Summary
The measurement of outcome in the treatment of acid-related diseases can be assessed against the background of various types of economic evaluation. Apart from endoscopic confirmation of healing (or nonhealing), the measurement of outcome has been based on recording separately the occurrence of various symptoms and possible adverse effects, as experienced and reported by the patients. Findings have been reported as the proportions of total patients who are free from some or all symptoms. These proportions may be noted in terms of prevalence or incidence.
However, unless one treatment dominates the others (is superior or at least is not worse) on all outcome measures, it is impossible to say conclusively which treatment is the best overall, provided of course that there is no difference between the treatments in the severity of symptoms and adverse effects.
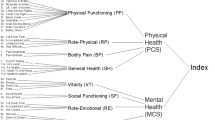
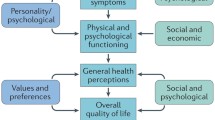
Decision trees have been built to combine some outcomes. However, in the future, measurements that give a more complete picture of the combined severity of various symptoms and adverse effects need to be adopted. These should help to assess how quickly and to what degree the severity of disease is affected by treatment. The emerging guidelines for economic evaluations encourage the measurement of outcome prospectively in terms of quality of life and/or survival, since for most drugs the ultimate outcome of therapy is to improve them, and expressing them as quality-adjusted life-years (QALYs) gained. Quality-of-life measures potentially appropriate for QALY calculations in acid-related diseases (EuroQol Group 1988; Kaplan & Anderson 1988; Rosser & Kind 1978; Sintonen & Pekurinen 1993) are briefly reviewed. Further, the use of decision analysis is encouraged to ensure that all relevant outcomes and their possibly changing probabilities over time are considered and quantified. Expressing results as proportions and numbers achieving specified targets (success) is recommended, because they are relatively easy to incorporate into economic evaluation. The use of cost-benefit analysis based on the human capital approach is discouraged, since there may not be an economic loss or benefit to society through a patient’s lack of, or return to, productive capacity.
Similar content being viewed by others
References
Barradell LB, McTavish D. Omeprazole: a pharmacoeconomic evaluation of its use in duodenal ulcer and reflux oesophagitis. PharmacoEconomics 3: 482–510, 1993
Bate CM. Cost effectiveness of omeprazole in the treatment of reflux oesophagitis. British Journal of Medical Economics 1: 53–61, 1991
Bate CM, Richardson PDI. Symptomatic and cost effectiveness of treatments for reflux oesophagitis: comparisons of omeprazole and histamine H2-receptor antagonists. British Journal of Medical Economics 2: 37–48, 1992
Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: development and final version of a health status measure. Medical Care 19: 787–805, 1981
Broome J. QALYs. Journal of Public Economics 50: 149–167, 1993
Commonwealth Department of Health, Housing and Community Services. Guidelines for the pharmaceutical industry on preparation of submissions to the Pharmaceutical Benefits Advisory Committee: including submissions involving economic analyses. Australian Government Publishing Service, Canberra, 1992
Drummond M. The role and importance of quality of life measurements in economic evaluations. British Journal of Medical Economics 4: 9–16, 1992
Drummond M, Rutten F, Brenna A, Pinto CG, Horisberger B, et al. Economic evaluation of pharmaceuticals: a European perspective. PharmacoEconomics 4: 173–186, 1993
Ebell MH. Peptic ulcer disease. American Family Physician 46: 217–227, 1992
EuroQol Group. EuroQol — a new facility for the measurement of health-related quality of life. Health Policy 16: 199–208, 1990
Garrett WR. Efficacy, safety, and cost issues in managing patients with gastroesophageal reflux disease. American Journal of Hospital Pharmacology 50 (Suppl. 1): 11–18, 1993
Gustavsson S, Holmberg L, Nyren O, Ohrvall U, Wells L. Risk of serious complications in patients with duodenal or prepyloric ulcers. Abstract. Gastroenterology 98: 54A, 1990
Harwood RH, Gompertz PH, Ebrahim S. Outcome measures should be relevant. British Medical Journal 304: 917, 1992
Heading RC. Epidemiology of oesophageal reflux disease. Scandinavian Journal of Gastroenterology 24 (Suppl. 168): 33–37, 1989
Hillman AL, Bloom BS, Fendrick AM, Schwartz JS. Cost and quality effects of alternative treatments for persistent gastroesophageal reflux disease. Archives of Internal Medicine 152: 1467–1472, 1992
Hunt SM, McKenna SP, McEwen J, Williams J, Papp E. The Nottingham Health Profile: subjective health status and medical consultations. Social Science and Medicine 15A: 221–229, 1981
Johannesson M, Johansson P-O, Jonsson B. Economic evaluation of drug therapy. A review of the contingent valuation method. PharmacoEconomics 5: 325–337, 1992
Jonsson B, Persson U. Cost-benefit analysis of cimetidine. Report 1982: 6, Institutet for halso-och sjukvardsekonomi, Lund 1982
Kaplan RM, Anderson JP. A general health model: update and applications. Health Services Research 23: 203–235, 1988
Lindberg G, Jonsson B. Omeprazol mer kostnadseffektivt an ranitidin vid refluxesofagit. Lakartidningen 89: 2530–2533, 1992
Mehrez A, Gafni A. Quality adjusted life years, utility theory and healthy years equivalents. Medical Decision Making 9: 142–149, 1989
Mehrez A, Gafni A. Preference based outcome measures for economic evaluation of drug interventions: quality adjusted life years (QALYs) versus healthy years equivalents (HYEs). PharmacoEconomics 1: 338–345, 1992
Nord E. Methods of quality adjustment of life years. Social Science and Medicine 34: 559–569, 1992
Parsonage M, Neuburger H. Discounting and health benefits. Health Economics 1: 71–76, 1992
Pauker SG, Kassirer JP. Decision analysis. New England Journal of Medicine 316: 250–258, 1987
Rosser R, Kind P. A scale of valuations of states of illness: is there a social consensus? International Journal of Epidemiology 7: 347–358, 1978
Sintonen H. (Ed.) EuroQol Conference Proceedings. Helsinki, October 1992, Discussion paper No 2, Kuopio University Publications E. Social Sciences 8. Kuopio University Printing Office, Kuopio, 1993
Sintonen H, Alander V. Comparing the cost-effectiveness of drug regimens in the treatment of duodenal ulcers. Journal of Health Economics 9: 85–101, 1990
Sintonen H, Lonnqvist J, Kiviruusu O. Cost-effectiveness and cost-utility analysis of two drug regimens in the treatment of depression. National Centre for Health Program Evaluation, Working Paper 37, Melbourne, 1994
Sintonen H, Pekurinen M. A generic 15 dimensional measure of health-related quality of life (15D). Journal of Social Medicine 26: 85–96, 1989
Sintonen H, Pekurinen M. A fifteen-dimensional measure of health-related quality of life (15D) and its applications. In Walker SR, Rosser RM (Eds) Quality of life assessments: key issues in the 1990s, pp. 185–195, Kluwer Academic Publishers, Dordrecht, 1993
Stewart AL, Hays RD, Ware JE. The MOS short-form general health survey: reliability and validity in a patient population. Medical Care 26: 727–735, 1988
Thompson MS, Read JL, Hutchings HC, Harris ED. The cost-effectiveness of auranofin: results of a randomized clinical trial. Journal of Rheumatology 15: 35–42, 1988
Veldhuyzen van Zanten SJO, Tytgat KMAJ, Pollak PT, Goldie J, Goodacre RL, et al. Can severity of symptoms be used as an outcome measure in trials on non-ulcer dyspepsia and Helicobacter pylori associated gastritis? Journal of Clinical Epidemiology 46: 273–279, 1993
Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF 36): I. Conceptual framework and item selection. Medical Care 30: 473–483, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sintonen, H. Outcome Measurement in Acid-Related Diseases. Pharmacoeconomics 5 (Suppl 3), 17–26 (1994). https://doi.org/10.2165/00019053-199400053-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-199400053-00005