Abstract
Background: NSAIDs are widely used for patients presenting with low back pain. A quick-release formulation of lornoxicam, a potent NSAID from the chemical class of oxicams, offers a faster onset of pain relief compared with the standard tablet formulation.
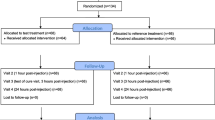
Methods: Time to onset of pain relief with lornoxicam was compared with the quick-release formulation of diclofenac potassium in acute low back pain in a randomised, double-blind, multicentre study. 220 patients received either lornoxicam 24mg or diclofenac potassium 150mg on day 1 followed by lornoxicam 8mg twice daily or diclofenac potassium 50mg twice daily for 5 days. Efficacy outcomes included time to onset of pain relief, as measured by the stopwatch method (primary outcome), pain intensity, pain relief, rescue medication, ability to perform daily activities and global evaluation of the study medication.
Results: The time to onset of pain relief ratios between diclofenac potassium/ lornoxicam was 1.03 (95% CI 0.91, 1.26) and 1.05 (95% CI 0.93, 1.29) in the intention-to-treat (ITT) and per-protocol (PP) analyses, respectively, demonstrating the non-inferiority of lornoxicam (defined by lower limits of the 95% CIs >0.80). Time to onset of pain relief was shorter with lornoxicam (30 minutes) compared with diclofenac potassium (36 minutes). The difference was not statistically significant (ITT analysis). A higher magnitude of analgesic effect associated with better global evaluation of the study medication for lornoxicam was also demonstrated. The drugs were equally well tolerated.
Conclusion: Lornoxicam administered as a quick-release formulation was shown to be non-inferior to the equivalent formulation of diclofenac potassium in terms of onset of pain relief and more effective on most of the major standard efficacy outcomes.









Similar content being viewed by others
References
Speed C. Low back pain. BMJ 2004; 328: 1119–21
Andersson GBJ. The epidemiology of spinal disorders. In: Frymoyer JW, editor. The adult spine; principles and practice. 2nd ed. Philadelphia (PA): Lippincott-Raven Publishers, 1997: 93–141
Jellema P, VanTulder MW, Van Poppel MN, et al. Lumbar supports for prevention and treatment of low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine 2001; 26(4): 377–86
Lahad A, Malter AD, Berg AO, et al. The effectiveness of four interventions for the prevention of low back pain. JAMA 1994, 1291
Hillman M, Wright A, Rajaratnam G, et al. Prevalence of low back pain in the community: implications for services provision in Bradford, UK. J Epidemiol Community Health 1996; 50: 347–52
Mayrhofer F, Siegmeth W, Singer F, et al. A multicenter, randomised, double-blind study comparing lornoxicam with conventional diclofenac in patients with chronic low back pain. Annals of Experimental and Clinical Medicine 1994; 5-6: 283–90
Schnitzer TJ, Ferraro A, Hunsche E, et al. A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain. J Pain Symptom Manage 2004; 28(1): 72–95
Bigos S, Bowyer O, Braen G. Acute low back pain problems in adults: clinical practice No. 14. AHCPR Publication No. 95-0643. Rockville (MD): Agency for Health Care Policy and Research, Public Health Services, US, Department of Health and Human Services, 1994
New Zealand Acute Low Back Pain Guide. ACC (Accident Rehabilitation and Compensation Insurance Corporation) and National Health Committee, Wellington, New Zealand 1997
Waddell G, McIntosh A, Hutchinson A, et al. Low back pain evidence review. London: Royal College of General Practitioners, 1999
Danish Institute for Health Technology Assessment (DIHTA). Low back pain: frequency, management and prevention from an HTA perspective. Copenhagen, Denmark: DIHTA. 1st ed. 1999
VanTulder MW, Scholten RJ, Koes BW, et al. Non steroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine 2000; 25(19): 2501–13
Balfour JA, Fitton A, Barradell LB. Lornoxicam: a review of its pharmacology and therapeutic potential in the management of painful inflammatory conditions. Drugs 1996; 51(4): 639–57
Radhofer-Welte S, Rabasseda X. Lornoxicam, a new potent NSAID with an improved tolerability profile. Drugs Today 2000; 36(1): 55–76
Hitzenberger G, Radhofer-Welte S, Takacs SF, et al. Pharmacokinetics of lornoxicam in man. Postgrad Med J 1990; 66:Suppl. 4: 22–6
Moller PL, Norholt SE. Analgesic efficacy of lornoxicam as a quick release tablet versus standard tablet in postoperative pain: a randomised, double-blind, placebo-controlled trial in the third molar surgery model. In preparation
Bakshi R, Jacobs LD, Lehnert S, et al. A double-blind, placebo-controlled trial comparing the analgesic efficacy of two formulations of diclofenac in postoperative pain. Curr Ther Res Clin Exp 1992; 3(52): 435–42
EMEA. Committee for Proprietary Medicinal products (CPMP). Note for guidance on statistical principles for clinical trials. ICH-E9. 1998 Mar, CPMP/ICH/363/96
Rainer F, Klein G, Mayerhofer S, et al. A prospective, multicentre, open-label, uncontrolled phase II study of the local tolerability, safety and efficacy of intramuscular chlortenoxicam in patients with acute low back pain. Eur J Clin Res 1996; 8: 1–13
EMEA. Committee for Proprietary Medicinal Products (CPMP). Note for guidance on clinical investigation of medicinal products for treatment of nociceptive pain. Nov 2002, CPMP/EWP/612/00
Max MB, Laska EM. Single-dose analgesic comparisons: advances in pain research and therapy. In Max MB, Portenoy RK, Laska EM, editors. The design of analgesic clinical trials. New York: Raven Press, 1991: 55–96
Acknowledgements
This study was supported by an unrestricted grant from Nycomed. The authors have no conflicts of interest directly relevant to the content of this study.
The authors thank all the co-investigators for their valuable contributions to the performance of this study. The co-investigators were: E.V. Podchufarova, A.I. Isaykin, D.V. Galanov, K.S. Glushkov, T.V. Mikhailova, T.A. Skoromets, A.P. Rachin, O.M. Ustennaya, Y.V. Yurieva, V.A. Bulanova, S.B. Ossipova, A.G. Raybov, E.V. Zakhovskaya.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yakhno, N., Guekht, A., Skoromets, A. et al. Analgesic Efficacy and Safety of Lornoxicam Quick-Release Formulation Compared with Diclofenac Potassium. Clin. Drug Investig. 26, 267–277 (2006). https://doi.org/10.2165/00044011-200626050-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200626050-00004