Abstract
Background and Objective
Patients with type 2 diabetes mellitus often have impaired renal function or may have impaired hepatic function, which can pose significant safety and tolerability issues for anti-hyperglycaemic pharmacotherapies. Therefore, the pharmacokinetics and tolerability of saxagliptin and its pharmacologically active metabolite, 5-hydroxy saxagliptin, in nondiabetic subjects with mild, moderate or severe renal or hepatic impairment, or end-stage renal disease (ESRD) were compared with saxagliptin and metabolite pharmacokinetics and tolerability in healthy adult subjects.
Methods
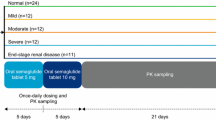
Two open-label, parallel-group, single-dose studies were conducted. Subjects received a single oral dose of saxagliptin 10 mg (Onglyza™).
Results
Compared with healthy subjects, the geometric mean area under the plasma concentration-time curve from time zero extrapolated to infinity (AUC∞) for saxagliptin was 16%, 41% and 108% (2.1-fold) higher in subjects with mild, moderate or severe renal impairment, respectively. AUC∞ values for 5-hydroxy saxagliptin were 67%, 192% (2.9-fold) and 347% (4.5-fold) higher in subjects with mild, moderate or severe renal impairment, respectively. As creatinine clearance (CLCR) values decreased, saxagliptin and 5-hydroxy saxagliptin AUC∞ generally increased or became more variable. Twenty-three percent of the saxagliptin dose (measured as the sum of saxagliptin and 5-hydroxy saxagliptin) was cleared by haemodialysis in a 4-hour dialysis session.
In the hepatic impairment study, the differences in exposure to saxagliptin and 5-hydroxy saxagliptin were less than 2-fold across all groups. As compared with healthy subjects matched for age, bodyweight, sex and smoking status, the AUC∞ values for saxagliptin were 10%, 38% and 77% higher in subjects with mild, moderate or severe hepatic impairment, respectively. These values were 22%, 7% and 33% lower, respectively, for 5-hydroxy saxagliptin compared with matched healthy subjects.
Conclusions
One-half the usual dose of saxagliptin 5mg (i.e. 2.5 mg orally once daily) is recommended for patients with moderate (CLCR 30–50 mL/min) or severe (CLCR <30 mL/min not on dialysis) renal impairment or ESRD, but no dose adjustment is recommended for those with mild renal impairment or any degree of hepatic impairment.






Similar content being viewed by others
References
WHO. Diabetes [fact sheet no. 312; online]. Available from URL: http://www.who.int/mediacentre/factsheets/fs312/en/ [Accessed 2010 Dec 1]
US Renal Data System. Annual data report: 2007. Atlas of chronic kidney disease and end-stage renal disease in the United States [online]. Available from URL: http://www.usrds.org/adr_2007.htm [Accessed 2010 Dec 1]
Bash LD, Selvin E, Steffes M, et al. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy. Arch Intern Med 2008; 168: 2440–7
Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology 2000; 32(4 Pt 1): 689–92
El-Serag HB, Tran T, Everharts JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004; 126: 460–8
Glucophage® (metformin hydrochloride): US prescribing information [online]. Available from URL: http://packageinserts.bms.com/pi/pi_glucophage.pdf [Accessed 2010 Dec 1]
Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA 2002; 287: 360–72
Odegard PS, Setter SM, Neumiller JJ. Considerations for the pharmacological treatment of diabetes in older adults. Diabetes Spectrum 2007; 20: 239–46
Rodighiero V. Effects of liver disease on pharmacokinetics: an update. Clin Pharmacokinet 1999; 37: 399–431
Tahrani AA, Piya MK, Barnett AH. Saxagliptin: a new DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Adv Ther 2009; 26: 249–62
Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab 2008; 10: 376–86
DeFronzo RA, Hissa M, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone. Diabetes Care 2009; 32: 1649–55
Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract 2009; 63: 1395–406
Jadzinsky M, Pfützner A, Paz-Pacheco E, et al. Saxagliptin given in combination with metformin as initial therapy improves glycemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab 2009; 11(6): 611–22
Hollander P, Li J, Allen E, et al. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab 2009; 94(12): 4810–9
Onglyza™ (saxagliptin): US prescribing information [online]. Available from URL: http://packageinserts.bms.com/pi/pi_onglyza.pdf [Accessed 2010 Dec 1]
De Fronzo R, Hissa MN, Garber AJ, et al. Once daily saxagliptin added to metformin provides sustained glycemic control and is well tolerated over 102 weeks in patients with T2D [abstract]. Diabetes 2009; 58 Suppl. 1: A147
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16(1): 31–41
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association, 1994
Boulton D, Goyal A, Li L, et al. The effects of age and gender on the single-dose pharmacokinetics and safety of saxagliptin in healthy subjects [abstract]. Diabetes 2008; 57 Suppl. 1: A164
Bristol-Myers Squibb/AstraZeneca. Saxagliptin clinical pharmacology summary. Princeton (NJ): Bristol-Myers Squibb, and Wilmington (DE): AstraZeneca, 2008(Data on file)
Testa R, Caglieris S, Risso D, et al. Monoethylglycinexylidide formation measurement as a hepatic function test to assess severity of chronic liver disease. Am J Gastroenterol 1997; 92: 2268–73
Uderman H. Placebo-controlled, ascending single-dose study to evaluate the safety, pharmacokinetics, and pharmacodynamics of BMS-47718 in healthy subjects. Lawrenceville (NJ): Bristol-Myers Squibb, 2005(Data on file)
European Medicines Agency. Onglyza (saxagliptin): summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001039/WC500044316.pdf [Accessed 2010 Dec 1]
Nowicki M, Rychlik I, Haller H, et al. Saxagliptin improves glycemic control and is well tolerated in patients with type 2 diabetes mellitus (T2DM) and renal impairment compared with placebo [abstract]. Diabetes 2010; 59 Suppl. 1: A149
Acknowledgements
This study was funded, designed and supervised by scientists at Bristol-Myers Squibb and AstraZeneca, the discoverers and developers of saxagliptin. David M. Kornhauser was an employee of Bristol-Myers Squibb until October 2006 and an employee of Icon Development Solutions until July 2007. Lorna Castaneda was an employee of Bristol-Myers Squibb until November 2009. Nimish N. Vachharajani is now an employee of Advinus Therapeutics (Bangalore, Karnataka, India). David W. Boulton, Li Li, Ernst U. Frevert, Angela Tang and Chirag G. Patel are current employees of Bristol-Myers Squibb and at the time that this work was conducted, all authors were employed by Bristol-Myers Squibb. The authors gratefully acknowledge the contributions of Thomas Marbury, MD (Orlando Clinical Research Center, Orlando, FL, USA); Ernesto Fuentes, MD (Elite Research Institute, Miami, FL, USA); and Sherwyn L. Schwartz, MD (Diabetes and Glandular Disease Research Associates, Inc., San Antonio, TX, USA) in the clinical conduct of the study, and the contribution of Daisy Whigan, MS (Bristol-Myers Squibb R&D, Princeton, NJ, USA) for her oversight of the bioanalytical work associated with this study. Technical and editorial assistance for this manuscript was provided by Gina Coviello, MS (Quintiles Medical Communications, Parsippany, NJ, USA).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Boulton, D., Li, L., Frevert, E.U. et al. Influence of Renal or Hepatic Impairment on the Pharmacokinetics of Saxagliptin. Clin Pharmacokinet 50, 253–265 (2011). https://doi.org/10.2165/11584350-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11584350-000000000-00000