Abstract
Background: All licensed medicines in the European Union must be provided with a Patient Information Leaflet that includes a list of all known side effects. Among patients who read the leaflet, the side effects section is the most often read. A UK government regulatory publication recommends providing medicine side effect risk information in a combined format, using verbal descriptors accompanied by numerical information.
Objectives: This study, with users of an existing popular patient information website, investigates the effectiveness of presenting medicine side effect risk information in different forms.
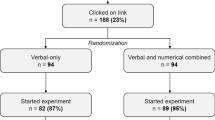
Design: Participants were randomly allocated to one of the three formats for representing risk information (verbal descriptors, e.g. ‘common’; absolute frequencies, e.g. ‘less than 1 in 10 people’; and a combination of verbal descriptors and frequency bands, e.g. ‘common (affects less than 1 in 10 people)’.
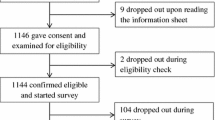
Methods: Participants (n= 187) were recruited from users of the Cancer Research UK patient information website. They were asked to imagine that they had to take a cancer treatment (tamoxifen), estimate the risks of four side effects occurring, and complete Likert scales relating to their satisfaction with the information supplied and perceived likelihood of various outcomes.
Results: Those in the absolute frequency format demonstrated greater accuracy in estimating the likelihood of having two of four side effects than the other two formats. They were also more accurate at estimating the likelihood of themselves or the average person having any side effect from taking tamoxifen. Participants in the absolute frequency format rated the risk to health from tamoxifen as lower than those in the other two formats, were more satisfied with the information they received than those in the verbal format, and felt there would be less impact of the information on tamoxifen use than those in the combined format.
Conclusions: These findings fail to confirm that the recommended use of combined descriptors for medicine side effects is unequivocally superior to absolute frequency alone. They also add weight to the growing body of research highlighting the deficiencies in using verbal descriptors for conveying side effect risk, and the strength of using absolute frequency descriptors.







Similar content being viewed by others
References
Department of Health. Patient and public involvement in health: the evidence for policy implementation. London: The Stationery Office, 2004 May
Department of Health. High quality care for all: NHS next stage review final report. London: The Stationery Office, 2008 Jun. Report no. CM 7432
Grime J, Blenkinsopp A, Raynor DK, et al. The role and value of written information for patients about individual medi-cines: a systematic review. Health Expect 2007; 10: 286–98
Berry DC, Gillie T, Banbury SP. What do patients want to know: an empirical approach to explanation generation and validation. Expert Syst Appl 1995; 8: 419–28
Berry DC, Michas IC, Gillie T, et al. What do patients want to know about their medicines and what do doctors want to tell them? A comparative study. Psychol Health 1997; 12: 467–80
Ziegler DK, Mosier MC, Buenaver M, et al. How much information about adverse effects of medication do patients want from physicians? Arch Intern Med 2001; 161: 706–3
Council of the European Communities. Directive 2001/83/ EC of the European Parliament and of the Council on the Community code relating to medicinal products for human use. OJEC 2001 Nov; 311: 67–128
Holmes ER, Desselle SP, Nath DM, et al. Ask the pharmacist: an analysis of online drug information services. Ann Pharmacother 2005 Apr; 39(4): 662–7
CancerHelp UK [online]. Available from URL: http://www.cancerhelp.org.uk [Accessed 2009 Jul 27]
Raynor DK, Knapp P, Berry DC. Side effects and patients. In: Lee A, editor. Adverse drug reactions. 2nd ed. London: Pharmaceutical Press, 2006: 23–41
European Commission. Draft guideline on the readability of the label and package leaflet of medicinal products for human use (revision). EC Pharmaceuticals Committee. Brussels: European Commission, 2006 Sep
Medicines and Healthcare products Regulatory Agency. Always read the leaflet: getting the best information with every medicine. Report of the Committee on Safety of Medicines Working Group on Patient Information. London: The Stationery Office, 2005. Report no. 180613 C6 7/05
Carrigan N, Raynor DK, Knapp P. Adequacy of patient information on adverse effects: an assessment of patient information leaflets in the UK. Drug Saf 2008; 31: 305–12
Berry DC, Raynor DK, Knapp P. Communicating risk of medication side effects: an empirical evaluation of EU re-commended terminology. Psychol Health Med 2003; 8
Berry DC, Knapp P, Raynor DK. Provision of information about drug side effects to patients. Lancet 2002; 359(9309): 853–4
Berry DC, Knapp P, Raynor DK. Is 15% very common? Informing people about the risks of medicine side effects. Int J Pharm Pract 2002; 10: 145–51
Berry DC, Holden W, Bersellini E. Interpretation of recommended risk terms: differences between doctors and lay people. Int J Pharm Prac 2004; 12: 117–24
Knapp P, Gardner PH, Carrigan N, et al. Perceived risk of medicine side effects in users of a patient information website: a study of the use of verbal descriptors, percentages and natural frequencies. Br J Health Psych 2009; 14: 579–94
Knapp P, Raynor DK, Berry DC. Comparison of two methods of presenting risk information to patients about the side effects of medicines. Qual Saf Health Care 2004; 13: 176–80
Berry DC, Raynor DK, Knapp P, et al. Patients’ understanding of risk with medication use: impact of European Commission Guidelines and other risk scales. Drug Saf 2003; 26(1): 1–11
Gigerenzer G, Edwards A. Simple tools for understanding risks: from innumeracy to insight. BMJ 2004; 327: 741–4
Bowskill R, Clatworthy J, Parham R, et al. Patients’ perceptions of information received about medication prescribed for bipolar disorder: implications for informed choice. J Affect Disord 2007; 100: 253–7
Berry DC, Hochhauser M. Verbal labels can triple perceived risk in clinical trials. Drug Inf J 2006; 40(3): 249–58
Rice RE. Influences, usage, and outcomes of internet health information seeking: multivariate results from the Pew surveys. Int J Med Inform 2006; 75: 8–28
British national formulary 56, September 2008 [online]. Available from URL: http://www.bnf.org/bnf/ [Accessed 2009 Jan 27]
Acknowledgements
This study was funded by the University of Leeds.
None of the authors have any conflicts of interest to declare that are relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knapp, P., Raynor, D.K., Woolf, E. et al. Communicating the Risk of Side Effects to Patients. Drug-Safety 32, 837–849 (2009). https://doi.org/10.2165/11316570-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11316570-000000000-00000