Abstract
Acute bacterial meningitis remains an important cause of morbidity and mortality in children. Children <2 years of age are particularly susceptible to infection with encapsulated bacteria due to their immature response to polysaccharide antigens. Conjugate vaccines, which induce T cell memory, can provide immunological protection for these children.
The Haemophilus influenzae type b (Hib) conjugate vaccine was the first such vaccine to become available. The efficacy of the vaccine has been quoted as being 98%. Its introduction was followed by a dramatic decrease in the incidence of all invasive Hib disease, including meningitis. This reduction was in part due to the ability of these vaccines to reduce nasopharyngeal carriage of the organism and thereby induce herd immunity.
Different Hib vaccines use a variety of protein carriers and differ in their immunogenicity and efficacy. The most suitable vaccine needs to be determined according to the local epidemiology of Hib disease. Commercial combination vaccines may lead to lower antibody levels. A recent increase in the incidence of Hib disease in the UK highlights the importance of continued surveillance and the need for booster vaccinations to ensure continued protection.
Conjugate vaccines to Streptococcus pneumoniae and Neisseria meningitidis have been developed. The introduction of a pneumococcal conjugate vaccine in the US has led to a decrease in the rate of infection by nearly 60% in children <5 years of age. A reduction in pneumococcal carriage may also modify disease epidemiology.
The UK introduced the conjugate meningococcal C vaccine into its infant schedule with a corresponding reduction in N. meningitidis group C disease. A recent decrease in the effectiveness of the vaccine, however, suggests a booster may be necessary in the future. Our present understanding of the immunology of conjugate vaccines is far from complete.
Developed countries have introduced conjugate vaccines into their immunisation schedules to prevent bacterial meningitis; however, their high cost precludes their use in many developing countries. Progress needs to be made in order to get these highly effective vaccines to those areas that need them.
Similar content being viewed by others
Acute bacterial meningitis is an important cause of morbidity and mortality among children in both developed and developing countries.[1,2] Despite potent antimicrobial agents and optimal intensive care, mortality rates and neurological sequelae remain high.[3,4]
Children <2 years of age are at high risk of bacterial meningitis caused by Haemophilus influenzae type b (Hib), Streptococcus pneumoniae and Neisseria meningitidis.[2] The advent of conjugate vaccines has launched a new era in vaccinology. In contrast to polysaccharide vaccines, conjugate vaccines produce a T-cell-dependent response. This leads to the development of immunological memory and protection in children <2 years of age.[5,6]
In the early 1990s, the introduction of the Hib conjugate vaccine led to changes in the epidemiological profile of invasive diseases, particularly meningitis, caused by Hib in developed countries.[7,8] More recently, pneumococcal and meningococcal serogroup C conjugate vaccines have been included in vaccine schedules.[9–11]
The aim of this article is to review the impact of Hib conjugate vaccines on the incidence of invasive Hib disease, especially meningitis. It will also discuss the potential of the newer conjugate pneumococcal and meningococcal vaccines in relieving the burden of disease caused by these organisms around the world.
1. Haemophilus influenzae Type b (Hib) Disease
Hib is an important cause of invasive infection in children <5 years of age, with 60% of such infection being meningitis. Overall fatality for Hib meningitis is 5%, with up to 11% of survivors having neurological sequelae.[12]
In the UK, the majority of invasive disease due to Hib occurs during the first 2 years of life,[13] whilst in the developing world the majority of such disease occurs even earlier.[14] It is difficult to know the true burden of Hib disease, especially in developing countries. Annually, it is estimated to cause at least three million cases of serious disease and many hundreds of thousands of deaths worldwide.[15]
1.1 Hib Conjugate Vaccine
The first generation capsular polysaccharide Hib vaccines were found to be ineffective in children <18 months of age, in whom most Hib disease occurred. The reason for this poor response was that bacterial polysaccharides do not generate memory B cells in young infants. To improve the immunogenicity of these vaccines, the capsular polysaccharide polyribosylribitol phosphate (PRP) was conjugated to an immunogenic carrier protein. Four such vaccines, conjugated to different carrier proteins, have been licensed for use in children:
-
diphtheria toxoid as the carrier protein (PRP-D), with moderate antibody response after second dose;
-
CRM197 mutant Corynebacterium diphtheria toxin protein as the carrier (HbOC), with good antibody response after second dose;
-
N. meningitidis protein outer membrane protein as the carrier (PRP-OMP), with moderate antibody response after first dose;
-
tetanus toxoid as the carrier protein (PRP-T), with good antibody response after second dose.
The PRP conjugated to tetanus toxoid (PRP-T) is the most widely used.[16]
1.2 Vaccine Efficacy
The efficacy of the Hib vaccine is approximately 98%;[17,18] however, the various Hib vaccines, despite being immunogenic and efficacious, are not identical in performance.[19–22] Infant vaccination with Hib conjugates usually occurs whilst there is persistence of maternal antibodies and this can modestly suppress the response to Hib conjugate vaccines.[23] This is particularly important in a population with high infection rates in the first few weeks of life. A reduced antibody response to the first dose of vaccine could affect efficacy, even if the peak response following a full course remained unaltered.
The carrier protein also influences priming. In a comparison study of three different Hib conjugate vaccines given at 2, 3 and 4 months, the priming response for a ‘booster’ at 1 year of age was lower in magnitude for PRP-T compared with other carrier proteins.[24] The relevance of this measure of priming to long-term vaccine efficacy remains unclear.
Hib vaccines can be administered on their own or in combination with other vaccines. Coadministration creates the potential for interaction with other vaccine antigens. This interaction may be physical, between individual components, or the immune response to one antigen may be altered by the immune response to another. This complex interaction is illustrated by the combination of Hib vaccine with diphtheria, tetanus and acellular pertussis (DTaP) vaccine that led to reduced antibody levels to Hib after primary immunisation.[25] This effect was not seen with simultaneous administration at separate sites.
The choice of conjugate, number of vaccine doses and their timing, and the local epidemiology of invasive Hib disease are all important in determining vaccine efficacy. PRP-T and PRP conjugated to diphtheria toxoid (PRP-D) have been shown to be highly efficacious in several clinical trials;[26–28] however, a PRP-D conjugate vaccine that had shown efficacy in Finland did not confer protection in an Alaskan population with high levels of disease in early life.[29] The introduction of a Hib vaccine featuring PRP conjugated to an N. meningitidis outer membrane protein complex (PRP-OMP) in 1991 did lead to a substantial decline in Hib disease, reflecting the earlier onset of immunity with this conjugate.[29] When the regimen was changed to a combination preparation of an Hib conjugate vaccine with a CRM197 mutant C. diphtheria toxin protein (commonly referred to as HbOC) combined with diptheria, tetanus and pertussis (DTP) vaccine to reduce the number of injections at each visit, there was an increase in invasive Hib disease related to the later onset of immunity following the two doses of HbOC.[29]
A recent systematic review examining the effects of conjugate Hib vaccine in preventing Hib disease or death in children <5 years, when compared with placebo/control, included four trials in a meta-analysis.[30] The relative risk for invasive Hib disease following vaccination was 0.2 (95% CI 0.07, 0.54) compared with no vaccination, but there was statistically significant unexplained variation between the effects in the four trials (p = 0.002). The meta-analysis found no difference in the size of effect with different vaccine types, number of doses, age at first vaccination or use in developed/developing countries, but the confidence intervals for estimates were wide. Hib-related mortality data, available from two of the trials, showed a nonsignificant trend towards benefit from vaccination, with a relative risk of 0.29 (95% CI 0.07, 1.20) with no adverse effects in the 257 000 infants included. The relative risk for all-cause mortality in the single trial from which such data were available was 1.01 (95% CI 0.38, 2.67). The authors concluded that the size of the effect could be anywhere in a 46–93% reduction in invasive Hib disease, before the effect of herd immunity was taken into account.
1.3 Implementation
After the introduction of the Hib vaccine in the UK in 1992, rates of invasive Hib disease, including meningitis, declined markedly in children aged 0–4 years.[18,31] The vaccine was used for routine vaccination in infants at 2, 3 and 4 months of age and was offered to those aged <4 years as part of a ‘catch-up’ programme; those aged >12 months received a single dose. Similar reductions in invasive Hib disease following Hib vaccine introduction were seen for Finland, Canada, Iceland, the US, Israel and Australia.[32] Peltola[33] reviewed the reduction in rates of Hib meningitis in Europe, whilst Laval et al.[34] examined the worldwide impact (table I). Reductions in rates of Hib meningitis have been reported from all countries using Hib conjugate vaccines in their immunisation schedules.[34–38] The dramatic decline in Hib-related bacterial meningitis was also due to an unexpected bonus of the vaccine programme: carriage was decreased in recipients, leading to herd immunity whereby unvaccinated children were protected because they were less likely to be exposed to the infection.[39,40] This led to a more rapid decline in disease following introduction of the vaccines than if there was no herd immunity effect.[41] Economic analysis from the US has shown that the national Hib vaccination campaign starting at 2 months of age was highly cost beneficial and resulted in substantial cost savings compared with no vaccination against Hib.[42] Similar conclusions were reached in Sweden[43] and France.[44]
1.4 The Need for a Booster Dose
Implementation of the Hib conjugate vaccines in those countries able to afford them has shown remarkable success.[53] Most countries adopted regimens involving three doses in infancy followed by a fourth, booster dose in the second year of life. In contrast, the UK regimen used an accelerated schedule in which the Hib vaccine is given at 2, 3 and 4 months, but no booster. Studies had suggested that Hib disease was close to elimination in the UK and there was persistence of satisfactory antibody levels to 4.5 years of age using this schedule.[54] Within a few years, administration of a combined Hib-DTP vaccine became routine as studies suggested similar immunogenicity to the separately administered components.[55] There was considerable concern when infants immunised with an Hib-DTaP combination vaccine had Hib antibody titres below protective levels following three doses.[56] These children showed good immunological memory with substantial booster responses to further conjugate doses. Those immunised with separate Hib conjugate vaccines had Hib antibody levels below the ‘protective thresholds’ by the end of the first year of life, without developing Hib disease.[25]
An increase in Hib disease in the UK from 0.66/100 000 children <5 years of age in 1998 to 2.96/100 000 in 2002,[57] led to the demonstration of lowered efficacy rates in children vaccinated during infancy compared with those vaccinated after their first year of life.[58] Effectiveness was higher in children vaccinated after 1 year of age, which was consistent with the lowered immunogenicity of Hib vaccines conjugated to either tetanus toxoid or modified diphtheria toxin administered according to the UK schedule compared with the response to a single dose in toddlers in the US.[59,60] Estimated effectiveness declined beginning 2 years after vaccination, and this decline was most marked in those immunised during infancy.[58] This decline correlated with the low antibody levels observed in pre-school children,[59] and suggested that the immunological memory demonstrated in UK infants aged 1 year[61] may not be sufficient to provide long-term protection to all children. The use of the combination Hib and DTaP vaccine[62] between January 2000 and August 2002, together with the lack of a booster dose, exacerbated the increase in invasive disease. The increase could not be attributed to colonisation, because there was a marked reduction in the prevalence of Hib colonisation over the period.[63] A comparison of vaccinated and unvaccinated children presenting with Hib meningitis demonstrated immunological memory in the vaccinated children, suggesting that immunological priming was not necessarily protective.[64] A booster programme to provide pre-school children with a fourth Hib dose was rapidly instituted[65] and aimed to boost the anti-PRP antibodies in those immunised, to increase herd immunity. A recent study from Finland found no increase in Hib meningitis with a schedule using vaccine at 4, 6 and 14–18 months.[45] Our present understanding of the relative importance of circulating antibody and of immunological memory in the prediction of protection provided by conjugate vaccines is therefore far from complete, but a booster dose in the second year of life seems to give longer protection.
1.5 The Resource-Poor Setting
The majority of Hib disease occurs in the countries that are the least able to purchase and implement routine immunisation. Despite the availability of Hib conjugate vaccines, approximately 132 million children worldwide have still not been immunised with this vaccine, most of them in resource-poor countries where Hib is the leading cause of bacterial meningitis.[1,66]
The introduction of these vaccines has been hampered by several factors. The most critical issue is cost. In South Africa, Hib vaccine costs six times more per dose than the total costs of a routine immunisation schedule with the DTP, polio, measles and Bacille Calmette-Guérin (BCG) vaccines.[30] Impeded increases in per capita spending on health due to unfavourable economic and financial situations make it unfeasible to introduce the Hib vaccine.[67] A possible way of combating the price issue is reducing the number of doses given; two primary doses could be given instead of three,[33] and although a booster dose is recommended in most countries, it may not be necessary in developing countries where most of the Hib disease occurs before 12–18 months of age;[14,68] however, the importance of control of carriage in controlling disease should not be underestimated.
The Global Alliance for Vaccines and Immunisation (GAVI) has been developed to provide opportunities to build consensus around policies, strategies and priorities in developing countries and assign responsibility to those that have a comparative advantage. Through the Vaccine Fund, GAVI aims to provide financial resources to purchase vaccines and to support the operational costs of immunisation. By the end of 2003, support from GAVI and the Vaccine Fund had ensured that almost 5 million children had been immunised against Hib.[69]
The conclusion of a recent Cochrane review suggested that there was insufficient evidence about the effects of Hib conjugate vaccine on either Hib-specific or on all-cause mortality from present randomised controlled trials[30] and, therefore, further data are needed on these outcome measures to help determine the cost effectiveness of vaccine introduction. This is important in the setting of developing countries, because they have complex health problems, including malaria, tuberculosis and HIV, all competing for priority against Hib. Despite these problems, public health policy-makers need to define the burden of Hib disease, be aware of the potential magnitude of social costs, and maximise efforts to incorporate this vaccine into routine health services.[68,70]
2. Pneumococcal Disease
Pneumococcus presents a more complex problem than Hib regarding the future control by immunisation. With over 90 serotypes,[71] there is some limit to the number that can be practically included in a conjugate vaccine. Nasopharyngeal carriage rates of those serotypes included in a conjugate vaccine are reduced for up to 2 years following immunisation in infancy,[72] although the difference between vaccinated and unvaccinated children is less apparent by 3–4 years of age due to natural aquisition of mucosal immune responses.[73] However, there is a compensatory rise in colonisation with serotypes not in the vaccine,[74] with a modest increase in otitis media caused by these serotypes.[75] Another feature of pneumococcus is that it is capable of capsular switching, which could be selected for by immunisation.[76] It is therefore clear that monitoring of epidemiological trends will be especially important with vaccines against pneumococcal disease, although data from the US are encouraging and indicate significant herd immunity and consistent effectiveness in vaccine recipients.[77]
S. pneumoniae is involved in a broader range of disorders than Hib. Annually, between 100 000 and 500 000 deaths are attributable to pneumococcal meningitis in children <5 years of age.[78] In recent years, resistance to one or more antimicrobials has developed in certain serotypes of S. pneumoniae, adding to the difficulty of treating patients with pneumococcal disease.[79]
2.1 Pneumococcal Conjugate Vaccine
For the US and Finland, the epidemiology of bacterial meningitis in children made S. pneumoniae the logical second target for conjugate vaccines after Hib.
In February 2000, a conjugate pneumococcal vaccine was approved in the US for use in children <2 years of age. Of the 90 serotypes identified so far, this vaccine contained only seven of them: 4, 6B, 9V, 14, 18C, 19F and 23F. These are responsible for up to 90% of invasive disease in the US, Canada, Africa and Europe.[80,81] Clinical trials in the US suggest vaccine efficacy levels of 97% against invasive pneumococcal disease, including meningitis, resulting from infection with the serotypes contained in the vaccine.[82]
The nine-valent vaccine currently under trial contains the seven-valent serotypes plus serotypes 1 and 5. It has been evaluated in large scale trials in South Africa and the Gambia,[83] whilst trials of an 11-valent vaccine (including serotypes 3 and 7V) are underway in Israel, Argentina and Chile.[84,85]
It is hoped that a reduction in the nasopharyngeal carriage of pneumococci will modify the disease epidemiology of S. pneumoniae.[86–88] The introduction of the seven-valent vaccine in the US led to a decrease in the rate of infection by nearly 60% in children aged <5 years, but also a decrease in adults aged ≥20 years who had not received the vaccine.[77] This study also demonstrated that vaccination could reduce the number of antibiotic-resistant pneumococcal infections.
3. Meningococcal Disease
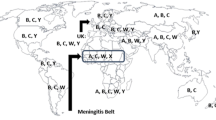
Meningococcal disease remains an important public health concern worldwide, especially in sub-Saharan Africa, where it occurs as regular epidemics.[89] Five of the 13 N. meningitidis groups cause most meningococcal disease: A, B, C, Y and W135. Serogroup A is responsible for epidemic disease in sub-Saharan Africa and in other developing countries. Serogroups B and C cause most of the infection in developed countries. The pattern of disease caused by serogroup B is typically sporadic. In the UK, 32% of the reports of invasive disease caused by N. meningitidis in 1996 were associated with serogroup C.[6]
3.1 Neisseria meningitidis Conjugate Vaccines
For the UK, the epidemiology of bacterial meningitis in children made N. meningitidis the second target after Hib. This was all the more important because of the steady rise in the incidence of invasive disease specifically due to N. meningitidis during the latter part of the 1990s.[90]
The polysaccharide vaccines against serogroups A and C were efficacious in older children and adults but much less immunogenic in children aged <5 years of age.[91] The conjugate vaccines, besides being more immunogenic, also offer herd immunity.
3.2 Group C
A dramatic reduction in the incidence of group C disease was observed after the introduction of the conjugate meningococcal group C vaccine into the UK immunisation programme.[92] Figures from 2002 showed that with a coverage of 89%, the incidence of serogroup C meningitis was reduced by 80% compared with historical data from before the introduction of the vaccine, whilst the number of deaths fell from 78 to 8 in the same period.[93] Cost-effectiveness analysis estimated a cost per life-year gained of £6259 (€10 726) for the meningococcal group C immunisation campaign in the UK, from its introduction in 1999 to 2001.[94] There has been a significant reduction in carriage rates of N. meningitidis group C in teenagers and young adults, coincident with introduction of immunisation.[95] Following the introduction of the meningococcal group C vaccine in the UK, it has been introduced in several other European countries, as well as in Canada. However, as with the Hib vaccine, protection has been age dependent, with the older age groups being found to have greater and longer lasting protection than those vaccinated in infancy.[96] Despite the rapid waning of vaccine effectiveness in infants, the number of cases of disease due to N. meningitidis group C in this age group remains low, probably due to herd immunity.[97] The implications of these findings would suggest that accelerated schedules may not be optimal for conjugate vaccines, and alternative vaccine schedules may need to be considered. This may require the administration of a booster dose in late infancy or a change in the age at which the final dose is given.
3.3 Group B
Despite the effectiveness of the meningococcal C conjugate vaccine campaign, only a portion of the worldwide meningococcal disease burden can be addressed by its use. Serogroup B meningococcus remains an important problem in Europe.[98,99] The serogroup B capsular polysaccharide is poorly immunogenic in humans because it resembles self antigen,[100] and an effective vaccine for widespread use is therefore still not available. Vaccines made from vesicles of meningococcal OMPs have been used successfully in Group B outbreaks. Their success, however, has been limited to outbreaks caused by a single circulating sero-subtype. Such a vaccine is being used in New Zealand at present, where a group B epidemic has been ongoing since 1991.[101] Several other OMPs have been identified and investigated but few offer promise for vaccine development.
3.4 Groups A, Y, W135
A vaccine focusing on the protection against serogroups A, C, Y and W135 meningococci offers the potential for addressing epidemic meningococcal disease in sub-Saharan Africa,[102] controlling the W135 strains associated with the Hajj,[103] and serogroup Y, which is particularly prevalent in the US.[104] Trials of the conjugate ACYW vaccine in infants have been disappointing,[105] despite promising results in adults and toddlers.[106,107]
In January 2005, a quadrivalent meningococcal conjugate vaccine was licensed for 11- to 55-year-olds (Menactra ®, manufactured by Sanofi Pasteur)Footnote 1. A single dose contains A, C, Y and W135 polysaccharides conjugated to diphtheria toxoid. Although not licensed for use 2- to 3-year-olds, Menactra was shown to be more immunogenic for all four serogroups than the polysaccharide vaccine Menomune ®, also manufactured by Sanofi Pasteur.[108] In addition, antibodies were found to persist for at least 2–3 years, although a large proportion of immunised children had suboptimal serum bactericidal antibody titres suggesting a lack of protection and the need for a booster dose.[109] Presently, routine immunisation of adolescents is recommended either at 11–12 years of age or before high school entry,[110] although an application has been filed with the US FDA to obtain approval for use in 2- to 10-year-olds.[111]
In Africa, the utilisation of two vaccines in an attempt to reduce the incidence of epidemic meningitis has begun. A heptavalent product (diphtheria-tetanus-whole pertussis [DTwP], hepatitis B, Hib, meningococcal conjugate A-C) is to be used in an expanded programme of immunisation, and a monovalent conjugate vaccine against meningococcus A will be used in the population aged 1–29 years.[112] The efficacy of this vaccine regimen against meningitis will need to be evaluated.
4. Future Use of Conjugate Vaccines
With an increasing number of conjugate vaccines becoming available, the potential for interaction with other vaccines increases. For example, a combination pneumococcal and meningococcal C vaccine showed reduced immunogenicity of the group C component and of concomitantly administered Hib and DTwP vaccines.[113] Studies confirming the safety and efficacy of combination conjugate vaccines are needed.
5. Conclusion
Polysaccharide conjugate vaccines have proven highly successful in preventing Hib meningitis, and show similar promise for S. pneumoniae and N. meningitidis. It will be important to continue to monitor and investigate possible vaccine failures. Surveillance of colonisation and disease isolates must remain an essential component of immunisation strategy. Combination vaccines that include conjugate vaccines will need to be studied carefully to ensure efficacy.
Advances in the primary prevention of bacterial meningitis have been made through the untargeted use of the conjugate vaccines in the immunisation schedules of wealthy countries. However, their high cost still precludes their use in many developing countries and progress needs to be made in order to get these highly effective vaccines to those areas that need them most.
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21 st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev 2000; 13(2): 302–17
Hausdorff W. Haemophilus, meningococcus and pneumococcus: comparative epidemiologic patterns of disease. Int J Clin Pract 2001; Suppl. 118: 2–4
Oostenbrink R, Maas M, Moons KG, et al. Sequelae after bacterial meningitis in childhood. Scand J Infect Dis 2002; 34(5): 379–82
Quagliarello VJ, Scheid WM. Treatment of bacterial meningitis. N Engl J Med 1997; 336(10): 708–16
Ada G. Vaccines and vaccination. N Engl J Med 2001; 345(14): 1042–53
Goldblatt D. Recent developments in bacterial conjugate vaccines. J Med Microbiol 1998; 47(7): 563–7
Adams WG, Deaver KA, Cochi SL, et al. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA 1993; 269(2): 221–6
Levine OS, Schwartz B, Pierce N, et al. Development, evaluation and implementation of Haemophilus influenzae type b vaccines for young children in developing countries: current status and priority actions. Pediatr Infect Dis J 1998; 17(9 Suppl.): S95–113
Eskola J, Anttila M. Pneumococcal conjugate vaccines. Pediatr Infect Dis J 1999; 18(6): 543–51
Salisbury D. Introduction of a conjugate meningococcal type C vaccine programme in the UK. J Paediatr Child Health 2001; 37(5): S34–6
Shinefield H. Pneumococcal conjugate vaccine and ongoing lessons. Int J Clin Pract 2001; Suppl. 118: 23–5
Department of Health. Salisbury DM, Begg NT, editors. Immunisation against infectious disease. London: HMSO, 1996
Booy R, Hodgson SA, Slack MP, et al. Invasive Haemophilus influenzae type b disease in the Oxford region (1985–91). Arch Dis Child 1993; 69(2): 225–8
Bijlmer HA, van Alphen L, Greenwood BM, et al. The epidemiology of Haemophilus influenzae meningitis in children under five years of age in The Gambia, West Africa. J Infect Dis 1990; 161(6): 1210–5
Global Programme for Vaccines and Immunization (GPV), World Health Organization. The WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec 1998; 73(10): 64–71
Kelly DF, Moxon ER, Pollard AJ. Haemophilus influenzae type b conjugate vaccines. Immunology 2004; 113(2): 163–74
The impact of Haemophilus influenzae immunisation on invasive infection in children. Commun Dis Rep CDR Wkly 1993; 3(51): 231
Booy R, Heath PT, Slack MP, et al. Vaccine failures after primary immunisation with Haemophilus influenzae type-b conjugate vaccine without booster. Lancet 1997; 349(9060): 1197–202
Granoff DM, Anderson EL, Osterholm MT, et al. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J Pediatr 1992; 121(2): 187–94
Decker MD, Edwards KM, Bradley R, et al. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr 1992; 120 (2 Pt 1): 184–9
Kayhty H, Eskola J, Peltola H, et al. Antibody responses to four Haemophilus influenzae type b conjugate vaccines. Am J Dis Child 1991; 145(2): 223–7
Schlesinger Y, Granoff DM. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. The Vaccine Study Group. JAMA 1992; 267(11): 1489–94
Englund JA, Glezen WP. Maternal immunization with Haemophilus influenzae type b vaccines in different populations. Vaccine 2003; 21(24): 3455–9
Granoff DM, Holmes SJ, Osterholm MT, et al. Induction of immunologic memory in infants primed with Haemophilus influenzae type b conjugate vaccines. J Infect Dis 1993; 168(3): 663–71
Eskola J, Ward J, Dagan R, et al. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 1999; 354(9195): 2063–8
Eskola J, Peltola H, Takala AK, et al. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in infancy. N Engl J Med 1987; 317(12): 717–22
Mulholland K, Hilton S, Adegbola R, et al. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants [published erratum appears in Lancet 1997; 350 (9076): 524]. Lancet 1997; 349(9060): 1191–7
Booy R, Moxon ER, MacFarlane JA, et al. Efficacy of Haemophilus influenzae type B conjugate vaccine in Oxford region. Lancet 1992; 340(8823): 847
Galil K, Singleton R, Levine OS, et al. Reemergence of invasive Haemophilus influenzae type b disease in a well-vaccinated population in remote Alaska. J Infect Dis 1999; 179(1): 101–6
Swingler G, Fransman D, Hussey G. Conjugate vaccines for preventing Haemophilus influenzae type b infections. Coch-rane Database Syst Rev 2003; (4): CD001729
Peltola H, Kallio MJ, Unkila-Kallio L. Reduced incidence of septic arthritis in children by Haemophilus influenzae type-b vaccination: implications for treatment. J Bone Joint Surg Br 1998; 80(3): 471–3
Heath PT. Haemophilus influenzae type b conjugate vaccines: a review of efficacy data. Pediatr Infect Dis J 1998; 17(9 Suppl.): S117–22
Peltola H. Haemophilus influenzae type b disease and vaccination in Europe: lessons learned. Pediatr Infect Dis J 1998; 17(9 Suppl.): S126–32
Laval CA, Pimenta FC, de Andrade JG, et al. Progress towards meningitis prevention in the conjugate vaccines era. Braz J Infect Dis 2003; 7(5): 315–24
Dickinson FO, Perez AE, Galindo MA, et al. Impact of vaccination against Haemophilus influenzae type b in Cuba [in Spanish]. Rev Panam Salud Publica 2001; 10(3): 169–73
Ribeiro GS, Reis JN, Cordeiro SM, et al. Prevention of Haemophilus influenzae type b (Hib) meningitis and emergence of serotype replacement with type a strains after introduction of Hib immunization in Brazil. J Infect Dis 2003; 187(1): 109–16
Agudelo CI, Munoz N, De la Hoz F. Rapid assessment of the impact of Haemophilus influenzae vaccine serotype b in Colombia [in Spanish]: Public Health Laboratories. Rev Panam Salud Publica 2000; 8(3): 181–4
Diaz JM, Catalan L, Urrutia MT, et al. Trends of etiology of acute bacterial meningitis in Chilean children from 1989 to 1998: impact of the anti-H influenzae type b vaccine [in Spanish]. Rev Med Chil 2001; 129(7): 719–26
Barbour ML, Mayon-White RT, Coles C, et al. The impact of conjugate vaccine on carriage of Haemophilus influenzae type b. J Infect Dis 1995; 171(1): 93–8
Hviid A, Melbye M. Impact of routine vaccination with a conjugate Haemophilus influenzae type b vaccine. Vaccine 2004; 22(3–4): 378–82
Takala AK, Santosham M, Meido-Hill J, et al. Vaccination with Haemophilus influenzae type b meningococcal protein conjugate vaccine reduces oropharyngeal carriage of Haemophilus influenzae type b among American Indian children. Pediatr Infect Dis J 1993; 12(7): 593–9
Zhou F, Bisgard KM, Yusuf HR, et al. Impact of universal Haemophilus influenzae type b vaccination starting at 2 months of age in the United States: an economic analysis. Pediatrics 2002; 110(4): 653–61
Trollfors B. Cost-benefit analysis of general vaccination against Haemophilus influenzae type b in Sweden. Scand J Infect Dis 1994; 26(5): 611–4
Livartowski A, Boucher J, Detournay B, et al. Cost-effectiveness evaluation of vaccination against Haemophilus influenzae invasive diseases in France. Vaccine 1996; 14(6): 495–500
Peltola H, Salo E, Saxen H. Incidence of Haemophilus influenzae type b meningitis during 18 years of vaccine use: observational study using routine hospital data. BMJ 2005; 330(7481): 18–9
Hanna JN. Impact of Haemophilus influenzae type b (Hib) vaccination on Hib meningitis in children in Far North Queensland, 1989 to 2003. Commun Dis Intell 2004; 28(2): 255–7
Ruocco G, Curto S, Savio M, et al. Vaccination against Haemophilus influenzae type b in Uruguay: experience and impact [in Spanish]. Rev Panam Salud Publica 1999; 5(3): 197–9
Dawson KG, Emerson JC, Burns JL. Fifteen years of experience with bacterial meningitis. Pediatr Infect Dis J 1999; 18(9): 816–22
Garpenholt O, Silfverdal SA, Hugosson S, et al. The impact of Haemophilus influenzae type b vaccination in Sweden. Scand J Infect Dis 1996; 28(2): 165–9
Teare EL, Fairley CK, White J, et al. Efficacy of Hib vaccine. Lancet 1994; 344(8925): 828–9
van Alphen L, Spanjaard L, van der Ende A, et al. Predicted disappearance of Haemophilus influenzae type b meningitis in Netherlands. Lancet 1994; 344(8916): 195
Zielen S, Ahrens P, Hofmann D. Efficacy of Hib vaccine. Lancet 1994; 344(8925): 828
Peltola H, Kilpi T, Anttila M. Rapid disappearance of Haemophilus influenzae type b meningitis after routine childhood immunisation with conjugate vaccines. Lancet 1992; 340(8819): 592–4
Heath PT, Bowen-Morris J, Griffiths D, et al. Antibody persistence and Haemophilus influenzae type b carriage after infant immunisation with PRP-T. Arch Dis Child 1997; 77(6): 488–92
Bell F, Martin A, Blondeau C, et al. Combined diphtheria, tetanus, pertussis, and Haemophilus influenzae type b vaccines for primary immunisation. Arch Dis Child 1996; 75(4): 298–303
Bell F, Heath P, Shackley F, et al. Effect of combination with an acellular pertussis, diphtheria, tetanus vaccine on antibody response to Hib vaccine (PRP-T). Vaccine 1998; 16(6): 637–42
Mc Vernon J, Moxon R, Heath P, et al. Haemophilus influenzae type b epiglottitis: article gives timely lesson [letter]. BMJ 2003; 326(7383): 284
Ramsay ME, McVernon J, Andrews NJ, et al. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J Infect Dis 2003; 188(4): 481–5
Heath PT, Booy R, Azzopardi HJ, et al. Antibody concentration and clinical protection after Hib conjugate vaccination in the United Kingdom. JAMA 2000; 284(18): 2334–40
Madore DV, Johnson CL, Phipps DC, et al. Safety and immunogenicity of Hib oligosaccharide-CRM197 conjugate vaccine in infants aged 15–23 months. Pediatrics 1990; 86: 527–34
Goldblatt D, Miller E, McCloskey N, et al. Immunological response to conjugate vaccines in infants: follow up study. BMJ 1998; 316(7144): 1570–1
McVernon J, Andrews N, Slack MP, et al. Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet 2003; 361(9368): 1521–3
McVernon J, Howard AJ, Slack MP, et al. Long-term impact of vaccination on Haemophilus influenzae type b (Hib) carriage in the United Kingdom. Epidemiol Infect 2004; 132(4): 765–7
McVernon J, Johnson PD, Pollard AJ, et al. Immunologic memory in Haemophilus influenzae type b conjugate vaccine failure. Arch Dis Child 2003; 88(5): 379–83
Heath PT, Ramsay ME. Haemophilus influenzae type b vaccine-booster campaign. BMJ 2003; 326(7400): 1158–9
Peltola H. Need for Haemophilus influenzae type b vaccination in Asia as evidenced by epidemiology of bacterial meningitis. Pediatr Infect Dis J 1998; 17(9 Suppl.): S148–51
World Health Organisation. Expert review of a tool for rapidly assessing Haemophilus influenzae type b (Hib) disease burden [online]. Available from URL: http://www.who.int/vaccines-documents/DocsPDF01/www.604.pdf [Accessed 2007 Jan 10]
Peltola H. Burden of meningitis and other severe bacterial infections of children in Africa: implications for prevention. Clin Infect Dis 2001; 32(1): 64–75
Vaccine fund progress report [online]. Available from URL: http://www.gavialliance.org [Accessed 2007 Apr 2]
Wenger JD, DiFabio J, Landaverde JM, et al. Introduction of Hib conjugate vaccines in the non-industrialized world: experience in four ‘newly adopting’ countries. Vaccine 1999; 18(7–8): 736–42
Henrichsen J. Typing of Streptococcus pnemoniae: past, present and future. Am J Med 1999; 107(1A): 50–4S
Mbelle N, Huebner RE, Wasas AD, et al. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis 1999; 180(4): 1171–6
Murdoch C, Lakshman R, Burkinshaw R, et al. Effect of heptavalent conjugate pneumococcal vaccine in infancy and booster polysaccharide vaccine at 13 months on nasopharyngeal carriage of S. pneumoniae in children aged 2–4 years [abstract]. Clin Infect Dis 2001; 33: 1148
Dagan R, Fraser D. Conjugate pneumococcal vaccine and antibiotic-resistant Streptococcus pneumoniae: herd immunity and reduction of otitis morbidity. Pediatr Infect Dis J 2000; 19(5 Suppl.): S79–87
Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med 2001; 344(6): 403–9
Gherardi G, Inostrozo JS, O’Ryan M, et al. Genotypic survey of recent beta-lactam-resistant pneumococcal nasopharyngeal isolates from asymptomatic children in Chile. J Clin Microbiol 1999; 37(11): 3725–30
Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348(18): 1737–46
Greenwood B. The epidemiology of pneumococcal infection in children in the developing world. Philos Trans R Soc Lond B Biol Sci 1999; 354(1384): 777–85
Whitney CG, Farley MM, Hadler J, et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med 2000; 343(26): 1917–24
Hausdorff WP, Bryant J, Paradiso PR, et al. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use (Pt I). Clin Infect Dis 2000; 30(1): 100–21
Hausdorff WP, Bryant J, Kloek C, et al. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use (Pt II). Clin Infect Dis 2000; 30(1): 122–40
Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000; 19(3): 187–95
Klugman KP, Madhi SA, Huebner RE, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003; 349(14): 1341–8
Mulholland K. Strategies for the control of pneumococcal diseases. Vaccine 1999; 17Suppl. 1: S79–84
Klugman KP. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect Dis 2001; 1(2): 85–91
Black SB, Shinefield HR, Hansen J, et al. Postlicensure evaluation of the effectiveness of seven valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2001; 20(12): 1105–7
Dagan R, Sikuler-Cohen M, Zamir O, et al. Effect of a conjugate pneumococcal vaccine on the occurrence of respiratory infections and antibiotic use in day-care center attendees. Pediatr Infect Dis J 2001; 20(10): 951–8
Dagan R, Melamed R, Muallem M, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis 1996; 174(6): 1271–8
Schwartz B, Moore PS, Broome CV. Global epidemiology of meningococcal disease. Clin Microbiol Rev 1989; 2 Suppl.: S118–24
Ramsay ME, Kaczmarski E, Rush M, et al. Changing patterns of case ascertainment and trends in meningococcal disease in England and Wales. Commun Dis Rep CDR Rev 1997; 7(4): R49–54
Gotschlich EC, Goldschneider I, Artenstein MS. Human immunity to the meningococcus. IV: immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med 1969; 129(6): 1367–84
Ramsay ME, Andrews N, Kaczmarski EB, et al. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 2001; 357(9251): 195–6
Trotter CL, Ramsay ME, Kaczmarski EB. Meningococcal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Commun Dis Public Health 2002; 5(3): 220–5
Trotter CL, Edmunds WJ. Modelling cost effectiveness of meningococcal serogroup C conjugate vaccination campaign in England and Wales. BMJ 2002; 324(7341): 809–14
Maiden MC, Stuart JM. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 2002; 359(9320): 1829–31
Trotter CL, Andrews NJ, Kaczmarski EB, et al. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 2004; 364(9431): 365–7
Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ 2003; 326(7385): 365–6
Cartwright K, Noah N, Peltola H. Meningococcal disease in Europe: epidemiology, mortality, and prevention with conjugate vaccines. Report of a European Advisory Board meeting, Vienna, Austria, 6–8 October, 2000. Vaccine 2001; 19(31): 4347–56
Connolly M, Noah N. Is group C meningococcal disease increasing in Europe: a report of surveillance of meningococcal infection in Europe 1993–6. European Meningitis Surveillance Group. Epidemiol Infect 1999; 122(1): 41–9
Segal S, Pollard AJ. The future of meningitis vaccines. Hosp Med 2003; 64(3): 161–7
Baker MG, Martin DR, Kieft CE, et al. A 10-year serogroup B meningococcal disease epidemic in New Zealand: descriptive epidemiology, 1991–2000. J Paediatr Child Health 2001; 37(5): S13–9
Pollard AJ, Maiden MC. Epidemic meningococcal disease in sub-Saharan Africa: towards a sustainable solution? Lancet Infect Dis 2003; 3(2): 68–70
Lingappa JR, Al-Rabeah AM, Hajjeh R, et al. Serogroup W-135 meningococcal disease during the Hajj, 2000 [published erratum appears in Emerg Infect Dis 2003; 9 (8): 1028]. Emerg Infect Dis 2003; 9(6): 665–71
Pollard AJ, Scheifele D. Meningococcal disease and vaccination in North America. J Paediatr Child Health 2001; 37(5): S20–7
Rennels M, King Jr J, Ryall R, et al. Dosage escalation, safety and immunogenicity study of four dosages of a tetravalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine in infants. Pediatr Infect Dis J 2004; 23(5): 429–35
Campbell JD, Edelman R, King Jr JC, et al. Safety, reactogenicity, and immunogenicity of a tetravalent meningococcal polysaccharide-diphtheria toxoid conjugate vaccine given to healthy adults. J Infect Dis 2002; 186(12): 1848–51
Rennels M, King Jr J, Ryall R, et al. Dose escalation, safety and immunogenicity study of a tetravalent meningococcal polysaccharide diphtheria conjugate vaccine in toddlers. Pediatr Infect Dis J 2002; 21(10): 978–9
Granoff DM, Harris SL. Protective activity of group C anticapsular antibodies elicited in two-year-olds by an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J 2004; 23(6): 490–7
Granoff DM, Morgan A, Welsch JA. Persistence of group C anticapsular antibodies two to three years after immunization with an investigational quadrivalent Neisseria meningitidis-diphtheria toxoid conjugate vaccine. Pediatr Infect Dis J 2005; 24(2): 132–6
Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunisation Practices (ACIP). MMWR Recomm Rep 2005; 54RR-7): 1–21
Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006; 19(1): 142–64
Pan American Health Organisation. Meningococcal conjugate vaccines for Africa. Washington, DC: Pan American Health Organisation, 2002
Buttery JP, Riddell A, McVernon J, et al. Immunogenicity and safety of a combination pneumococcal-meningococcal vaccine in infants: a randomized controlled trial. JAMA 2005; 293(14): 1751–8
Acknowledgements
No sources of funding were used to assist in the preparation of this review. Dr Riordan has acted as a consultant for GSK vaccines on the topics of rotavirus and human papilloma vaccines in Europe, and has also received a grant from this source as a local investigator in the Rotavirus study in the UK. Dr Makwana has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Makwana, N., Riordan, F.A.I. Bacterial Meningitis. CNS Drugs 21, 355–366 (2007). https://doi.org/10.2165/00023210-200721050-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200721050-00001