Abstract
The National Institute for Health and Clinical Excellence (NICE) invited the manufacturer of alitretinoin (Basilea Pharmaceuticals Ltd, Basel, Switzerland) to submit evidence for the clinical and cost effectiveness of this drug for the treatment of patients with severe chronic hand eczema (CHE), as part of the Institute’s single technology appraisal (STA) process. The Centre for Reviews and Dissemination and the Centre for Health Economics at the University of York were commissioned to act as the Evidence Review Group (ERG). This article provides a description of the company submission, the ERG review and NICE’s subsequent decisions.
The ERG produced a critical review of the evidence for the clinical and cost effectiveness of the technology based upon the manufacturer’s submission to NICE. The ERG also independently searched for relevant evidence and modified the manufacturer’s decision analytic model to examine the impact of altering some of the key assumptions.
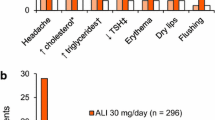
The main clinical effectiveness data were derived from a single-placebo randomized controlled trial (RCT) of daily treatment with alitretinoin for 12–24 weeks, with follow-up for a further 24 weeks, in patients with severe CHE unresponsive to topical corticosteroids. A significantly greater proportion of patients achieved ‘clear’ or ‘almost clear’ hands by week 24 with alitretinoin than those using placebo: 48% with alitretinoin 30mg (p < 0.001); 28% with alitretinoin 10 mg (p < 0.005); 17% with placebo. Most patients who responded remained in remission during the 24-week follow-up period. The most commonly reported adverse event was dose-dependent headache, with rates of 20% in the alitretinoin 30 mg group and 11% in the alitretinoin 10mg group, respectively. Serious adverse events were rare, although alitretinoin was associated with increases in both total cholesterol and triglycerides. No direct or indirect comparisons of alitretinoin with any of the relevant treatment comparators (psoralen +UVA [PUVA], ciclosporin or azathioprine) were available.
In the manufacturer’s original submission to NICE, the base-case incremental cost-effectiveness ratios (ICERs) reported for alitretinoin were £8614 per QALY versus ciclosporin, −£469 per QALY versus PUVA (with alitretinoin dominant) and £10 612 per QALY versus azathioprine (year 2007–8 values). In response to a request from the ERG, the manufacturers provided a revised model that compared alitretinoin only with placebo, for which the ICER was reported to be £12 931. However, the omission of adverse events entirely from this revised model, in combination with a number of other factors, led the ERG to conclude that the model underestimated the costs of treatment associated with alitretinoin. Estimates of health-related quality of life (HR-QOL) were the primary source of uncertainty, with the use of values from an alternative source producing ICERs of around £30 000 per QALY gained.
The ERG concluded that, although the evidence presented indicates that alitretinoin is efficacious in the treatment of severe CHE, it gives little indication of alitretinoin’s efficacy relative to likely alternative treatment options or its efficacy and safety in the longer term. Although the ICERs estimated by the manufacturer suggested that alitretinoin may be cost effective for use in the UK NHS, utilizing the alternative HR-QOL estimates resulted in a 2-fold increase in the ICER. Thus, there was considerable uncertainty as to the true ICER of alitretinoin versus the relevant treatment comparators. The Appraisal Committee recommended that alitretinoin be provided to those patients with severe CHE and a Dermatology Life Quality Index (DLQI) score of at least 15. They recommended that treatment be stopped as soon as an adequate response was observed, or if CHE remained severe at 12 weeks, or if response was inadequate at 24 weeks.


Similar content being viewed by others
Notes
Precise DLQI numbers cannot be reported here, as these data were submitted by the manufacturer as academic in confidence.
References
National Institute for Health and Clinical Excellence. Guide to the single technology appraisal (STA) process. London: NICE, 2006 [online]. Available from URL: http://www.nice.org.uk/page.aspx?o=STAprocessguide [Accessed 2009Mar 1]
National Institute for Health and Clinical Excellence. Eczema (chronic): alitretinoin [online]. Available from URL: http://www.nice.org.uk/guidance/index.jsp?action=byID&o=12035 [Accessed 2009 Dec 2]
Diepgen TL, Agner T, Aberer W, et al. Management of chronic hand eczema. Contact Dermatitis 2007 Oct; 57 (4): 203–10
Agner T, Andersen KE, Brandao FM, et al. Hand eczema severity and quality of life: a cross-sectional, multicentre study of hand eczema patients. Contact Dermatitis 2008; 59 (1): 43–7
Cvetkovski RS, Rothman KJ, Olsen J, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. Br J Dermatol 2005; 152 (1): 93–8
Meding B, Wrangsjo K, Jarvholm B. Fifteen-year follow-up of hand eczema: persistence and consequences. Br J Dermatol 2005; 152 (5): 975–80
Halpern SM, Anstey AV, Dawe RS, et al. Guidelines for topical PUVA: a report of a workshop of the British photodermatology group. Br J Dermatol 2000 Jan; 142 (1): 22–31
Akhaven A, Rudikoff D. The treatment of atopic dermatitis with systemic immunosuppresive agents. Clin Dermatol 2003; 21 (3): 225–40
Basilea Pharmaceuticals Ltd. Toctino 10 mg and 30mg soft capsules. SPC. electronic Medicines Compendium (eMC), 2008 [online]. Available from URL: http://emc.medicines.org.uk/medicine/21177/SPC/Toctino+10mg+and+30mg+soft+capsules/ [Accessed 2009 Dec 2]
National Institute for Health and Clinical Excellence. Eczema (chronic) alitretinoin: manufacturer submission [online]. Available from URL: http://www.nice.org.uk/guidance/index.jsp?action=folder&o=43972 [Accessed 2009 Dec 2]
Ruzicka T, Lynde CW, Jemec GBE, et al. Efficacy and safety of oral alitretinoin (9-cis retinoic acid) in patients with severe chronic hand eczema refractory to topical corticosteroids: results of a randomized, double-blind, placebo-controlled, multicentre trial. Br J Dermatol 2008; 158 (4): 808–17
Ruzicka T, Lahfa M, Lynde CH, et al. Re-treatment study of alitretinoin (9-cis retinoic acid) in severe chronic hand eczema refractory to topical treatment [abstract no. P-280]. 16th Congress of the European Academy of Dermatology and Venereology (EADV); 2007 May 1620; Vienna
Ruzicka T, Larsen FG, Galewicz D, et al. Oral alitretinoin (9-cis-retinoic acid) therapy for chronic hand dermatitis in patients refractory to standard therapy: results of a randomized, double-blind, placebo-controlled, multicenter trial. Arch Dermatol 2004; 140 (12): 1453–9
Reference sheet: excerpts from clinical study report. Safety and efficacy of alitretinoin in severe refractory chronic hand dermatitis [protocol BAP00200/report BAP00983; reference number DOF-ALI08021]. Basel: Basilea Pharmaceuticals Ltd, 2008. (Data on file)
Reference sheet: Augustin quality of life in patients with hand eczema [unpublished abstract; academic in confidence; reference number DOF-ALI08018]. Basel: Basilea Pharmaceuticals Ltd, 2008. (Data on file)
National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. London: NICE, 2008 [online]. Available from URL: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf [Accessed 2009 Dec 2]
Woolacott N, Hawkins N, Mason A, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technology Assessment 2006; 10 (46): 1–233
National Institute for Health and Clinical Excellence. Final appraisal determination: alitretinoin for the treatment of severe chronic hand eczema. London: NICE, 2009 Jul [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/EczemaFAD.pdf [Accessed 2009 Dec 2]
Acknowledgements
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project number 08/87/01) and will be published as part of a compendium of ERG articles in Health Technology Assessment. See the HTA programme website (http://www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the Appraisal Committee’s review and incorporates additional information and comment from the authors on the STA process and iterations of the NICE guidance not covered by the HTA report. This summary has not been externally peer reviewed by PharmacoEconomics.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of NICE or the Department of Health.
The authors have no conflicts of interest that are directly relevant to the content of this summary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodgers, M., Griffin, S., Paulden, M. et al. Alitretinoin for Severe Chronic Hand Eczema. Pharmacoeconomics 28, 351–362 (2010). https://doi.org/10.2165/11532160-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11532160-000000000-00000