Abstract
Panitumumab (Vectibix®) is a recombinant, fully human, IgG2 anti-epidermal growth factor receptor (EGFR) monoclonal antibody. This article reviews the clinical efficacy of intravenous panitumumab in combination with chemotherapy in the first- and second-line treatment of metastatic colorectal cancer and as monotherapy in chemotherapy-refractory metastatic colorectal cancer, as well as summarizing its pharmacological properties and tolerability. Panitumumab is indicated for use in patients with wild-type rather than mutant KRAS tumours.
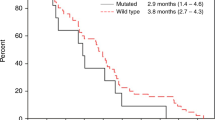
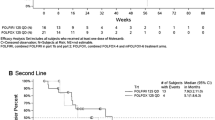
The efficacy of intravenous panitumumab 6 mg/kg administered every 2 weeks was examined in randomized, open-label, multicentre, phase III trials in patients with metastatic colorectal cancer. When administered as first- or second-line treatment in combination with chemotherapy, panitumumab plus chemotherapy prolonged progression-free survival to a significantly greater extent than chemotherapy alone in patients with wild-type KRAS tumours; no significant between-group difference in overall survival was seen in the second-line treatment trial. In patients with mutant KRAS tumours, progression-free survival was significantly shorter with panitumumab plus oxaliplatin-based chemotherapy than with oxaliplatin-based chemotherapy alone in the first-line treatment trial, with no significant difference between patients receiving panitumumab plus irinotecan-based chemotherapy (FOLFIRI) and those receiving FOLFIRI alone in the second-line treatment trial. In chemotherapy-refractory patients with metastatic colorectal cancer, panitumumab monotherapy plus best supportive care prolonged progression-free survival to a significantly greater extent than best supportive care alone in both the overall population and in patients with wild-type KRAS tumours, but not in those with mutant KRAS tumours; there was no significant between-group difference in overall survival.
Panitumumab has an acceptable tolerability profile when administered as monotherapy or in combination with chemotherapy. It is associated with the skin-related toxicities characteristic of EGFR inhibitors and appears to have a low risk of immunogenicity.
In conclusion, in patients with wild-type KRAS tumours, panitumumab is a useful option in combination with chemotherapy for the first- and second-line treatment of metastatic colorectal cancer or as monotherapy for the treatment of chemotherapy-refractory metastatic colorectal cancer.





Similar content being viewed by others
References
Center MM, Jemal A, Smith RA, et al. Worldwide variations in colorectal cancer. CA Cancer J Clin 2009 Nov–Dec; 59(6): 366–78
Mancl EE, Kolesar JM, Vermeulen LC. Clinical and economic value of screening for Kras mutations as predictors of response to epidermal growth factor receptor inhibitors. Am J Health Syst Pharm 2009 Dec 1; 66(23): 2105–12
Yang X-D, Jia X-C, Corvalan JRF, et al. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res 1999 Mar 15; 59(6): 1236–43
Modjtahedi H, Essapen S. Epidermal growth factor receptor inhibitors in cancer treatment: advances, challenges and opportunities. Anticancer Drugs 2009 Nov; 20(10): 851–5
Ortega J, Vigil CE, Chodkiewicz C. Current progress in targeted therapy for colorectal cancer. Cancer Control 2010 Jan; 17(1): 7–15
Yang X-D, Jia X-C, Corvalan JRF, et al. Development of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer therapy. Crit Rev Oncol Hematol 2001; 38: 17–23
Banck MS, Grothey A. Biomarkers of resistance to epidermal growth factor receptor monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res 2009 Dec 15; 15(24): 7492–501
Foon KA, Yang X-D, Weiner LM, et al. Preclinical and clinical evaluations of ABX-EGF, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys 2004 Mar 1; 58(3): 984–90
Yang X-D, Jia X-C, Corvalan JR, et al. Therapeutic potential of ABX-EGF, a fully human anti-EGF receptor monoclonal antibody, for cancer treatment [abstract no. 183]. 36th Annual Meeting of the American Society of Clinical Oncology; 2000 May 20–23; New Orleans (LA)
Freeman D, McDorman K, Bush T, et al. Mono- and combination-therapeutic activity of panitumumab (ABX-EGF) on human A431 epidermoid and HT-29 colon carcinoma xenografts: correlation with pharmacodynamic parameters [abstract no. 313]. 16th EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; 2004 Sep 28–Oct 1; Geneva
Foltz IN, King CT, Liang M, et al. Panitumumab induces internalization of the epidermal growth factor receptor (EGFr) [abstract no. B43]. 17th AACR-NCI EORTC International Conference on Molecular Targets and Cancer Therapeutics; 2005 Nov 14–18; Philadelphia (PA)
Amgen Inc. Vectibix® (panitumumab) solution for intravenous infusion: US prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125147s080lbl.pdf [Accessed 2010 Jan 14]
Giannopoulou E, Antonacopoulou A, Matsouka P, et al. Autophagy: novel action of panitumumab in colon cancer. Anticancer Res 2009 Dec; 29(12): 5077–82
Stephenson JJ, Gregory C, Burris H, et al. An open-label clinical trial evaluating safety and pharmacokinetics of two dosing schedules of panitumumab in patients with solid tumors. Clin Colorectal Cancer 2009 Jan; 8(1): 29–37
Lynch DH, Yang X-D. Therapeutic potential of ABX-EGF: a fully human anti-epidermal growth factor receptor monoclonal antibody for cancer treatment. Semin Oncol 2002 Feb; 29 (1 Suppl. 4): 47–50
Doi T, Ohtsu A, Tahara M, et al. Safety and pharmacokinetics of panitumumab in Japanese patients with advanced solid tumors. Int J Clin Oncol 2009 Aug; 14(4): 307–14
Rowinsky EK, Schwartz GH, Gollob JA, et al. Safety, pharmacokinetics, and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol 2004 Aug 1; 22(15): 3003–15
European Medicines Agency. Vectibix (panitumumab) 20 mg/mL concentrate for solution for infusion: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/humandocs/PDFs/EPAR/vectibix/H-741-PI-en.pdf [Accessed 2010 Jan 14]
Weiner LM, Belldegrun AS, Crawford J, et al. Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin Cancer Res 2008 Jan 15; 14(2): 502–8
Ma P, Yang B-B, Wang Y-M, et al. Population pharmacokinetic analysis of panitumumab in patients with advanced solid tumors. J Clin Pharmacol 2009 Oct; 49(10): 1142–56
Yang B-B, Hecht JR, Malik I, et al. Pharmacokinetics (pk) of panitumumab and irinotecan were not altered after first-line panitumumab therapy with irinotecan, 5-fluorouracil, and leucovorin (IFL) in metastatic colorectal cancer (mCRC) patients (pts) [abstract no. 311P]. Ann Oncol 2004; 15 Suppl. 3: iii83
Siena S, Cassidy J, Tabernero J, et al. Randomized phase III study of panitumumab (pmab) with FOLFOX4 compared to FOLFOX4 alone as first-line treatment (tx) for metastatic colorectal cancer (mCRC): PRIME trial [abstract no. 283]. 2010 Gastrointestinal Cancers Symposium; 2010 Jan 22–24; Orlando (FL)
Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009 Feb 10; 27(5): 672–80
Berlin J, Posey J, Tchekmedyian S, et al. Panitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancer. Clin Colorectal Cancer 2007 Mar; 6(6): 427–32
Köhne C-H, Mineur L, Greil R, et al. Primary analysis of a phase II study (20060314) combining first-line panitumumab (pmab) with FOLFIRI in the treatment of patients with metastatic colorectal cancer [abstract no. 414 plus poster]. 2010 Gastrointestinal Cancers Symposium; 2010 Jan 22–24; Orlando (FL)
Peeters M, Price TJ, Hotko YS, et al. Randomized phase III study of panitumumab (pmab) with FOLFIRI versus FOLFIRI alone as second-line treatment (tx) in patients (pts) with metastatic colorectal cancer (mCRC): patient-reported outcomes (PRO) [abstract no. 282]. 2010 Gastrointestinal Cancers Symposium; 2010 Jan 22–24; Orlando (FL)
Cohn AL, Smith DA, Neubauer MA, et al. Results from panitumumab (pmab) regimen evaluation in colorectal cancer to estimate primary response to treatment (PRECEPT): second-line treatment with pmab and FOLFIRI by tumor KRAS status [abstract no. 4067 plus poster]. 45th Annual Meeting of the American Society of Clinical Oncology; 2009 May 29–Jun 2; Orlando (FL)
Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010 Mar 10; 28(8): 1351–7
Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007 May 1; 25(13): 1658–64
Hecht JR, Patnaik A, Berlin J, et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer 2007 Sep 1; 110(5): 980–8
Hecht JR, Mitchell E, Neubauer MA, et al. Lack of correlation between epidermal growth factor receptor status and response to panitumumab monotherapy in metastatic colorectal cancer. Clin Cancer Res 2010 Apr 1; 16(7): 2205–13
Muro K, Yoshino T, Doi T, et al. A phase 2 clinical trial of panitumumab monotherapy in Japanese patients with metastatic colorectal cancer. Jpn J Clin Oncol 2009 May; 39(5): 321–6
National Comprehensive Cancer Network. NCCN clinical practice guidelines in onoclogy: colon cancer. v.2.2010 [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf [Accessed 2010 Feb 22]
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: rectal cancer. V.2.010 [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf [Accessed 2010 Feb 22]
Douillard J, Siena S, Cassidy J, et al. Randomized phase 3 study of panitumumab with FOLFOX4 compared to FOLFOX4 alone as 1st-line treatment (tx) for metastatic colorectal cancer (mCRC): the PRIME trial [abstract no. 10LBA]. 15th European Cancer Conference and the 34th Congress of the European Society for Medical Oncology; 2009 Sep 20–24; Berlin
Amgen. PRIME: panitumumab randomized trial in combination with chemotherapy for metastatic colorectal cancer to determine efficacy [ClinicalTrials.gov identifier NCT00364013]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2010 Jan 8]
Peeters M, Price T, Hotko Y, et al. Randomized phase 3 study of panitumumab (pmab) with FOLFIRI vs FOLFIRI alone as second-line treatment (tx) in patients with metastatic colorectal cancer (mCRC) [abstract no. 14LBA]. 15th European Cancer Conference and the 34th Congress of the European Society for Medical Oncology; 2009 Sep 20–24; Berlin
Amgen. Comparison of treatment effect of chemotherapy with panitumumab to chemotherapy alone [ClinicalTrials.gov identifier NCT00339183]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2010 Jan 8]
Van Cutsem E, Siena S, Humblet Y, et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol 2008 Jan; 19(1): 92–8
Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008 Apr 1; 26(10): 1626–34
Siena S, Peeters M, Van Cutsem E, et al. Association of progression-free survival with patient-reported outcomes and survival: results from a randomised phase 3 trial of panitumumab. Br J Cancer 2007 Dec 3; 97(11): 1469–74
Amado RG, Wolf M, Freeman D, et al. Association of KRAS mutational status and efficacy of panitumumab monotherapy for the treatment (TX) of metastatic colorectal cancer (MCRC): results of pooled data from 4 clinical studies [abstract no. 359P]. 33rd Congress of the European Society for Medical Oncology; 2008 Sep 12–16; Stockholm
Carcereny E, Castellvi-Bel S, Alonso V, et al. EGFR polymorphisms as predictors of clinical outcome in patients with advanced colorectal cancer (ACRC) treated with cetuximab and panitumumab [abstract no. 4124]. 44th Annual Meeting of the American Society of Clinical Oncology; 2008 May 30–Jun 2; Chicago (IL)
Siena S, Sartore-Bianchi A, Di Nicolantonio F. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst 2009 Oct 7; 101(19): 1308–24
Moroni M, Veronese S, Benvenuti S, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol 2005 May; 6(5): 279–86
Sartore-Bianchi A, Moroni M, Veronese S, et al. Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol 2007 Aug 1; 25(22): 3238–45
Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008 Dec 10; 26(35): 5705–12
Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res 2009 Mar 1; 69(5): 1851–7
Perkins G, Lièvre A, Ramacci C, et al. Additional value of EGFR downstream signaling phosphoprotein expression to KRAS status for response to anti-EGFR antibodies in colorectal cancer. Int J Cancer. Epub 2010 Jan 4
Douillard J, Peeters M, Kohne C, et al. Safety of panitumumab (pmab) in combination with chemotherapy (ct) from five clinical trials in 812 patients (pts) with metastatic colorectal cancer (mCRC) and wild-type (WT) KRAS tumors [abstract no. 409]. 2010 Gastrointestinal Cancers Symposium; 2010 Jan 22–24; Orlando (FL)
Starcevic M, Weeraratne D, Lofgren JA, et al. Panitumumab immunogenicity in two phase III trials of patients with metastatic colorectal cancer (mCRC) treated with panitumumab plus FOLFIRI or FOLFOX4 [abstract no. 433]. 2010 Gastrointestinal Cancers Symposium; 2010 Jan 22–24; Orlando (FL)
Peeters M, Siena S, Van Cutsem E, et al. Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer 2009 Apr 1; 115(7): 1544–54
Panitumumab: Japanese prescribing information. Takeda, 2010
European Medicines Agency. Erbitux (cetuximab) 2mg/mL solution for infusion: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/humandocs/PDFs/EPAR/erbitux/H-558-PI-en.pdf [Accessed 2010 Jan 21]
ImClone Systems Incorporated and Bristol-Myers Squibb Company. Erbitux® (cetuximab) solution for intravenous infusion: US prescribing information [online]. Available from URL: http://packageinserts.bms.com/pi/pi_erbitux.pdf [Accessed 2010 Apr 27]
Weiner LM. Fully human therapeutic monoclonal antibodies. J Immunother 2006 Jan/Feb; 29(1): 1–9
VanCutsem EJD, Oliveira J. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008 May; 19 Suppl. 2: ii33–4
Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2010 Nov 15; 357(20): 2040–8
Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009 Feb 5; 360(6): 563–72
Marshall JL. Vascular endothelial growth factor plus epidermal growth factor receptor dual targeted therapy in metastatic colorectal cancer: synergy or antagonism? J Oncol. Epub 2009 Dec 6
Spanish Cooperative Group for Gastointestinal Tumour Therapy. Safety and efficacy study of FOLFOX4+ panitumumab vs FOLFIRI+ panitumumab in subjects WT KRAS colorectal cancer and liver-only metastases (PLANET) [ClinicalTrials.gov identifier NCT00885885]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2010 Feb 26]
Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009 Apr 20; 27(12): 2091–6
Graham CN, Borker R, Oppe M, et al. Cost-effectiveness of panitumumab plus best supportive care (BSC) compared with BSC alone in chemorefractory metastatic colorectal cancer (MCRC) patients with wild-type (WT) KRAS tumor status in the Netherlands [abstract no. 387P]. 33rd Congress of the European Society for Medical Oncology; 2008 Sep 12–16; Stockholm
Tan EH, Chan A. Evidence-based treatment options for the management of skin toxicities associated with epidermal growth factor receptor inhibitors. Ann Pharmacother 2009 Oct; 43(10): 1658–66
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: A.B. Benson III, Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, USA; J.-L. Canon, Medical Oncology, Grand Hopital de Charleroi, Charleroi, Belgium; J. Cassidy, Cancer Research UK, Glasgow, Scotland; A. Hendlisz, Medical Oncology Clinic, Institut Jules Bordet, Brussels, Belgium; T.J. Price, Department of Oncology, The Queen Elizabeth Hospital and University of Adelaide, Adelaide, South Australia, Australia; W. Scheithauer, Division of Clinical Oncology, Department of Internal Medicine I and Cancer Center, Medical University Vienna, Vienna, Austria; J.-L. Van Laethem, Department of Gastroenterology–GI Cancer Unit, Erasme University Hospital, Brussels, Belgium.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘panitumumab’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘panitumumab’ and ‘colorectal cancer’. Searches were last updated 27 April 2010.
Selection: Studies in patients with metastatic colorectal cancer who received panitumumab. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Panitumumab, metastatic colorectal cancer, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Keating, G.M. Panitumumab. Drugs 70, 1059–1078 (2010). https://doi.org/10.2165/11205090-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11205090-000000000-00000