Abstract
The fields of pharmacogenetics and pharmacogenomics have become important practical tools to progress goals in medical and pharmaceutical research and development. As more screening tests are being developed, with some already used in clinical practice, consideration of cost-effectiveness implications is important. A systematic review was performed on the content of and adherence to pharmacoeconomic guidelines of recent pharmacoeconomic analyses performed in the field of pharmacogenetics and pharmacogenomics.
Economic analyses of screening strategies for genetic variations, which were evidence-based and assumed to be associated with drug efficacy or safety, were included in the review. The 20 papers included cover a variety of healthcare issues, including screening tests on several cytochrome P450 (CYP) enzyme genes, thiopurine S-methyltransferase (TMPT) and angiotensin-converting enzyme (ACE) insertion deletion (ACE I/D) polymorphisms.
Most economic analyses reported that genetic screening was cost effective and often even clearly dominated existing non-screening strategies. However, we found a lack of standardization regarding aspects such as the perspective of the analysis, factors included in the sensitivity analysis and the applied discount rates. In particular, an important limitation of several studies related to the failure to provide a sufficient evidence-based rationale for an association between genotype and phenotype.
Future economic analyses should be conducted utilizing correct methods, with adherence to guidelines and including extensive sensitivity analyses. Most importantly, genetic screening strategies should be based on good evidence-based rationales. For these goals, we provide a list of recommendations for good pharmacoeconomic practice deemed useful in the fields of pharmacogenetics and pharmacogenomics, regardless of country and origin of the economic analysis.




Similar content being viewed by others
References
Sadée W. Pharmacogenomics. BMJ 1999; 319: 1286–1289
Farrall M, Moms AP. Gearing up for genome-wide gene-association studies. Hum Mol Genet 2005 Oct 15,14 Spec No. 2: R157–R162
Swen JJ, Huizinga TW, Gelderblom H, et al. Translating pharmacogenomics: challenges on the road to the clinic. PLoS Med 2007 Aug; 4 (8): e209
Ess SM, Schneeweiss S, Szucs TD. European healthcare policies for controlling drug expenditure. Pharmacoeconomics 2003; 21 (2): 89–103
Dervieux T, Bala MV. Overview of the pharmacoeconomics of pharmacogenetics. Pharmacogenomics 2006 Dec; 7 (8): 1175–1184
Flowers CR, Veenstra D. The role of cost-effectiveness analysis in the era of pharmacogenomics. Pharmacoeconomics 2004; 22 (8): 481–493
Phillips KA, Van Bebber SL. A systematic review of cost-effectiveness analyses of pharmacogenomic interventions. Pharmacogenomics 2004 Dec; 5 (8): 1139–1149
Rogowski W. Genetic screening by DNA technology: a systematic review of health economic evidence. Int J Technol Assess Health Care 2006; 22 (3): 327–337
Schroter M, Zollner B, Schafer P, et al. Comparison of three HCV genotyping assays: a serological method as a reliable and inexpensive alternative to PCR based assays. J Clin Virol 2001 Dec; 23 (1–2): 57–63
Chaix-Couturier C, Holtzer C, Phillips KA, et al. HIV-1 drug resistance genotyping: a review of clinical and economic issues. Pharmacoeconomics 2000 Nov; 18 (5): 425–433
Hughes DA, Vilar FJ, Ward CC, et al. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics 2004 Jun; 14 (6): 335–342
Atthobari J, Bos JM, Boersma C, et al. Adherence of pharmacoeconomic studies to national guidelines in the Netherlands. Pharm World Sci 2005 Oct; 27 (5): 364–370
International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomic guidelines around the world [online]. Available from URL: http://www.ispor.org/PEguidelines/index.asp [Accessed 2008 May 19]
Schalekamp T, Boink GJ, Visser LE, et al. CYP2C9 genotyping in acenocoumarol treatment: is it a cost-effective addition to international normalized ratio monitoring? Clin Pharmacol Ther 2006 Jun; 79 (6): 511–520
You JH, Chan FW, Wong RS, et al. The potential clinical and economic outcomes of pharmacogenetics-oriented management of warfarin therapy: a decision analysis. Thromb Haemost 2004 Sep; 92 (3): 590–597
Schalekamp T, van Geest-Daalderop JH, de Vries-Goldschmeding H, et al. Acenocoumarol stabilization is delayed in CYP2C93 carriers. Clin Pharmacol Ther 2004 May; 75 (5): 394–402
Desta Z, Zhao X, Shin JG, et al. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet 2002; 41 (12): 913–958
Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin Pharmacol Ther 2007 Apr; 81 (4): 521–528
Lehmann DF, Medicis JJ, Franklin PD. Polymorphisms and the pocketbook: the cost-effectiveness of cytochrome P450 2C19 genotyping in the eradication of Helicobacter pylori infection associated with duodenal ulcer. J Clin Pharmacol 2003 Dec; 43 (12): 1316–1323
Chou WH, Yan FX, de Leon J, et al. Extension of a pilot study: impact from the cytochrome P450 2D6 polymorphism on outcome and costs associated with severe mental illness. J Clin Psychopharmacol 2000 Apr; 20 (2): 246–251
Marra CA, Esdaile JM, Anis AH. Practical pharmacogenetics: the cost effectiveness of screening for thiopurine s-methyl-transferase polymorphisms in patients with rheumatological conditions treated with azathioprine. J Rheumatol 2002 Dec; 29 (12): 2507–2512
Oh KT, Anis AH, Bae SC. Pharmacoeconomic analysis of thiopurine methyltransferase polymorphism screening by polymerase chain reaction for treatment with azathioprine in Korea. Rheumatology (Oxford) 2004 Feb; 43 (2): 156–163
Dubinsky MC, Reyes E, Ofman J, et al. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn’s disease treated with azathioprine or 6-mer-captopurine. Am J Gastroenterol 2005 Oct; 100 (10): 2239–2247
Priest VL, Begg EJ, Gardiner SJ, et al. Pharmacoeconomic analyses of azathioprine, methotrexate and prospective pharmacogenetic testing for the management of inflammatory bowel disease. Pharmacoeconomics 2006; 24 (8): 767–781
Winter J, Walker A, Shapiro D, et al. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment Pharmacol Ther 2004 Sep 15; 20 (6): 593–599
Tavadia SM, Mydlarski PR, Reis MD, et al. Screening for azathioprine toxicity: a pharmacoeconomic analysis based on a target case. J Am Acad Dermatol 2000 Apr; 42 (4): 628–632
van den Akker-van Marie ME, Gurwitz D, Detmar SB, et al. Cost-effectiveness of pharmacogenomics in clinical practice: a case study of thiopurine methyltransferase genotyping in acute lymphoblastic leukemia in Europe. Pharmacogenomics 2006 Jul; 7 (5): 783–792
Kim SK, Jun JB, El-Sohemy A, et al. Cost-effectiveness analysis of MTHFR polymorphism screening by polymerase chain reaction in Korean patients with rheumatoid arthritis receiving methotrexate. J Rheumatol 2006 Jul; 33 (7): 1266–1274
Lehman AF, Steinwachs DM. Translating research into practice: the Schizophrenia Patient Outcomes Research Team (PORT) treatment recommendations. Schizophr Bull 1998; 24 (1): 1–10
Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet 2000 May 6; 355 (9215): 1615–1616
Perlis RH, Ganz DA, Avorn J, et al. Pharmacogenetic testing in the clinical management of schizophrenia: a decision-analytic model. J Clin Psychopharmacol 2005 Oct; 25 (5): 427–434
Meckley LM, Veenstra DL. Screening for the alpha-adducin Gly460Trp variant in hypertensive patients: a cost-effectiveness analysis. Pharmacogenet Genomics 2006 Feb; 16 (2): 139–147
Maitland-van der Zee AH, Klungel OH, Strieker BH, et al. Pharmacoeconomic evaluation of testing for angiotensin-converting enzyme genotype before starting beta-hydroxy-beta-methylglutaryl coenzyme A reductase inhibitor therapy in men. Pharmacogenetics 2004 Jan; 14 (1): 53–60
Scharplatz M, Puhan MA, Steurer J, et al. What is the impact of the ACE gene insertion/deletion (I/D) polymorphism on the clinical effectiveness and adverse events of ACE inhibitors? Protocol of a systematic review. BMC Med Genet 2004 Sep 10; 5: 23
Scharplatz M, Puhan MA, Steurer J, et al. Does the angiotensin-converting enzyme (ACE) gene insertion/deletion polymorphism modify the response to ACE inhibitor therapy? A systematic review. Curr Control Trials Cardiovasc Med 2005 Oct 24; 6: 16
Costa-Scharplatz M, van Asselt AD, Bachmann LM, et al. Cost-effectiveness of pharmacogenetic testing to predict treatment response to angiotensin-converting enzyme inhibitor. Pharmacogenet Genomics 2007 May; 17 (5): 359–368
Veenstra DL, Harris J, Gibson RL, et al. Pharmacogenomic testing to prevent aminoglycoside-induced hearing loss in cystic fibrosis patients: potential impact on clinical, patient, and economic outcomes. Genet Med 2007 Oct; 9 (10): 695–704
European Central Bank [online]. Available from URL: http://www.ecb.int/ [Accessed 2008 May 19]
Berger ML, Bingefors K, Hedblom EC, et al. Health care cost, quality, and outcomes: ISPOR book of terms. Lawrenceville (NJ): International Society for Pharmacoeconomics and Outcomes Research, 2003
Bos JM, Postma MJ. Using pharmacoeconomics for policy making: is rational decision making enhanced by applying thresholds for cost-effectiveness? Expert Rev Pharmacoecon Outcomes Res 2004; 4 (3): 247–250
Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002; 23: 377–401
Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press, 2005
Watson ME, Pimenta JM, Spreen WR, et al. HLA-B*5701 and abacavir hypersensitivity. Pharmacogenetics 2004 Nov; 14 (11): 783–784
Yates CR, Krynetski EY, Loennechen T, et al. Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance. Ann Intern Med 1997 Apr 15; 126 (8): 608–614
Roberts RL, Barclay ML, Gearry RB, et al. A multiplexed allele-specific polymerase chain reaction assay for the detection of common thiopurine S-methyltransferase (TPMT) mutations. Clin Chim Acta 2004 Mar; 341 (1–2): 49–53
Arranz MJ, Munro J, Osborne S, et al. Difficulties in replication of results. Lancet 2000 Oct 14; 356 (9238): 1359–1360
PHARMAC. Prescription for pharmacoeconomic analysis: methods for cost utility analysis. 2007 May [online]. Available from URL: http://www.pharmac.govt.nz/2007/06/19/PFPAFinal.pdf [Accessed 2008 May 19]
Gold M, Siegel J, Russell L. Cost-effectiveness in health and medicine. New York: Oxford University Press, 1996
Danser AH, Batenburg WW, van den Meiracker AH, et al. ACE phenotyping as a first step toward personalized medicine for ACE inhibitors: why does ACE genotyping not predict the therapeutic efficacy of ACE inhibition? Pharmacol Ther 2007 Mar; 113 (3): 607–618
Gerhard T, Beitelshees AL, Johnson JA, et al. Screening for the alpha-adducin Gly460Trp variant in hypertensive patients: a cost-effectiveness analysis [letter]. Pharmacogenet Genomics 2006 Aug: 16 (8): 613
Tang HY, Hutcheson E, Neill S, et al. Genetic susceptibility to aminoglycoside ototoxicity: how many are at risk? Genet Med 2002 Sep; 4 (5): 336–345
Clayton D, McKeigue PM. Epidemiological methods for studying genes and environmental factors in complex diseases. Lancet 2001 Oct 20; 358 (9290): 1356–1360
Chakravarti A, Little P. Nature, nurture and human disease. Nature 2003 Jan 23; 421 (6921): 412–414
Vandenbroucke JP. When are observational studies as credible as randomised trials? Lancet 2004 May 22; 363 (9422): 1728–1731
Glick HA, Doshi JA, Sonnad SS, et al. Economic evaluation in clinical trials. New York: Oxford University Press, 2007
Meijerman I, Sanderson LM, Smits PH, et al. Pharmacogenetic screening of the gene deletion and duplications of CYP2D6. Drug Metab Rev 2007; 39 (1): 45–60
Ingle JN. Pharmacogenomics of tamoxifen and aromatase inhibitors. Cancer 2007 Dec 10; 112 (S3): 695–699
Becker ML, Visser LE, Trienekens PH, et al. Cytochrome P450 2C9 *2 and *3 polymorphisms and the dose and effect of sulfonylurea in type II diabetes mellitus. Clin Pharmacol Ther 2008 Feb; 83 (2): 288–292
Kirchheiner J, Roots I, Goldammer M, et al. Effect of genetic polymorphisms in cytochrome p450 (CYP) 2C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet 2005; 44 (12): 1209–1225
van Kuilenburg AB, De Abreu RA, van Gennip AH. Pharmacogenetic and clinical aspects of dihydropyrimidine dehydrogenase deficiency. Ann Clin Biochem 2003 Jan; 40 (Pt 1): 41–45
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004 May 20; 350 (21): 2129–2139
Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 2005 Jun 2; 352 (22): 2285–2293
Bodin L, Verstuyft C, Tregouet DA, et al. Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood 2005 Jul 1; 106 (1): 135–140
Schalekamp T, Brasse BP, Roijers JF, et al. VKORC1 and CYP2C9 genotypes and acenocoumarol anticoagulation status: interaction between both genotypes affects overanticoagulation. Clin Pharmacol Ther 2006 Jul; 80 (1): 13–22
Schalekamp T, Brasse BP, Roijers JF, et al. VKORC1 and CYP2C9 genotypes and phenprocoumon anticoagulation status: interaction between both genotypes affects dose requirement. Clin Pharmacol Ther 2007 Feb; 81 (2): 185–193
Ingelman-Sundberg M. Pharmacogenomic biomarkers for prediction of severe adverse drug reactions. N Engl J Med 2008 Feb 7; 358 (6): 637–639
Acknowledgements
This work was supported by the applied GENomic stratEgies for Treatment and Prevention of Cardiovascular death in Uraemia and End stage REnal disease (GENECURE) project (www.genecure.eu), a Specific Targeted Research or Innovation Project, funded by the European Commission under the Sixth Framework Programme as FP6−037696. GENECURE is led by Professor Dr G.J. Navis, University Medical Center Groningen in Groningen, the Netherlands; its goal is to elucidate the genomic basis of cardiovascular complications in renal disease. GENECURE is hosted by the Renal Genome Network (ReGeNet) project (www.regenet.eu), a pan-European network of clinicians and scientists from academia and industry seeking to generate and facilitate genetic and genomic studies to the clinical benefit of the renal patient.
This article has been prepared with the assistance of the European Union. The content of this review is the sole responsibility of the authors and can in no way be taken to reflect the views of the European Union. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1. Search Strategy
1.1 Search Limits
-
1.
Publication year: January 2000 up until December 2007
-
2.
Studies in humans
-
3.
No language restrictions.
1.2 Keywords
Pharmacoeconomic terms: ‘cost-effectiveness’ OR ‘cost effectiveness’ OR ‘costeffectiveness’ OR ‘cost-utility’ OR ‘cost utility’ OR ‘costutility’ OR ‘cost-benefit’ OR ‘cost benefit’ OR ‘costbenefit’ OR ‘cost-minimization’ OR ‘cost minimization’ OR ‘costminimization’ OR ‘cost-minimisation’ OR ‘cost minimisation’ OR ‘costminimisation’ OR ‘Pharmacoeconomics’ OR ‘Pharmacoeconomic’ OR ‘Pharmaco-economic’ OR ‘Pharmaco-economics’ OR ‘Pharmaco economic’ OR ‘Pharmaco economics’.
Pharmacogenetic terms: ‘pharmaco-genetics’ OR ‘pharmacogenetics’ OR ‘pharmaco genetics’ OR ‘pharmaco-genetic’ OR ‘pharmacogenetic’ OR ‘pharmaco genetic’ OR ‘pharmacopharmacogenomics’ OR ‘pharmacogenomics’ OR ‘pharmaco genomics’ OR ‘pharmacopharmacogenomic’ OR ‘pharmacogenomic’ OR ‘pharmaco genomic’ OR ‘genotyping’ OR ‘genetic screening’ OR ‘genetic testing’ OR ‘genotyped’ OR ‘polymorphism screening’.
1.3 Search Strategy
-
1.
Search pharmacoeconomic terms
-
2.
Search pharmacogenetic terms
-
3.
Combine pharmacoeconomic and pharmacogenetic terms
-
4.
Exclude reviews, editorials and other non-research articles
-
5.
Screen title and/or abstract for pharmacoeconomic studies on pharmacogenetic or pharmacogenomic screening strategies.
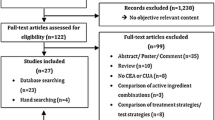
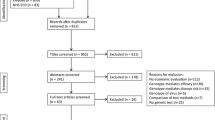
1.4 PubMed Search Results
-
1.
Pharmacoeconomic terms: 18 546
-
2.
Pharmacogenetic terms: 27 680
-
3.
Combined terms: 285
-
4.
Non-research articles excluded: 156
-
5.
Final selection: 20.
1.5 ISI Search Results
-
1.
Pharmacoeconomic terms: 22 810
-
2.
Pharmacogenetic terms: 28 085
-
3.
Combined terms: 211
-
4.
Exclusion of non-research: 144
-
5.
Final selection: 18, (compared to the PubMed search, Meckley and Veenstra,[32] Desta et al.[17] were not located).
1.6 EMBASE Search Results
Rights and permissions
About this article
Cite this article
Vegter, S., Boersma, C., Rozenbaum, M. et al. Pharmacoeconomic Evaluations of Pharmacogenetic and Genomic Screening Programmes. Pharmacoeconomics 26, 569–587 (2008). https://doi.org/10.2165/00019053-200826070-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200826070-00005