease (PD), Lewy body dementia, or studies that used the Unified Parkinson Disease Rating Scale (UPDRS) were excluded from the systematic review, as stroke- and PD-related motor impairments (spasticity and Parkinsonian rigidity) have different clinical presentation and other pathophysiological origin than dementia-related motor impairments.
Data extraction and study quality
The data were independently extracted by two reviewers. A standardized data extraction form was utilized in the review of each study, and the following data were extracted: first author, year of publication, study design, study population (age, gender, and type of dementia) and content/context regarding paratonia (i.e., definition and clinical criteria, diagnosis and assessment, pathogenesis, and therapy and interventions). The methodological quality of each study was assessed to determine to what extent each publication addressed the possibility of bias in its design. Critical appraisal tools were used to examine the study design to ensure that the criteria were met [20]. The tools determined whether a criterion was met, whether it was unclear if the criterion was met, or whether that criterion was not applicable. One point was allocated when a criterion was met. The number of points was summed and compared to the maximum number of points possible. If an item was not applicable, the maximum number of points was reduced by one (Table 2). The assessment of bias was performed independently by two reviewers. Disagreements were resolved by discussion until a consensus was reached.
Table 2
Data extraction Table
| First author/year of publication | Design | Population, n = age, gender and dementia type | Definition/Clinical criteria | Diagnosis and assessment | Pathogenesis | Therapy and interventions | Critical appraisal score Items positive/total items |
| Bautmans et al. (2008) [28] | RCT | n = 15, age range: 77–98, male n = 6, severe AD | Form of hypertonia characterized by an active unintentional resistance against passive movement. Based on consensus definition by Hobbelen et al. 2006 [47]. | Presenting paratonia with altered neck posture (cervical antero-position, extension or kyphosis) and known dysphagia. | – | Cervical spine mobilization, caused by postural head and cervical neck changes due to paratonia, to improve swallowing capacity. | 10/13 |
| Benassi et al. (1990) [33] | Cross-sectional | n = 398, age range: 67–87, dementia (n = 27, male n = 14) | Primitive reflex. | Limp placement maneuver, examiner passively lifts the patients arm with the instruction to relax. When the arm stays elevated this indicates paratonia. | Cortical disinhibition sign. | – | 3/7 |
| Bennet et al. (2002) [32] | Cohort, 5.8 years | n = 77, age range: 60–86, male n = 58, VaD | – | – | Frontal system dysfunction. | – | 8/9 |
| Beversdorf and Heilman (1998) [11] | Cross-sectional and test | n = 25, age mean: 70.1(8.7), male n = 9, AD (n = 20), FTD (n = 2), LBD (n = 1), pseudodementia (n = 1), isolated memory impairment (n = 1) | Alteration of tone to passive movement, divided into oppositional paratonia (Gegenhalten) and facilitory paratonia. | Subjective impressions of both assistance and resistance offered to passive flexion and extension about the elbow versus Modified Kral procedure; With this procedure the patient is in seated position. The examiner will flex and extend the right arm between full flexion and 90 degrees of extension three times with the patient instructed to relax completely. At the end of the third cycle, the patient’s arm will be released at the level of the patient’s leg and the examiner’s hand withdrawn. The patient’s response must be scored as follows: 0 = no movement; 1 = right arm flexes, but insufficiently to raise the hand off of the leg; 2 = right arm flexes, lifting the arm off of the leg but less than halfway to full flexion;3 = right arm flexes at least halfway, but not fully; 4 = right arm flexes fully, or repeated cycles of flexion and extension occur. | Frontal lobe dysfunction. | – | 3/7 Score on Test accuracy appraisal; 7/9 |
| Critchley (1956) [22] | Narrative review | – | Gegenhalten. A uniform resistance to passive manipulations, especially in limbs and the neck. Often with a “mental” component, i.e., rigidity may vary directly with the amount of force used by the examiner or the subject is not relaxing. | – | Cerebrovascular degeneration. | – | 5/6 |
| Damasceno et al. (2005) [34] | Cross-sectional | n = 174, AD (n = 30, age: mean 70.9 (9.1), male n = 11 | Primitive reflex, Gegenhalten. | Subjective impressions of resistance to passive, irregular extension and flexion of arms and legs. | Cortico-subcortical neuronal loss. | – | 5/7 |
| Drenth et al. (2017) [40] | Prospective study, 6 months on psychometric properties test | n = 152, age: mean 83.5 (8.2), dementia | Form of hypertonia with an involuntary variable resistance during passive movement. Based on consensus definition by Hobbelen et al. 2006 [47]. | MyotonPro (handheld device objectively measuring muscle properties) versus MAS-P, PAI, passive movement of extremities. Following criteria must be met;-An involuntary variable resistance during passive movement-No clasp-knife phenomenon-Resistance to passive movement is in any direction-Resistance must be felt in either one limb in two movement directions or in two different limbs-Degree of resistance correlates with the speed of movement (e.g., a low resistance to slow movement and a high resistance to fast movement). | Peripheral biomechanical muscle changes due to AGEs. | – | 10/10 |
| Drenth et al. (2017) [30] | Cohort, one-year | n = 144, age: mean 80.7 (7.7), AD or AD/VaD | Form of hypertonia characterized by an active unintentional resistance against passive movement. Based on consensus definition by Hobbelen et al. 2006 [47]. | PAI; passive movement of extremities (see above) MAS-P. | Peripheral biomechanical muscle changes due to AGEs. | Preventing or postponing paratonia by decreasing AGE formation and accumulation through glycemic control (physical activity, diet) | 9/9 |
| Duret et al. (1989) [29] | RCT | n = 14, age: range 56–83, male n = 6, dementia | Rigidity, extrapyramidal sign. | Modifies Columbia rating scale for rigidity. | Lewy bodies, neuronal loss or gliosis in substantia nigra. Rigidity in AD differs pharmacologically from Parkinson rigidity. | – | 11/13 |
| Franssen et al. (1991) [35] | Cross-sectional | n = 135, age: mean 72.6 (9.5), male n = 45, no memory impairment n = 27, mild cognitive impairment n = 20, AD n = 88 | Primitive reflex. Paratonic rigidity defined as stiffening of a limb in response to contact with the examiner’s hand and an involuntary resistance to passive changes in position and posture. | Graded with the amount of passive force necessary to elicit stiffening. | Cortical inhibition loss. | – | 6/7 |
| Franssen et al. (1993) [46] | Case series | n = 56, age: mean 74.6 (9.6), male n = 16, AD | Primitive reflex, paratonic rigidity or Gegenhalten. Defined as a sudden increase in muscle tone of an extremity in reaction to an external perturbation of that extremity, resulting in an involuntary resistance to passive movement. | Resistance elicited with sudden passive changes in position and/or speed and regularity of movement of the upper extremity. | Frontal lobe pathology. | – | 89 |
| Hobbelen et al. (2003) [25] | Pilot RCT | n = 15, age: range 70–9, male n = 1 | Oppositional paratonia a form of hypertonia in late stage dementia. | Middelveld-Jacobs criteria; passive movement of extremities, trunk and head. Following criteria must be met:-Increase in muscle tone during passive movement of the extremities, head or trunk-Occurring at flexion and extension, independent of the starting position of the joints-Increasing with rapid movement-Decreasing slow movement-Ranging from light tovery fierce. MAS-P. | – | - Passive movement therapy-Stabilizing cushions | 11/13 |
| Hobbelen et al. (2006) [47] | Delphi procedure for consensus definition | – | Paratonia is a form of hypertonia with an involuntary variable resistance during passive movement. The nature of paratonia may change with progression of dementia (e.g., from active assistance to active resistance). The degree of resistance depends on the speed of movement.The degree of paratonia is proportional to the amount of force applied and increases with progression of dementia. The resistance to passive movement is in any direction and there is no clasp-knife phenomenon. | – | – | – | 6/6 |
| Hobbelen et al. (2008) [42] | Cross-sectional on psychometric properties test in 3 phases | Phase 1: n = 79, age: range 67–99, male n = 17, AD/VaD (n = 57), VaD (n = 17), LBD (n = 4), other (n = 1)Phase 2: n = 86, age: range 65–96, male n = 26, AD/VaD (n = 60), VaD (n = 22), LBD (n = 2), other (n = 2)Phase 3: n = 24, age: range 78–95, male n = 4, AD/VaD (n = 16), VaD (n = 6), other (n = 1) | Consensus definition Hobbelen et al. 2006 [47]. | PAI passive movement of extremities (see above). | – | – | 6/6 |
| Hobbelen et al. (2011) [14] | Cohort, one year | n = 204, age: mean 79.8 (7.5), male n = 93, AD (n = 91), VaD (n = 50), AD/VaD (n = 36), LBD (n = 14), other (n = 13) | Consensus definition Hobbelen et al. 2006 [47]. | PAI passive movement of extremities (see above). | Unclear-Frontal lobe pathology-Substantia nigra pathology-Peripheral biomechanical changes-Anti psychotic medication may induce paratonia-like rigidity.-Diabetes and vascular damage are risk factors | – | 10/10 |
| Hobbelen et al. (2012) [26] | RCT | n = 102 (intervention n = 48, control n = 54), age: range 67–98, male n = 19, AD (n = 64), VaD (n = 18), AD/VaD (n = 11), LBD (n = 4), other (n = 5) | Consensus definition Hobbelen et al. 2006 [47]. | PAI passive movement of extremities (see above). MAS-P. | – | Passive movement therapy. | 12/13 |
| Jenkyn et al. (1977) [21] | Cross-sectional | n = 76, age: range 16–80, male n = 43, cognitive impaired; mild (n = 25), moderate (n = 23), marked (n = 10), no impairment (n = 18) | Primitive reflex, Gegenhalten. Paratonia can be differentiated from the full range, regular rigidity, or cog-wheeling of Parkinsonism and theclasp-knife phenomenon of spasticity. | Passive extension and flexion of the limbs.An irregular opposition to the examiner’s movementsafter instructions to relax resulted in a ‘catching’sensation is considered abnormal.Limp placement maneuver, examiner passively lifts the patients arm with the instruction to relax. Any delay in dropping of the arm was considered abnormal after Parkinsonian rigidity and spasticity had been excluded. | Cerebral dysfunction, cortical inhibition loss. | – | 7/7 |
| Kleiner-Fisman et al. (2014) [27] | RCT | n = 10 (intervention n = 5, control n = 5 with crossover), age: mean 85.3 (8.0), male n = 1, AD (n = 9), FTD (n = 1) | Increasing muscle resistance reflexively when a limb is moved passively (severity can fluctuate depending on level of relaxation), unto constant muscle contraction in advanced stages. | PAI passive movement of extremities (see above). Severity by joint range of motion. | Originate in the central nervous system. | Botulinum toxin injection | 12/13 |
| Kurlan et al. (2000) [12] | Narrative review/ Viewpoint | – | In paratonic rigidity, there is resistance to passive movement of a limb whereby the degree of resistance varies depending on the speed of movement. Resistance increases when the limb is moved more rapidly and decreases or even disappears when it is moved more slowly. Cogwheeling can occur with parkinsonian or paratonic rigidity and does not help to distinguish the two types of rigidity. The presence of mitgehen suggests that the quality of rigidity is paratonic. | – | – | – | 6/6 |
| Marinelli et al. (2017) [43] | Cross-sectional on psychometric properties test | n = 10, age: range 63–84, male n = 6, MCI (n = 7), AD (n = 1), AD/VaD (n = 1), Fld (n = 1) | Oppositional (Gegenhalten) with resistance to passive movement or facilitory (mitgehen) occurring when the action is in the same direction of passive movement. | Paratonia scale; subjective impressions of both assistance and resistance offered to passive flexion and extension about the elbow.Modified Kral procedure (see above) versus electromyography. | Defective response inhibition associated with frontal lobe lesions. | – | 8/10 |
| O’Keeffe et al. (1996) [36] | Cross-sectional | n = 110, age: mean 78 (5)AD (n = 55, male n = 21) Control (n = 55) | Primitive reflexParatonic rigidity (Gegenhalten). | Presence or absence of Gegenhalten noted during irregular passive extension and flexion of the elbow and wrist joints. | Frontal lobe pathologyExtrapyramidal neural loss. | – | 6/7 |
| Paulson and Gottlieb (1968) [23] | Narrative review | n = 85, age: mean 62.3 (?), dementia | Primitive reflex.Increase in rigidity in both the motor and psychological areas. Paratonic rigidity (Gegenhalten). Marked increase in tone which the examiner may think is voluntary, but usually is not consciously determined.Hypertonus is accentuated by rapid passive movements of the limb and distinguished it from spasticity (clasp-knife) as well as from cogwheel rigidity of Parkinsonism. | – | Central nervous degeneration by age and disease. | – | 5/6 |
| Peralta and Cuesta. (2017) [50] | Narrative review | AD | Gegenhalten. Catatonia like sign. Motor abnormality of severe mental disorder. | – | – | – | 6/6 |
| Pauc and Young (2012) [48] | Narrative review | Dementia | Involuntary resistance to passive movement (Gegenhalten).A primitive reflex occurring naturally in neonates which should disappear as the brain develops. | – | Neuronal loss occurring with normal ageing and the onset of dementia. | – | 3/6 |
| Ries (2018) [49] | Narrative review | AD, VaD, AD/VaD, LBD, PDD, FTD | Consensus definition Hobbelen et al. 2006 [47]. | PAI passive movement of extremities (see above). | – | Facilitating the movement by giving some counterpressure in the direction of the movement that the patient must move into | 6/6 |
| Risse et al. (1990) [31] | Cohort until death | n = 28, male n = 28, (probable) AD | Resistance to movement with increased muscle tone throughout the range of motion while not on antipsychotic drugs. Gegenhalten, patient appears to be actively resisting passive movement in any direction. | – | No neuro-pathologic (substantia nigra neural loss) evidence for Parkinson disease. | – | 4/9 |
| Souren et al. (1997) [16] | Narrative review | AD | Type of rigidity that occurs on contact. Changing resistance to passive alterations in position and posture. The degree of paratonia increases when the patient is repeatedly urged to relax. Can be elicited or augmented by state of anxiety, anger and agitation. | Moving passively an extremity (usually the forearm) repeatedly, first slowly and evenly, and, subsequently in a more rapid, brusque and irregular fashion. | Symptom of congenital or acquired diffuse brain damage.Resembles primitive reflex in healthy infants, therefore can be considered as the return of an infantile stabilization reflex mechanism, parallel to the patient’s motor function decline. | Paratonia serves as a physiologic and psychological stabilizing mechanism, protecting the patient against overwhelming external stimuli. Approach and behavioral advice are given, such as gentle movements, eyes and voice contact to reduce anxiety and agitation.Active physical activity to prevent or postpone contractures. | 6/6 |
| Tyrell and Rossor. (1988) [38] | Cross-sectional | n = 10, age; range 53–82, male n = 2, AD (n = 4), AD/VaD (n = 1), Progressive dyspraxia (n = 3), CVD (n = 2) | Paratonic rigidity (Gegenhalten) an increase in muscle tone which occurs in response to passive movement, proportional in degree to the stimulus applied. | Rigidity detected on passive movement of the limb, occurring in absence of cogwheeling or a spastic catch, that is not exacerbated by movement in the contralateral fist.Limb placement maneuver (see above). | – | – | 3/7 |
| Tyrell et al. (1990) [37] | Cross-sectional | n = 20, age: mean 59.1 (8.5), AD | Extrapyramidal sign, rigidity. | – | No evidence for dopaminergic nigrostriatal dysfunction. | – | 4/7 |
| Van Deun et al. (2016) [41] | Cross-sectional on psychometric properties test | n = 16, age: mean 85.5 (6.8), male n = 3, dementia with paratonia | Consensus definition Hobbelen et al. 2006 [47]. | PAI passive movement of extremities (see above).MAS-P.MyotonPro (handheld device objectively measuring muscle properties). | – | – | 9/10 |
| Van Deun et al. (2018) [45] | Survey | Physiotherapists in nursing homes (n = 242) | Consensus definition Hobbelen et al. 2006 [47]. | – | – | Positioning and soft passive mobilization were most applied.Key points for paratonia approach were relaxation, positioning, active movement stimulation. | 7/9 |
| Van Deun et al. (2018) [39] | Cross-sectional | Healthy control (n = 60, age: mean 82.07 (5.53), male n = 30). Mild dementia (n = 31, age: mean 80.48 (4.87), male n = 15). Moderate dementia (n = 31, age: mean 87.81 (4.94), male n = 3) | Consensus definition Hobbelen et al. 2006 [47]. | PAI passive movement of extremities (see above).MyotonPro (handheld device objectively measuring muscle properties). | – | – | 7/8 |
| Van Deun et al. (2019) [44] | CT with AB/BA crossover | n = 22, age: mean 84.8 (7.3), AD (n = 10), VaD (1), FTD (n = 1), other (n = 10) | Consensus definition Hobbelen et al. 2006 [47]. | PAI passive movement of extremities (see above).MAS-P.MyotonPro (handheld device objectively measuring muscle tone). | – | -Harmonic movement techniques.-Supporting cushions | 6/9 |
| Vahia et al. (2007) [13] | Prevalence study | n = 80, age: mean 75.5 (7.0), male n = 17, AD | External stimulus dependent increase in muscle tone that is absent at rest. | Graded with the amount of passive force necessary to elicit stiffening. | Disorder of frontal lobe origin. | – | 4/9 |
| Villeneuve et al. (1974) [24] | Cross-sectional | n = 155, Organic brain syndrome (including dementia) (n = 56), age: mean 76 (7.2), male n = 56, Functional psychoses (n = 51), age: mean 70.9 (5.8), male n = 33, Schizophrenia (n = 16), age: mean 76.3 (8.8), male n = 0, Healthy controls (n = 32), age: mean 83.2 (4.9), male n = 11 | Primitive reflex. Gegenhalten, a particular form of rigidity, also called negativism or opposition hypertonia.The marked increase in tone is not consciously determined and is accentuated when a rapid movement is imparted to a limb. It is different form spasticity (clasp-knife) and from cogwheel rigidity of Parkinsonism. | Any resistance of the forearm to passive movement from the examiner is considered as a hypertonic manifestation. | Central nervous degeneration by age and disease (frontal lobe, metabolic, vascular cerebral, deep coma) | – | 5/8 |
AD, Alzheimer Disease; AGE, advanced glycation end-products; CVD, cardiovascular disease; FTD, frontotemporal dementia; LBD, Lewy body dementia; MAS-P, Modified Ashworth Scale for paratonia severity; MCI, mild cognitive impairment; PAI, Paratonia Assessment Instrument; PDD, Parkinson’s disease dementia; RCT, randomized controlled trial; VaD, vascular dementia.
RESULTS
Search results and study quality
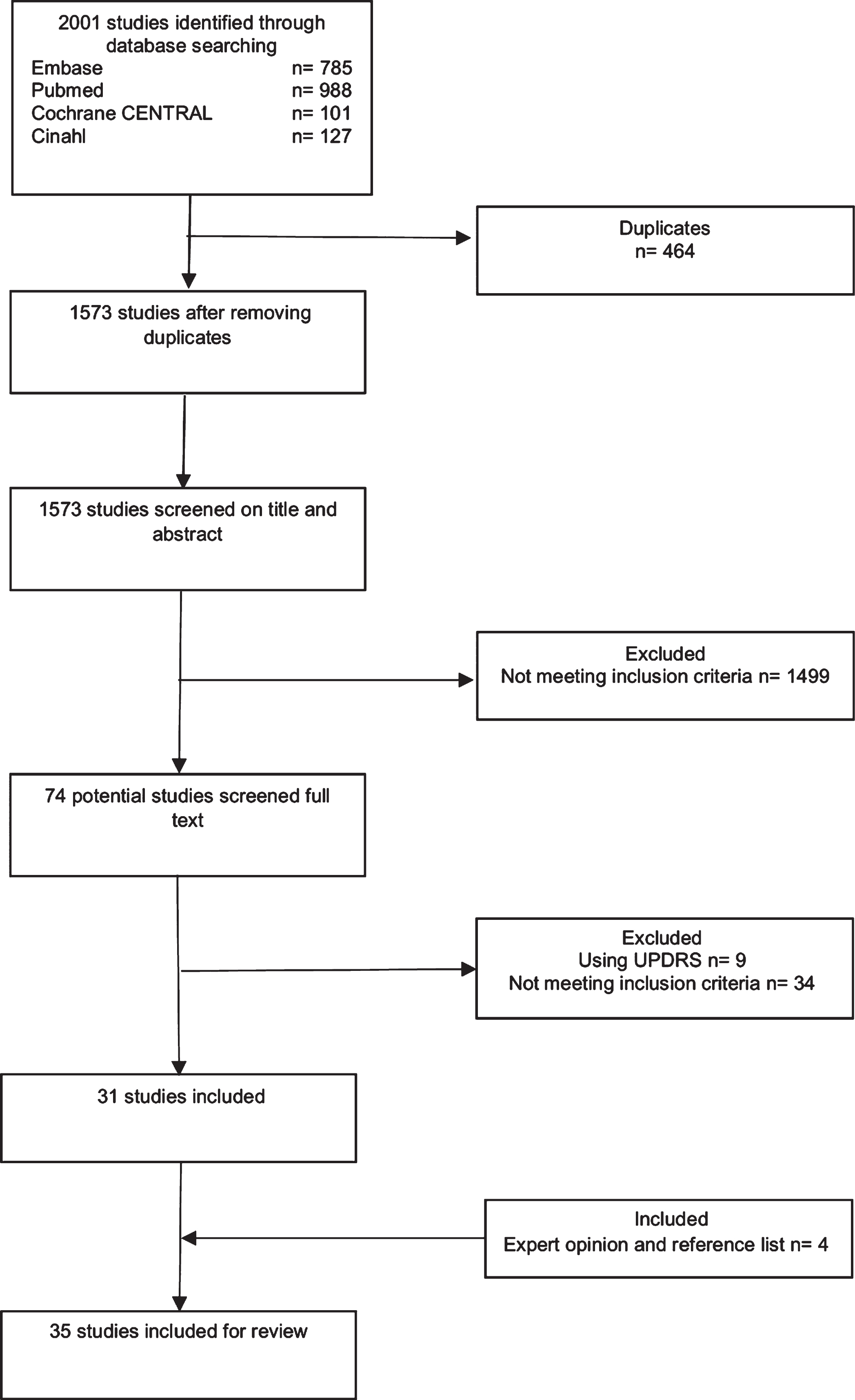
After duplicates were eliminated, 1,573 publications were identified using key words. After an initial screening of titles and abstracts, 1,499 studies were excluded because they did not meet the inclusion criteria, and the full texts of 74 studies were reviewed. An examination of the available reference lists of these 74 studies did not reveal additional studies. Nine studies were excluded because they used the UPDRS, and 34 studies were excluded because they did not meet the inclusion criteria. Additionally, two studies [21, 22] were included on recommendation by external experts and examination of those references yielded in two more studies [23, 24]. Thus, 35 studies were included in this systematic review (Fig. 2 and Table 2). For detailed information on the critical appraisal, see Supplementary Table 2.
Fig. 2
Flowchart of selection process.
Data extraction
The extracted data is summarized in Table 2.
Study design
The study designs included randomized controlled trials (RCTs) [25–29] (n = 5), cohort studies [14, 30–32] (n = 4), cross-sectional studies [11, 21, 24, 33–39] (n = 10), studies on the psychometric properties of measurement instruments [40–43] (n = 4), a clinical trial with crossover [44] (n = 1), a prevalence study [13] (n = 1), a survey [45] (n = 1), a case series [46] (n = 1), a Delphi procedure [47] (n = 1), and narrative review [12, 16, 22, 23,48–50] (n = 7).
Definition and clinical criteria of paratonia
With the exception of one study [32], all studies gave a definition of paratonia and/or described the clinical criteria.
Two studies [22, 38] published a definition of paratonia based on the study by Kleist [9]; they defined paratonia as “an increase in muscle tone which occurs in response to passive movement, proportional in degree to the stimulus applied”. Vahia et al. [13] extended this definition to include that an “external stimulus-dependent increase in muscle tone is absent at rest”.
Two publications [29, 37] reported that dopaminergic nigrostriatal dysfunction was present in PD; the authors further hypothesized that dementia-related rigidity was an “extrapyramidal sign”.
In seven studies [21, 23, 24, 33, 34, 36, 48], the presence of paratonia was described as paratonic rigidity (Gegenhalten) associated with the appearance of other primitive reflexes, such as snout, suck, palmomental, grasp and glabellar reflexes, which are normally present in infancy and disappear as the nervous system matures. Two studies [23, 24] emphasized that this perceived increase in tone is not consciously determined by the patient. Franssen et al. [35] also described paratonic rigidity (Gegenhalten) as a primitive reflex and further defined it as “the stiffening of a limb in response to contact with the examiner’s hand and an involuntary resistance to passive changes in position and posture”. Another study [46] defined paratonia as “a sudden increase in muscle tone of an extremity in reaction to an external perturbation of that extremity, resulting in an involuntary resistance”. The flexed posture with contractures of the extremities, which is seen in the last stage of dementia is described by Paulson and Gottlieb [23] as a related phenomenon to paratonic rigidity, occurring over time after an initial phase of Gegenhalten.
One study [25] defined oppositional paratonia as “a form of hypertonia in late stage dementia”. Two studies [11, 43] stated that paratonia can be divided into two separate entities: “facilitory paratonia”, in which the patient moves along with the examiner (keeping control of the movement, or inability to relax), and “oppositional paratonia”, in which the subject appears to resist the passive movement of the examiner.
Five studies [12, 21, 23, 24, 47] distinguish Parkinsonian rigidity from paratonia by noting that in paratonia the resistance increases when the limb is moved more rapidly and decreases or even disappears when the limb is moved more slowly. Furthermore, Kurlan et al. [12] describes that the presence of “oppositional” paratonia suggests that the quality of rigidity is paratonic and that cogwheeling can occur with parkinsonian or paratonic rigidity and cannot be used to distinguish the two types of rigidity. Four studies [21, 23, 24, 47] state that paratonia is different form spasticity (clasp-knife) and from cogwheel rigidity of Parkinsonism.
Risse et al. [31] further specified that “paratonia is resistance to movement with increased muscle tone throughout the range of motion while not on antipsychotic drugs”. Souren et al. [16] noted that “paratonia occurs on contact and can be elicited or augmented by a state of anxiety, anger and agitation”.
One study [50] described paratonia as “a catatonia like sign and a motor abnormality of severe mental disorder”. Catatonia is a neuropsychiatric psychomotor syndrome characterized not only by a broad range of movement disorders but also by affectivity disturbance and complex behaviors, including the disturbance of will. Paratonia is usually suggested to occur with other catatonic symptoms, such as automatic obedience, motor perseveration, echopraxia and primitive reflexes.
Thirteen studies [14, 26, 45, 47, 49, 27, 28, 30, 39–42, 44] included the consensus definition of paratonia that was proposed by Hobbelen et al. [47] in 2006. This definition was derived using a Delphi process with 8 international experts.
The Delphi panel agreed on the following definition of Paratonia:
“a form of hypertonia with an involuntary variable resistance during passive movement. The nature of paratonia may change with progression of the dementing illness (e.g., active assistance) is more common early in the course of degenerative dementias, whilst active resistance is more common later in the course of the disease). The degree of resistance varies depending on the speed of movement (e.g., a low resistance to slow movement and a high resistance to fast movement). The degree of paratonia is proportional to the amount of force applied. Paratonia increases with progression of dementia. Furthermore, the resistance to passive movement is in any direction and there is no clasp-knife phenomenon” [47].
With this operational definition of paratonia, the distinction between paratonia, spasticity and Parkinsonian rigidity is possible. The distinction between (oppositional) paratonia and stroke-spasticity is based upon two factors; 1) the absence of a catch/ clasp-knife response and 2) that resistance to movement in paratonia can be in any direction in contrast to a hemiplegic unilateral, focal, upper motor neuron pattern resistance in stroke spasticity [51]. The distinction between (oppositional) paratonia and Parkinsonian rigidity is based upon the velocity dependency of paratonia. In Parkinsonian rigidity the resistance is not dependent on the speed of the movement (lead pipe phenomenon) [52].
Diagnosis of paratonia
We identified 25 studies [11, 13, 29, 30, 33–36, 38–41, 14,42–44, 46, 49, 16, 21, 24–28] that described the process of diagnosing a patient with paratonia. The subjective impression of resistance (oppositional paratonia) to sudden, irregular passive changes in position and/or speed in the extension and flexion of arms and legs was described in 7 studies [16, 21, 24, 34–36, 46]. Three studies [23, 24, 38] expanded this description with that resistance occurs in the absence of cogwheeling or a spastic catch. Tyrrell and Rossor [38] supplemented this with that the resistance is not exacerbated by movement in the contralateral fist. Three studies [21, 33, 38] describe the limb placement maneuver, whereby the examiner passively lifts the patient’s arm with the instruction to relax. When the arm remains elevated or shows any delay in dropping, this indicates paratonia. Vahia et al. [13] indicated paratonia to be the amount of passive force necessary to elicit stiffening. The modified Columbia rating scale for rigidity to identify rigidity in patients with dementia was used by Duret et al. [29]. In 2003, Hobbelen et al. [25] used specific criteria [53] for diagnosing paratonia; during passive movement of the extremities, trunk and head an increase in muscle tone must occur at flexion and extension, independent of the starting position of the joints, and a rapid movement must show an increase of tone and a slow movement a decrease. One study [28] described paratonia when there was an altered neck position (cervical antero position, extension or kyphosis) in patients with severe dementia. The psychometric properties were not reported for the abovementioned described procedures.
Ten studies [14, 26, 30, 39–42, 44, 49, 54] used the validated Paratonia Assessment Instrument (PAI), based on the consensus definition of Hobbelen et al. in 2006 [47]. With this assessment instrument, an examiner can establish the presence (or absence) of paratonia by successively moving all four limbs passively in flexion and extension while the participant is in a sitting position (Fig. 3) or supine in bed. The PAI is a tool to confirm the presence of paratonia and distinguish it from other forms of increased tone, but it has not been validated to determine whether it captures change over time or grades the magnitude of the severity of paratonia. The PAI has only expert validity and face validity. In a three-stage study examining 87, 97, and 24 participants with an established form of dementia, interobserver reliability as measured by Cohen’s kappa ranged from 0.63 to 1.
Fig. 3
The PAI procedure: The examiner starts with a slow movement of the limb, after which the movement is accelerated. A construct of five criteria allows a categorical diagnosis of paratonia, i.e., paratonia can only be diagnosed when all five criteria are present; 1) an involuntary variable resistance; 2) a degree of resistance that varies depending on the speed of the movement (e.g., a low resistance to slow movements and a high resistance to fast movement; 3) resistance to passive movement can be felt in any direction (no distinct pattern); 4) no clasp-knife phenomenon; and 5) resistance is present in 2 movement directions in 1 limb or in 2 different limbs. With permission from Cambridge University Press reprinted from; Diagnosing paratonia in the demented elderly: reliability and validity of the Paratonia Assessment Instrument (PAI) [42].
![The PAI procedure: The examiner starts with a slow movement of the limb, after which the movement is accelerated. A construct of five criteria allows a categorical diagnosis of paratonia, i.e., paratonia can only be diagnosed when all five criteria are present; 1) an involuntary variable resistance; 2) a degree of resistance that varies depending on the speed of the movement (e.g., a low resistance to slow movements and a high resistance to fast movement; 3) resistance to passive movement can be felt in any direction (no distinct pattern); 4) no clasp-knife phenomenon; and 5) resistance is present in 2 movement directions in 1 limb or in 2 different limbs. With permission from Cambridge University Press reprinted from; Diagnosing paratonia in the demented elderly: reliability and validity of the Paratonia Assessment Instrument (PAI) [42].](https://content.iospress.com:443/media/jad/2020/78-4/jad-78-4-jad200691/jad-78-jad200691-g003.jpg)
Facilitory paratonia can be diagnosed with the paratonia scale, which establishes the subjective impressions of both assistance and resistance offered to passive flexion and extension about the elbow [11, 43]. Facilitory paratonia can also be diagnosed with the modified Kral procedure as described by Beversdorf and Heilman [11].
Marinelli et al. [43] validated a quantitative me-thod to assess and visualize facilitory and oppositional paratonia in elbow flexor and extensor muscles using surface electromyography (EMG) and metro-nome-controlled passive movements. In a group of outpatients with cognitive impairment (n = 10), the researchers found that facilitory paratonia was more prevalent than oppositional paratonia and had a higher amplitude in elbow flexors than in extensors. Both facilitory and oppositional paratonia increased over time during continuous passive flexion and extension elbow movements, differently from discontinuous movements, underlining the role of move-ment repetition in increasing paratonia. Information on the psychometric properties is absent in the mentioned procedures.
Two studies [40, 41] investigated a device called the MyotonPro to objectively assess the presence and severity of paratonia. The MyotonPro is a small handheld device that measures mechanical muscle properties and can differentiate between muscle tone and biomechanical properties (stiffness, elasticity, and viscoelasticity). Van Deun et al. [41] reported low-to-high interrater reliability (ICC: 0.43–0.73), moderate-to-high between-series intrarater reliability (ICC: 0.57–0.86), and poor-to-moderate intrarater reliability and agreement (ICC = 0.23–0.47) in a group of patients with paratonia (n = 16). Validity, reliability and responsiveness were studied by Drenth et al. [40] in a cohort of people with dementia (with paratonia n = 70, without n = 82). In the total population of their study, they found evidence that MyotonPro is able to differentiate between people with and without paratonia (area under the curve ranging from 0.60 to 0.67), with sensitivity estimates ranging from 70% –60%, specificity estimates ranging from 54% –61% and moderate to high inter- and intrarater reliability (ICC: 0.57–0.75 and ICC: 0.54–0.71, respectively).
Assessment of paratonia severity
Beversdorf an Heilman [11] published the first semiquantitative paratonia rating scale, thus enabling researchers to rate oppositional or facilitory paratonia. In 1999, a modified Ashworth scale (MAS-P) was validated and found to be reliable for assessing the severity of oppositional paratonia (intrarater reliability, Kendall’s Tb 0.62–0.80; interrater reliability, Kendall’s Tb 0.72–0.77) [55]. Six studies [25, 26, 30, 40, 41, 44] used the MAS-P to assess the severity of paratonia (after being identified with the PAI). With the MAS-P, muscle tone is assessed by the perceived resistance during passive movement and rated on a 5-point scale ranging from 0 to 4, (0 = no resistance to passive movement, 1 = slight resistance, 2 = more marked resistance, 3 = considerable resistance, and 4 = severe resistance) [55].
The severity of paratonia can also be assessed by the MyotonPro device [40, 41]. The MyotonPro is able to record changes over time in patients with paratonia, and the minimal detectable change (MDC) and minimal detectable important change (MDIC) were calculated (Supplementary Table 3) [40]. The MDIC was established with an anchor-based method in which longitudinal changes in the MyotonPRO outcomes after six months were related to an external criterion for important change (the “anchor”), and the worsening of movement opposition during activities of daily living (ADL) was measured with the Clinical Global Impression (CGI) of change scale. Because the MDC values surpassed the MCID, the ability to detect small relevant changes in this population is complicated [40].
Pathogenesis
Central nervous system (CNS)
Five studies [14, 22, 27, 35, 48] suggested that the pathogenesis of paratonia is related to damage to cortical and subcortical motor structures. Impaired response inhibition associated with frontal lobe lesions was suggested in 10 studies [11, 13, 21, 23, 24, 32, 33, 36, 43, 46]. One study [16] hypothesized that paratonia may be a re-manifestation of an infantile stabilization reflex mechanism. In these studies, however, these possible pathways were only briefly mentioned, and no scientific data that described the relationship between paratonia and changes in specific brain areas were presented. Although the involvement of the basal ganglia and possibly the substantia nigra in the pathogenesis of paratonia was mentioned in 4 studies [29, 31, 36, 37], there was no evidence that dopaminergic nigrostriatal dysfunction is a mechanism for the pathophysiologic substrate of paratonia.
Peripheral biomechanical changes
Peripheral biomechanical changes induced by advanced glycation end-products (AGEs) contributing to the etiology of paratonia were described in 2 studies [14, 30]. AGEs are mediated by the nonenzymatic condensation of a reducing sugar with proteins (especially collagen tissue) and are spontaneously produced in human tissues which increases with aging [56]. In many age-related diseases, the accumulation of AGEs is a significant contributing factor in degenerative processes, especially in cardiovascular diseases, diabetes mellitus (DM), and Alzheimer’s disease (AD) [56, 57].
Hobbelen et al. [14] hypothesized that AGEs could be involved in the development of paratonia by the formation of cross-links in muscle collagen due to DM, resulting in tissue stiffness. In a one-year follow-up study in people with early, moderate and severe dementia (n = 174), they found that after adjusting for several known confounders people with dementia and DM (n = 39) had a significantly higher risk (OR = 10.7; 95% CI: 2.2–51.7) of developing paratonia than people those with dementia but without DM.
Drenth et al. [30] published a one-year longitudinal study examining the relationship between AGEs and paratonia in people with early-stage dementia (n = 118). After adjusting for several known confounders, they found that AGE levels were associated with the presence (OR = 3.47, 95% CI: 1.87–6.44, p < 0.001) and severity (β=0.17, 95% CI: 0.11 –0.23, p < 0.001) of paratonia. The authors hypothesized that AGE-induced impaired skeletal muscle function, due to collagen cross-linking and intramuscular inflammation, causes perceived resistance during passive movement in early-stage paratonia; these findings indicate that these peripheral biomechanical changes play a role in initiating movement stiffness in the early stages, whereas CNS pathology accelerates paratonia as dementia progresses.
Interventions
Five studies [25–28, 44] addressed therapeutic interventions to treat paratonia.
Hobbelen et al. [25] performed a pilot study of 15 nursing home residents with dementia and paratonia to investigate the effects of stabilizing cushions and passive movement therapy (PMT). Subjects were randomized to one of three groups: group 1 received PMT 3 times a week, group 2 used stabilizing cushions only, and group 3 was a control group receiving standard care. The results showed a modest short-term (15 minutes after one treatment) benefit of cushions in the arms and positive short-term effects of PMT. However, the long-term effects (after three weeks) were positive only in the control group. This pilot study provided the groundwork for a larger multicenter randomized clinical trial of PMT [26] in which 101 nursing home residents with dementia and moderate-to-severe paratonia were randomly assigned to either a PMT group or a control group. The PMT group received PMT 3 times a week over 4 weeks. The main outcomes were the severity of paratonia as measured by the MAS-P and caregiver burden as measured by the modified patient-specific complaint score list. The study showed that PMT increased the severity of paratonia and did not reduce caregiver burden.
Bautmans et al. [28] used a randomized controlled crossover design to study gentle cervical spine mobilization in elderly nursing home residents with altered neck posture (cervical antero-position, extension, or kyphosis) due to paratonia and known dysphagia (n = 15). Patients were randomized into 2 groups: group one started with one week of cervical spine mobilization, followed by one-week of wash-out and one week of control treatment (i.e., socializing visits). The other group started with one week of control treatment, followed by one-week of wash-out and one week of cervical spine mobilization. The primary outcome measures were feasibility (attendance, hostility to therapy, and complications) and dysphagia limit (maximal volume of water that can be swallowed in a single movement (0–20 ml)). The results show that gentle cervical spine mobilization was feasible and improved swallowing capacity after one session (p = 0.01) and after one week of treatment (p = 0.03).
Kleiner-Fishman et al. [27] used a blinded, randomized, placebo-controlled crossover design to study the application of botulinum toxin in patients with advanced cognitive impairment and paratonia (n = 10). Patients were randomized to one of two treatment cycle protocols: active drug then placebo vs placebo followed by active drug. Each treatment cycle lasted for 16 weeks to allow for the complete washout of toxin. Patients received up to 300 units of incobotulinumtoxin A in the upper extremities. The primary outcome measure was the modified Carer Burden Scale and the secondary outcome measure was joint passive range of motion (PROM) as measured by a goniometer. The results demonstrated that botulinum toxin was safe and efficacious in reducing caregiver burden (p = 0.02) and increasing the PROM around different joints (p = 0.02 to < 0.001).
Van Deun et al. [44] used a crossover design to study the short-term effects of supporting cushions (SC) and harmonic techniques (HT) in nursing home residents with moderate-to-severe paratonia (n = 22). The primary outcome measure was muscle tone as measured by the MyotonPro. The other outcome measures were elbow and knee PROM, which were measured by a goniometer, and pain, which was measured by a validated instrument to assess pain in nonverbal people with dementia (the PACSLAC-D) and by the visual analog scale (VAS) to assess the comfort of the respondent. The HT intervention consisted of pulsating/oscillating movements applied to the upper limbs, lower limbs, and trunk. To maximize the harmonic oscillating effect of the HT, balloons were placed under the limbs of the participant to provide additional movement resonance. The results demonstrated short-term improvement in PROM after both SC and HT interventions (p = 0.028 to p < 0.001) and decreased upper limb muscle tone after the SC intervention (p = 0.028). However, upper limb muscle tone increased after the HT intervention (p = 0.032). Improvements in pain (p = 0.003) and caregiver-reported VAS (p = 0.001 to p = 0.019) were observed after the HT intervention.
Four other studies [16, 30, 45, 49] outlined current practices to address paratonia or examined potential beneficial interventions. A survey among Belgian long-term care (LTC) facilities assessed the current standard of care for paratonia in late-stage dementia [45]. It highlighted that the most common interventions being utilized in Belgian LTC facilities included stabilizing cushions, multisensory stimulation (snoezelen), pulsation/rocking, active physical activity, active and passive mobilization techniques (PMTs), stretching, casting, heat application, massage and relaxation baths. Souren et al. [16] hypothesized that paratonia served as a physiologic and psychological stabilizing mechanism that protected against overwhelming external stimuli. Thus, the authors proposed the use of behavioral interventions intended to reduce anxiety and agitation, such as gentle movements, eye contact and soothing voice, and physical activity to prevent or delay contractures. Ries [49] suggested the use of counterpressure movements as an additional strategy to reduce paratonia. Drenth et al. [30] postulated that AGE-induced impairments in muscle function contributed to paratonia. Thus, they hypothesized that interventions to decrease AGE formation and accumulation could be viable for preventing and reducing paratonia; such interventions could be achieved by optimizing glycemic control through physical activity and diet.
DISCUSSION
General
This systematic review indicates that there has been promising research activity and progress in the field of paratonia. Although the literature search yielded only 35 articles from the last 64 years (the oldest article was from 1956), 13 studies were from the last 10 years and were written by 4 different research groups (Italy, Canada, Belgium, and the Netherlands). It is unclear why there appears to be a lack of scientific interest in paratonia despite the fact that paratonia has a devastating effect on the quality of life of people with late stage dementia. We hypothesize that in the four countries in which research on paratonia has been performed in the last decade, there is greater intersection between geriatric care and scientific research with infrastructure in place making it more feasible to perform studies in this population. Further, scientific research in the field of dementia is often focused on pre-clinical or early stage disease where paratonia is absent or mild. There has been limited focus on late stage disease targeting palliative approaches to reduce suffering and enhance quality of life.
We excluded 9 articles that used the UPDRS to diagnose rigidity or paratonia in people with dementia. It is important to highlight this, as diagnosing paratonia in dementia with an instrument that is developed to assess the motor aspects of PD may mislead clinicians to diagnose parkinsonism and to use interventions that target PD rather than paratonic rigidity; these diseases have different underlying etiologies and therapies. The UPDRS should not be used for people with dementia other than people with Lewy body dementia or PD dementia.
Definition, diagnosis, and assessment of severity
Most recent studies in this review used the consensus definition of paratonia that was proposed in 2006. This working definition led to additional research, including the creation of the PAI, which is a standardized, validated method to clinically identify paratonia and differentiate it from other forms of increased muscle tone, such as parkinsonian rigidity and spasticity [42].
One study [50] described paratonia as a catatonia-like symptom. Cognitive factors that affect muscle tone when a catatonic posture is assumed (i.e., perseveration of limb placement) are mentioned in the literature, but are differentiated from paratonia [21, 22]. Muscle tone in catatonia requires further characterization as it is difficult to determine whether the alteration of muscle tone occurs only during passive movements, or whether it persists when the examiner is no longer manipulating the limb. For example, “waxy flexibility” is found when the patient maintains a posture imposed by the examiner, which is not considered a feature of paratonia. Further research is necessary to determine whether the limb placement maneuver detects paratonia or detects a catatonic posture.
Studies of the MyotonPro indicated that this device can be used to objectively quantify paratonia severity. However, because of the inherent variability in movement resistance in paratonia, the authors suggested that the outcomes from the MyotonPRO should be interpreted with care [40, 41]. The promising results of the MyotonPRO studies provide optimism for further study. It is conceivable that the MyotonPro could be used in the clinical diagnosis of paratonia. The MyotonPro may be used as an alternative for the subjective MAS-P; however, future studies should focus on additional guidelines for MyotonPro measurements, such as multiple measurements. This could possibly allow for adjustment for diurnal variation in paratonia and increase the clinical interpretation and improve reproducibility [40].
Until objective measuring instruments are available to measure the presence and severity of para-tonia, it is recommended that, subsequent studies on clinal correlations, causes and consequences of paratonia, should include multiple outcome measures such as motor function, ADL, pain, behavior, and diurnal variation of paratonia.
One possible objective strategy for the diagnosis and quantification of paratonia involves recording electromyographic muscle activation related to paratonia to differentiate facilitory from oppositional paratonia [43]. Surface electromyography is the most appropriate way of demonstrating muscle activation, as it provides optimal temporal resolution, the possibility to distinguish among muscle groups and the agonist-antagonist interplay. In a pilot study, [43] demonstrated that paratonic activity can be quantitatively measured and that its oppositional and facilitory features can be distinguished. Quantitative analysis confirmed that paratonia is a velocity-dependent phenomenon and that it increases following movement repetition, which is the underlying premise of the PAI [42]. Interestingly, paratonia is the only known condition in which movement repetition induces an increase in muscle activation. In fact, in spasticity, repetitive muscle stretching induces a gradual reduction in muscle reflex activation, while in parkinsonian rigidity, the activity remains the same. The contrast between paratonia and these other forms of hypertonia can be used to clearly distinguish oppositional paratonia from spasticity and parkinsonian rigidity.
Pathogenesis
Although a CNS-based etiology of paratonia seems likely in severe paratonia, additional research on the relation between the development of paratonia and AGEs indicates that a peripheral biomechanical component cannot be ruled out. It can be hypothesized that several central and peripheral pathways take place simultaneously and reinforce or interact with each other. It is currently assumed that facilitory paratonia is the first stage of paratonia and that at some stage, a transition into oppositional paratonia occurs. However, it is not known whether facilitory and oppositional paratonia are two aspects of the same phenomenon or even if the two types of paratonia share the same neural mechanisms.
There may be several risk factors for paratonia: The longitudinal study of Hobbelen et al. [14], demonstrated that in 51 participants that developed paratonia, those with Vascular Dementia had the highest Hazard Ratio (3.1). The severity of cognitive decline and the presence of DM were statistically significant risk factors for developing paratonia. Drenth et al. [30] showed that the level of AGE accumulation is also a risk factor for developing paratonia.
An increase in tissue stiffness due to AGE cross-linking is associated with a loss of viscoelasticity; the tissue becomes stiffer, and after contraction, the muscle may not return to its original baseline tone. A decrease in elasticity suggests that the tissue becomes weak and that is has difficulty returning to its original form after deformation. If we assume that a smaller dissipation of mechanical energy (which was registered in the MyotonPro studies) is associated with a higher elasticity in the tissue, it is plausible that when tissue stiffness and intrinsic tension increase due to AGE crosslinking, the mechanical energy increases (similar to a tightly tensioned spring), as in passive movement. It remains unclear in what way AGEs cause motor decline in early-stage dementia.
In patients suffering from paratonia, hypertonia and active opposition during ADL can vary and may fluctuate. Although factors such as aggression, agitation, anxiety, pain, confusion and sudden external stimuli (e.g., light, sound) have been reported to potentially influence the severity of paratonia in observational studies [13, 16], further research is necessary to investigate the exact relationship of these factors and their impact on paratonia. In addition to memory loss, other cortical functions decline in dementia, including impaired sensory input [58]. This could elicit primitive fight/flight responses, which include increased muscle tone. It has been hypothesized that in people with dementia, the loss of the sense of proprioception is compensated for by extreme joint positions and an increase in muscular tension to gain additional tactile sensory information [59]. Although aging is generally associated with a decline in the sense of proprioception [60], no studies have been published addressing the impact of dementia on proprioception.
Another putative theory to explore regarding the origin of paratonia is the age-associated increase in the coactivation of antagonist muscles. Some studies have demonstrated that aging results in the heightened activation of antagonist muscles during voluntary movements [61]. The activation of agonist and antagonist muscle pairs is postulated to be organized around a dual system of cortically and spinally mediated reciprocal inhibition that is affected by age. It could be hypothesized that reciprocal inhibition is also modified or augmented with increasing cognitive impairment as dementia progresses. This could be an explanation for the perceived resistance to movement, especially when considering the inability to relax. To our knowledge, however, the only study using EMG recordings in paratonia [43] did not reveal the coactivation of agonist and antagonist muscles. Further neurophysiological studies could shed light on the role of cortical and spinal circuitry.
Intervention
An important practical question for managing people with paratonia in daily clinical practice is “what are effective interventions to reduce muscle tone in order to decrease caregiver burden, reduce or delay contractures, and improve quality of life?”
Our findings are very limited:
1. In severe paratonia: One pilot study [25] (n = 5) showed a positive effect of stabilizing cushions on muscle tone, and one study [44] (n = 22) showed a positive effect of harmonic techniques on range of motion. Both studies had important methodological shortcomings. One study [28] showed promising results with the use of cervical spine mobilization to improve swallowing capacity, but the sample size for this study was also small (n = 15); therefore, it is not possible to make definitive recommendations at this time until methodologically rigorous studies are conducted.
2. In moderate paratonia, promising results were shown in a randomized placebo-controlled pilot using botulinum toxin to reduce involuntary postures due to paratonia [27]. This method has the potential to be a significant palliative treatment for reducing complications due to paratonia, including contractures and pressure ulcers if the results of the pilot trial can be reproduced in large-scale trials that are in progress (personal communication).
3. No research has been published regarding how to prevent paratonia. Intensive glycemic control and reducing oxidative stress by nutrition and physical activity may be key methods for decreasing AGE formation [62] and potentially prevent paratonia. Physical inactivity has been found to be associated with high AGE levels in older people [63]. The mechanism of how exercises modulate the development of AGEs, paratonia is an important research avenue to pursue.
Strength and limitations
A limitation of this study is that we only found 35 studies. The wide variety of different types of research into paratonia prevented us from pooling the data and performing a meta-analysis. Further, much of the data available were of low quality, and there were very few randomized placebo-controlled trials. On the other hand, we performed an extensive literature search using all possible search terms to identify studies of paratonia, and we found 1,577 studies. We also diligently adhered to the PRISMA guidelines in performing this review to avoid potential biases. The fact that so few studies with varying designs were eligible for our review indicates that there is still little research on paratonia. This study indicates that high-quality research is urgently needed to allow for definitive conclusions regarding the pathophysiology of paratonia and to develop possible preventive and therapeutic interventions to alleviate the effects of paratonia.
This comprehensive review highlights the fact that paratonia is poorly recognized and health professionals lack clear guidance as to management. In future, creating a practical and operational decision tree in the management of paratonia might be helpful for help professionals to refer to; in the interim the PAI as part of the general assessment in people with dementia should be encouraged for use in recognizing paratonia as it is a valid and reliable tool to differentiate paratonia from Parkinson’s rigidity and spasticity [42].
Future directions of research
We propose a paratonia research agenda comprising 3 main topics: 1) Delineation of the pathogenesis of paratonia; 2) Validation and refinement of instruments and techniques to capture and measure the severity of paratonia that can detect changes due to trial interventions; and 3) Methodologically rigorous clinical trials of therapeutics.
Research on paratonia needs to bridge several research questions, areas, techniques and disciplines. To improve the quality of life of people with dementia, intensive collaboration between different fields of research is imperative.
Conclusion
This systematic review of the literature on paratonia outlines what is currently known about paratonia with respect to defining, detecting, and treating it. There was also preliminary research to suggest the underlying mechanisms of paratonia. Although paratonia has obvious devastating impacts on health and quality of life, the amount of research to date has been limited. However, in the last decade, there appears to have been increased research on paratonia, which hopefully will increase the momentum to further advance the field.
ACKNOWLEDGMENTS
This work, performed by the International Joint Research Group ‘Move in Age’ was supported by regular funds of the Research Group Healthy Ageing, Allied Healthcare and Nursing, Hanze University Groningen, the Department of General Practice and Elderly Care Medicine, University Medical Center Groningen, the Frailty in Ageing Research Group and Gerontology Department, Vrije Universiteit Brussels and ZuidOostZorg, Organisation for Elderly Care, Drachten.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-0691r1).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-200691.
REFERENCES
[1] | American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5), Washington, DC. |
[2] | Scarmeas N , Hadjigeorgiou GM , Papadimitriou A , Dubois B , Sarazin M , Brandt J , Albert M , Marder K , Bell K , Honig LS , Wegesin D , Stern Y ((2004) ) Motor signs during the course of Alzheimer disease. Neurology 63: , 975–982. |
[3] | Dumurgier J , Artaud F , Touraine C , Rouaud O , Tavernier B , Dufouil C , Singh-Manoux A , Tzourio C , Elbaz A ((2017) ) Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci Med Sci 72: , 655–661. |
[4] | Buracchio T , Dodge HH , Howieson D , Wasserman D , Kaye J ((2010) ) The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol 67: , 980–986. |
[5] | Mc Ardle R , Morris R , Wilson J , Galna B , Thomas AJ , Rochester L ((2017) ) What can quantitative gait analysis tell us about dementia and its subtypes? A structured review. J Alzheimers Dis 60: , 1295–1312. |
[6] | Kueper JK , Speechley M , Lingum NR , Montero-Odasso M ((2017) ) Motor function and incident dementia: A systematic review and meta-analysis. Age Ageing 46: , 729–738. |
[7] | Friedlander LH ((1828) ) Arznygelahrtheit. Allg Lit Zeiting, pp. 257-258. |
[8] | Dupré E ((1910) ) Débilité mentale et débilité motrice associées. Rev Neurol (Paris) 20: , 54–56. |
[9] | Kleist K ((1927) ) Gegenhalten (motorischer Negativismus) Zwanga greifen und Thalamus Opticus. Monatschr Psychiat Neurol 65: , 317. |
[10] | Kral VA ((1949) ) Ueber eine iterative bewegunsstörung bei stirnhirnläsionen. Monatsschr Psychiatr Neurol 118: , 257–272. |
[11] | Beversdorf DQ , Heilman KM ((1998) ) Facilitory paratonia and frontal lobe functioning. Neurology 51: , 968–971. |
[12] | Kurlan R , Richard IH , Papka M , Marshall F ((2000) ) Movement disorders in Alzheimer’s disease: More rigidity of definitions is needed. Mov Disord 15: , 24–29. |
[13] | Vahia I , Cohen CI , Prehogan A , Memon Z ((2007) ) Prevalence and impact of paratonia in Alzheimer disease in a multiracial sample. Am J Geriatr Psychiatry 15: , 351–353. |
[14] | Hobbelen JHSM , Tan FE , Verhey FR , Koopmans RT , de Bie RA ((2011) ) Prevalence, incidence and risk factors of paratonia in patients with dementia: A one-year follow-up study. Int Psychogeriatr 23: , 1051–1060. |
[15] | Gilhooly KJ , Gilhooly MLM , Sullivan MP , McIntyre A , Wilson L , Harding E , Woodbridge R , Crutch S ((2016) ) A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatr 16: , 106. |
[16] | Souren LE , Franssen EH , Reisberg B ((1997) ) Neuromotor changes in Alzheimer’s disease: Implications for patient care. J Geriatr Psychiatry Neurol 10: , 93–98. |
[17] | Shamseer L , Moher D , Clarke M , Ghersi D , Liberati A , Petticrew M , Shekelle P , Stewart LA ((2015) ) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation.g. BMJ 350: , 7647. |
[18] | Schardt C , Adams MB , Owens T , Keitz S , Fontelo P ((2007) ) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7: , 16. |
[19] | Ouzzani M , Hammady H , Fedorowicz Z , Elmagarmid A ((2016) ) Rayyan—a web and mobile app for systematic reviews. Syst Rev 5: , 210. |
[20] | Joanna Briggs Institute, Critical Appraisal Tools. https://joannabriggs.org/critical-appraisal-tools |
[21] | Jenkyn LR , Walsh DB , Culver CM , Reeves AG ((1977) ) Clinical signs in diffuse cerebral dysfunction. J Neurol Neurosurg Psychiatry 40: , 956–966. |
[22] | Critchley M ((1956) ) Neurologic changes in the aged. J Chronic Dis 3: , 459–477. |
[23] | Paulson G GG ((1968) ) Developmental reflexes: The reappearance of foetal and neonatal reflexes in aged patients. Brain 91: , 37–52. |
[24] | Villeneuve A , Turcotte J , Bouchard M , Côté JM , Jus A ((1974) ) Release phenomena and iterative activities in psychiatric geriatric patients. Can Med Assoc J 110: , 147–153. |
[25] | Hobbelen JHSM , de Bie RA , van Rossum E ((2003) ) Het effect van passief bewegen op de mate van paratonie. Een partieel geblindeerde gerandomiseerde klinische trials. Ned Tijdschr Fysiother 6: , 132–137. |
[26] | Hobbelen JHSM , Tan FES , Verhey FRJ , Koopmans RTCM , de Bie RA ((2012) ) Passive movement therapy in severe paratonia: A multicenter randomized clinical trial. Int Psychogeriatr 24: , 834–844. |
[27] | Kleiner-Fisman G , Khoo E , Moncrieffe N , Forbell T , Gryfe P , Fisman D ((2014) ) A randomized, placebo controlled pilot trial of botulinum toxin for paratonic rigidity in people with advanced cognitive impairment. PLoS One 9: , e114733. |
[28] | Bautmans I , Demarteau J , Cruts B , Lemper J-C , Mets T ((2008) ) Dysphagia in elderly nursing home residents with severe cognitive impairment can be attenuated by cervical spine mobilization. J Rehabil Med 40: , 755–760. |
[29] | Duret M , Goldman S , Messina D , Hildebrand J ((1989) ) Effect of L-dopa on dementia-related rigidity. Acta Neurol Scand 80: , 64–67. |
[30] | Drenth H , Zuidema SU , Krijnen WP , Bautmans I , van der Schans C , Hobbelen H ((2017) ) Advanced glycation end-products are associated with the presence and severity of paratonia in early stage Alzheimer disease. J Am Med Dir Assoc 18: , 636.e7–636.e12. |
[31] | Risse SC , Lampe TH , Bird TD , Nochlin D , Sumi SM , Keenan T , Cubberley L , Peskind E , Raskind MA ((1990) ) Myoclonus, seizures, and paratonia in Alzheimer disease. Alzheimer Dis Assoc Disord 4: , 217–225. |
[32] | Bennett HP , Corbett AJ , Gaden S , Grayson DA , Kril JJ , Broe GA ((2002) ) Subcortical vascular disease and functional decline: A 6-year predictor study. J Am Geriatr Soc 50: , 1969–1977. |
[33] | Benassi G , D’Alessandro R , Gallassi R , Morreale A , Lugaresi E ((1990) ) Neurological examination in subjects over 65 years: An epidemiological survey. Neuroepidemiology 9: , 27–38. |
[34] | Damasceno A , Delicio AM , Mazo DFC , Zullo JFD , Scherer P , Ng RTY , Damasceno BP ((2005) ) Primitive reflexes and cognitive function. Arq Neuropsiquiatr 63: , 577–582. |
[35] | Franssen EH , Reisberg B , Kluger A , Sinaiko E , Boja C ((1991) ) Cognition-independent neurologic symptoms in normal aging and probable Alzheimer’s disease. Arch Neurol 48: , 148–154. |
[36] | O’Keeffe ST , Kazeem H , Philpott RM , Playfer JR , Gosney M , Lye M ((1996) ) Gait disturbance in Alzheimer’s disease: A clinical study. Age Ageing 25: , 313–316. |
[37] | Tyrrell PJ , Sawle G V , Ibanez V , Bloomfield PM , Leenders KL , Frackowiak RS , Rossor MN ((1990) ) Clinical and positron emission tomographic studies in the “extrapyramidal syndrome” of dementia of the Alzheimer type. Arch Neurol 47: , 1318–1323. |
[38] | Tyrrell P , Rossor M ((1988) ) The association of gegenhalten in the upper limbs with dyspraxia. J Neurol Neurosurg Psychiatry 51: , 995–997. |
[39] | Van Deun B , Van Den Noortgate N , Van Bladel A , Palmans T , Cambier D ((2019) ) The impact of paratonia on fine and gross motor function in older adults with mild and moderate dementia. Alzheimer Dis Assoc Disord 33: , 54–61. |
[40] | Drenth H , Zuidema SU , Krijnen WP , Bautmans I , van der Schans C , Hobbelen H ((2018) ) Psychometric properties of the MyotonPRO in dementia patients with paratonia. Gerontology 64: , 401–412. |
[41] | Van Deun B , Hobbelen JSM , Cagnie B , Van Eetvelde B , Van Den Noortgate N , Cambier D ((2018) ) Reproducible measurements of muscle characteristics using the MyotonPRO device: Comparison between individuals with and without paratonia. J Geriatr Phys Ther 41: , 194–203. |
[42] | Hobbelen JSM , Koopmans RTCM , Verhey FRJ , Habraken KM , de Bie RA ((2008) ) Diagnosing paratonia in the demented elderly: Reliability and validity of the Paratonia Assessment Instrument (PAI).. Int Psychogeriatr 20: , 840–852. |
[43] | Marinelli L , Mori L , Pardini M , Beversdorf D , Cocito L , Curra A , Fattapposta F , Ghilardi MF , Abbruzzese G , Trompetto C ((2017) ) Electromyographic assessment of paratonia. Exp Brain Res 235: , 949–956. |
[44] | Van Deun B , Van Den Noortgate N , Van Bladel A , De Weerdt K , Cambier D ((2019) ) Managing paratonia in persons with dementia: Short-term effects of supporting cushions and harmonic techniques. J Am Med Dir Assoc 20: , 1521–1528. |
[45] | Van Deun B , Van den Noortgate N , Cinthia S , Van Bladel A , Dirk C ((2018) ) Paratonia in Flemish nursing homes: Current state of practice. Am J Alzheimers Dis Other Demen 33: , 205–214. |
[46] | Franssen EH , Kluger A , Torossian CL , Reisberg B ((1993) ) The neurologic syndrome of severe Alzheimer’s disease. Relationship to functional decline. Arch Neurol 50: , 1029–1039. |
[47] | Hobbelen JSM , Koopmans RTCM , Verhey FRJ , Van Peppen RPS , de Bie RA ((2006) ) Paratonia: A Delphi procedure for consensus definition. J Geriatr Phys Ther 29: , 50–56. |
[48] | Pauc R , Young A ((2012) ) Paratonia and gegenhalten in childhood and senescence. Clin Chiropr 15: , 31–34. |
[49] | Ries JD ((2018) ) Rehabilitation for individuals with dementia; facilitating success. Curr Geriatr Rep 7: , 59–70. |
[50] | Peralta V , Cuesta MJ ((2017) ) Motor abnormalities: From neurodevelopmental to neurodegenerative through “functional” (neuro)psychiatric disorders. Schizophr Bull 43: , 956–971. |
[51] | Thibaut A , Chatelle C , Ziegler E , Bruno M-A , Laureys S , Gosseries O ((2013) ) Spasticity after stroke: Physiology, assessment and treatment. Brain Inj 27: , 1093–1105. |
[52] | Shahed J , Jankovic J ((2007) ) Motor symptoms in Parkinson’s disease. Handb Clin Neurol 83: , 329–342. |
[53] | Middelveld-Jacobs IM , Boogerd van den MECP ((1986) ) Paratonie een vorm van hypertonie in het psychogeriatrisch verpleeghuis. Ned Tijdschr Fysiother 96: , 85–87. |
[54] | Hobbelen JSM , Verhey FRJ , Bor JHJ , de Bie RA , Koopmans RTCM ((2007) ) Passive movement therapy in patients with moderate to severe paratonia; study protocol of a randomised clinical trial (ISRCTN43069940).. BMC Geriatr 7: , 30. |
[55] | Waardenburg H , Elvers JWH , van Vechgel F , Oostendorp RAB ((1999) ) Is Paratonie Betrouwbaar te meten? Een betrouwbaarheid onderzoek voor het meten van paratonie met de VAS en de gemodificeerde tonusschaal van Ashworth. Ned Tijdschr Physiother 2: , 30–35. |
[56] | Rahmadi A , Steiner N , Munch G ((2011) ) Advanced glycation endproducts as gerontotoxins and biomarkers for carbonyl-based degenerative processes in Alzheimer’s disease. Clin Chem Lab Med 49: , 385–391. |
[57] | Gasser A , Forbes JM ((2008) ) Advanced glycation: Implications in tissue damage and disease. Protein Pept Lett 15: , 385–391. |
[58] | Scherder E ((2016) ) Aging and dementia. Neuropsychology, motor skills, and pain, VU University Press, Amsterdam. |
[59] | Rakt J van der ((1997) ) The development of a fetal position in psychogeriatric patients; a hypothesis. Fysiother Ouderenzorg, pp. 2-6. |
[60] | Verschueren SMP , Brumagne S , Swinnen SP , Cordo PJ ((2002) ) The effect of aging on dynamic position sense at the ankle. Behav Brain Res 136: , 593–603. |
[61] | Hortobagyi T , Devita P ((2006) ) Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc Sport Sci Rev 34: , 29–35. |
[62] | Magalhães PM , Appell HJ , Duarte JA ((2008) ) Involvement of advanced glycation end products in the pathogenesis of diabetic complications: The protective role of regular physical activity. Eur Rev Aging Phys Act 5: , 17–29. |
[63] | Drenth H , Zuidema SU , Krijnen WP , Bautmans I , Smit AJ , Schans C van der , Hobbelen H ((2018) ) Advanced glycation end-products are associated with physical activity and physical functioning in the older population. J Gerontol A Biol Sci Med Sci 73: , 1545–1551. |



