Abstract
Background: Hand-held electronic devices may provide a simple reproducible means by which quality of life (QOL) may be documented in patients with cancer. However, the QOL scales that are routinely used were originally validated when used with paper and pencil data collection. Patient-reported outcomes acquired using hand-held electronic devices (electronic patient-reported outcomes [ePRO]) may not be the same as those acquired using paper and pencil, so validation of this method of data collection is needed.
Objectives: This study aimed to compare the results of e-PRO and paper and pencil collection of Functional Assessment of Cancer Therapy-Lung (FACT-L) and EuroQol-5 Dimension (EQ-5D) QOL data in patients with advanced non-small cell lung cancer (NSCLC), and to ascertain patients’ preferences for the different modes of collection.
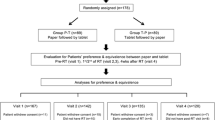
Methods: This randomized, single-cohort, crossover study was performed in a tertiary referral hospital cancer center. Fifty patients with previously treated locally advanced or metastatic NSCLC were randomized in a 1: 1 ratio to complete either paper versions of the questionnaires (FACT-L and EQ-5D) followed by the e-PRO versions, or the e-PRO questionnaire followed by the paper versions.
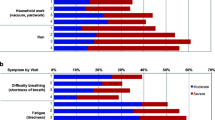
Results: The majority (88%) of the FACT-L and all (100%) of the EQ-5D individual question responses were within +1 point of each other when data collection via e-PRO and via pencil and paper were compared. There was no significant difference between the mean total FACT-L scores obtained using the two methods; however, 29% of patients had a difference between FACT-L total scores obtained with the two methods that was greater than ±6 points. The mean completion time was shorter for the paper and pencil method than the e-PRO method (p < 0.0001). However, most patients stated that they preferred the e-PRO method over paper and pencil (60% vs 12%).
Conclusion: This study suggests that the mode of administration of the FACT-L and EQ-5D had a relatively small effect on the mean responses given to the questionnaires in patients with advanced NSCLC. However, at the individual patient level, data varied considerably between the different modes of administration. Therefore, the group results obtained using the e-PRO should be similar to the originally validated paper method, with the advantages of improved patient acceptability and ease of reliable interfacing with trial databases.






Similar content being viewed by others
References
Non Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995; 311: 899–909
Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 1995; 12: 199–220
Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) study 5592. J C Epidemiol 2002; 55: 285–95
EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208
International Society for Pharmacoeconomics and Outcomes Research. Patient reported outcomes special interest group [online]. Available from URL: http://www.ispor.org/sigs/PRO_e.asp [Accessed 2007 Jul 25]
Taenzer PA, Speca M, Atkinson MJ, et al. Computerised quality-of-life screening in an oncology clinic. Cancer Pract 1997; 5: 168–75
Hollen PJ, Gralla RJ, Kris MG, et al. A comparison of visual analogue and numerical rating scale formats for the Lung Cancer Symptoms Scale (LCSS): does format affect patient ratings of symptoms and quality of life? Qual Life Res 2005; 14: 837–47
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 327: 307–10
Velikova G, Wright EP, Smith AB, et al. Automated collection of quality-of life data: a comparison of paper and computer touchscreen questionnaires. J Clin Oncol 1999; 17: 998–1007
Hollen P, Gralla R, Kuruvilla P, et al. Evaluating quality of life and patient reported outcomes in non-small cell lung cancer: a prospective study using a handheld computerized form of the validated LCSS instrument in clinical trials and clinical practice. 11th World Lung Cancer Conference; 2005 Jul 3–6; Barcelona
Acknowledgments
AstraZeneca UK provided funding for the conduct of the study, and were involved in study design, management, analysis, and the preparation and review of the manuscript.
CRF Inc. provided the electronic diaries.
Drs Watkins and Meddis are employees of Astra-Zeneca UK. The remaining authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ring, A.E., Cheong, K.A., Watkins, C.L. et al. A Randomized Study of Electronic Diary versus Paper and Pencil Collection of Patient-Reported Outcomes in Patients with Non-Small Cell Lung Cancer. Patient-Patient-Centered-Outcome-Res 1, 105–113 (2008). https://doi.org/10.2165/01312067-200801020-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/01312067-200801020-00006