Summary
Abstract|Atomoxetine (Strattera™) is a selective norepinephrine reuptake inhibitor and nonstimulant that has shown greater efficacy than placebo in attention deficit hyperactivity disorder (ADHD) in adults. In two large, well controlled, 10-week trials in adults with ADHD, improvements in ADHD symptoms, as assessed by investigator- and patient-rated scores, were greater with oral atomoxetine (60, 90 or 120 mg/day) than with placebo. Mean reductions in the total ADHD symptom score on the investigator-rated Conners' Adult ADHD Rating Scale (CAARS) in atomoxetine versus placebo recipients were 28.3% versus 18.1% and 30.1% versus 19.6%, respectively. Mean reductions in the scores on the Clinician Global Impression of Severity Scale, patient-rated CAARS and Wender-Reimherr Adult Attention Deficit Disorder Scale were also significantly greater with atomoxetine than with placebo. Continued efficacy was demonstrated in a noncomparative, 34-week extension phase
Atomoxetine was generally well tolerated in clinical trials; withdrawal rates due to adverse events in atomoxetine-treated versus placebo-treated patients participating in the two major trials were 7.8% versus 4.3% and 9.3% versus 2.4% (p < 0.05 for the latter trial). Adverse events reported significantly more frequently with atomoxetine than placebo included dry mouth, insomnia, nausea, decreased appetite, constipation, dizziness, sweating, dysuria, sexual problems and palpitations. Modest increases in heart rate and blood pressure were well tolerated and gradually decreased on cessation of treatment. Atomoxetine was not associated with QT interval prolongation.
Atomoxetine can be administered once or twice daily. Its subjective-effects profile is different to that of methylphenidate and atomoxetine is not associated with abuse or diversion; it is therefore not a controlled substance in the US. This also means repeat prescriptions during long-term treatment can be more conveniently processed.
Conclusion: Atomoxetine is an effective and generally well tolerated treatment for adults with ADHD. It is a nonstimulant and is the first ADHD treatment to be approved specifically for adult use based on its efficacy in well controlled adult trials. It can be administered as a single daily dose or split into two evenly divided doses. It carries negligible risk of abuse or diversion and is not a controlled substance. Atomoxetine is a valuable new treatment option for adults with ADHD and is particularly useful in patients who are at risk for substance abuse or who do not wish to take a controlled substance.
Pharmacodynamic Properties| The mechanism of action of atomoxetine in attention deficit hyperactivity disorder (ADHD) is thought to be related to its selective inhibition of norepinephrine reuptake. The dissociation constant (Ki) for atomoxetine inhibition of radioligand binding in animal and human cell membranes transfected with human norepinephrine transporters was 5 nmol/L compared with 77 and 1451 nmol/L for binding to serotonin and dopamine transporters
Atomoxetine has demonstrated selective inhibition of the presynaptic uptake of norepinephrine in adrenergic neurons in animals. A study in humans showed marked inhibition of norepinephrine uptake (p = 0.054 vs placebo). Atomoxetine demonstrated selectivity as serotonin uptake into platelets isolated from study participants was unaffected.
In the prefrontal cortex of the rat brain, atomoxetine increased extracellular levels of norepinephrine and dopamine (but not serotonin) and in the subcortical areas it increased extracellular norepinephrine but not dopamine. Increased norepinephrine transmission in these areas may play a role in the efficacy of atomoxetine in ADHD and may indicate the potential for the alleviation of symptoms of comorbid depression and anxiety by atomoxetine. The lack of increase in dopamine transmission in the subcortical areas may indicate a low potential for atomoxetine to produce tics, have psychomimetic effects or lead to abuse.
Atomoxetine had no appreciable affinity for various neurotransmitter receptors in the rat or human brain, suggesting that it has a low potential for adverse effects and/or drug interactions.
There were modest increases in heart rate and systolic blood pressure (BP) and no QT interval prolongation in atomoxetine recipients in the pivotal trials. Small but statistically significant increases from baseline in heart rate, BP and bodyweight were observed during the 34-week extension phase.
The subjective-effects profile of atomoxetine is distinct from that of methyl-phenidate. Atomoxetine was rated significantly higher than placebo for ‘bad’ and ‘sick’ effects, which indicates that it is unlikely to be associated with abuse. Methylphenidate was rated higher than placebo for stimulant effects and dysphoric or psychomimetic effects.
Pharmacokinetic Profile| The oxidative metabolism of atomoxetine to its major active metabolite, 4-hydroxyatomoxetine, is via the genetically polymorphic cytochrome P450 (CYP) 2D6 pathway. Two subpopulations of metabolisers have been identified and, although dosages in clinical practice are not adjusted for genotype, most pharmacokinetic data are available as distinct values for each metaboliser subtype (extensive metabolisers [EMs] and poor metabolisers [PMs])
Atomoxetine is rapidly absorbed from the gastrointestinal tract after oral administration and has an absolute bioavailability of 94% in PMs and 63% in EMs. The median time to reach maximum plasma concentrations (Cmax) at steady state was approximately 1–2 hours in both groups. Food slowed the rate, but not the extent, of absorption of atomoxetine and the drug can be administered with or without food. At steady state, Cmax for atomoxetine was almost 6-fold higher in PMs than in EMs and mean area under the plasma concentration-time curve was approximately 8-fold higher.
The steady-state volume of distribution of atomoxetine after intravenous administration is 0.85 L/kg and is similar for PMs and EMs. The apparent volume of distribution was 1.02 L/kg in PMs and 2.33 L/kg in EMs. At therapeutic concentrations, 98% of atomoxetine is bound to plasma protein (principally to albumin).
The same metabolites of atomoxetine are formed (4-hydroxyatomoxetine and N-desmethylatomoxetine) regardless of CYP2D6 status, but the proportions of circulating metabolites differ according to the metabolic status of individuals. In EMs, atomoxetine and 4-hydroxyatomoxetine (equipotent with atomoxetine for norepinephrine transporter inhibition) are the principal circulating compounds. Because in PMs the rate of formation of 4-hydroxyatomoxetine is slower, the principal circulating compounds are atomoxetine and N-desmethylatomoxetine (a relatively inactive metabolite). The exposure of PMs to atomoxetine is, however, approximately 8- to 10-fold that of EMs, which is primarily due to the slower rate of formation of 4-hydroxyatomoxetine, but also the reduced overall rate of plasma clearance of atomoxetine.
Plasma elimination half-life (t½) of atomoxetine in PMs is approximately 4-fold longer than in EMs (20 vs 5 hours) indicating increased systemic exposure to atomoxetine in PMs. The mean apparent plasma clearance of atomoxetine at steady state was approximately 0.036 L/h/kg for PMs versus 0.373 L/h/kg for EMs.
Atomoxetine is eliminated from the body mainly via excretion of its glucuronidated metabolites in the urine (>80%) with approximately 13–22% and 1–2% eliminated in the faeces of PMs and EMs, respectively. Less than 3% is eliminated as unchanged drug.
Although Cmax of atomoxetine was not increased in patients with hepatic impairment, there was increased systemic exposure in that population due to decreased atomoxetine clearance, necessitating dosage adjustment. Higher exposure to atomoxetine was observed in patients with end-stage renal disease, but there was no difference when the exposure was corrected for mg/kg dose.
Selective CYP2D6 inhibitors (paroxetine, fluoxetine or quinidine) increase the exposure to atomoxetine. The pharmacokinetic values for atomoxetine in EMs concomitantly receiving a CYP2D6 inhibitor are, predictably, similar to those observed in PMs. asTherapeutic Efficacy| Orally administered atomoxetine (60, 90 or 120 mg/day) has shown efficacy in two well controlled 10-week trials in adults with ADHD (n = 267 and 248 in the intention-to-treat analyses of study I and II). 80% of patients in study I and 75% in study II received 90 or 120 mg/day (approximately equal numbers receiving each dosage). Mean reductions in total ADHD symptom score on the investigator-rated Conners' Adult ADHD Rating Scale (CAARS) [primary endpoint] in atomoxetine versus placebo recipients were 28.3% versus 18.1% in study I and 30.1% versus 19.6% in study II (p < 0.01 for both). Mean reductions in the patient-rated CAARS total ADHD symptom score were 19.4% versus 11.5% in study I and 20.9% versus 14.5% in study II (p < 0.01 for both).
Emotional dysregulation (indicated by at least moderate scores for temper, affective lability and emotional over-reactivity on the Wender-Reimherr Adult Attention Deficit Disorder Scale) was evident at baseline in one-third of patients in the major studies and showed a greater improvement with atomoxetine than with placebo (42% vs 19% improvement, p = 0.001).
An open-label extension phase (mean 34 weeks) of the pivotal trials demonstrated continued efficacy of atomoxetine, and improved clinical responses with higher bodyweight-based dosages.
No head-to-head comparisons of atomoxetine with any of the stimulants or other effective treatments for ADHD have been conducted.
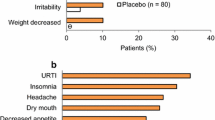
Tolerability| Atomoxetine was generally well tolerated in 10-week clinical trials and no serious treatment-related adverse events were reported. The adverse effect-related withdrawal rates for atomoxetine versus placebo recipients from the two major trials were 7.8% versus 4.3% (study I) and 9.3% versus 2.4% (p < 0.05) [study II]. Treatment-related withdrawals (reported by more than one patient) among the 270 atomoxetine-treated patients were due to insomnia (n = 3), chest pain (n = 2), palpitations (n = 2) and urinary retention (n = 2). Hypotension and hypertension were among events responsible for one withdrawal each
The most common adverse events that were reported significantly more frequently with atomoxetine than placebo included dry mouth (21.2% vs 6.8%), insomnia (20.8% vs 8.7%), nausea (12.3% vs 4.9%), decreased appetite (11.5% vs 3.4%), constipation (10.8% vs 3.8%), dizziness (6.3% vs 1.9%), sweating (5.2% vs 0.8%), dysuria (5% versus 0.4%), palpitations (3.7% vs 0.8%) and sexual problems.
There was a modest but significant increase in heart-rate and systolic BP with atomoxetine versus placebo but these increases were generally well tolerated and gradually decreased on cessation of treatment. The incidence of tachycardia was not significantly greater in atomoxetine recipients than in placebo recipients. Atomoxetine was not associated with QT interval prolongation.
Dosage and Administration| The starting dosage recommended in the published prescribing information for atomoxetine in adults with ADHD is 40mg orally once daily in the morning (or as an evenly divided dose twice daily) which can then be increased to a target dose of 80 mg/day or a maximum of 100 mg/day if necessary
Atomoxetine should be used with caution in patients with hypertension, tachycardia or cerebral or cardiovascular disease. Dosage adjustment may be necessary for patients with hepatic impairment or those receiving CYP2D6 inhibitors (paroxetine, fluoxetine or quinidine). The concomitant use of atomoxetine and monoamine oxidase inhibitors is contraindicated and the drug is not recommended in patients with narrow angle glaucoma.




Similar content being viewed by others
Notes
Use of tradenames is for product identification purposes only and does not imply endorsement.
References
Spencer TJ, Biederman J, Wilens TE, et al. Overview and neurobiology of attention-deficit/hyperactivity disorder. J Clin Psychiatry 2002; 63 Suppl. 12: 3–9
Biederman J, Spencer T. Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder. Biol Psychiatry 1999; 46(9): 1234–42
Weiss M, Murray C. Assessment and management of attention-deficit hyperactivity disorder in adults. CMAJ 2003 Mar 18; 168(6): 715–22
Horrigan JP. Present and future pharmacotherapeutic options for adult attention deficit/hyperactivity disorder. Expert Opin Pharmacother 2001 Apr; 2(4): 573–86
Higgins ES. A comparative analysis of antidepressants and stimulants for the treatment of adults with attention-deficit hyperactivity disorder. J Fam Pract 1999 Jan; 48(1): 15–20
Wilens TE, Biederman J, Spencer TJ. Pharmacotherapy of attention deficit hyperactivity disorder in adults. CNS Drugs 1998; 9(5): 347–56
Spencer T, Biederman J, Wilens TE, et al. Adults with attention-deficit/hyperactivity disorder: a controversial diagnosis. J Clin Psychiatry 1998; 59 Suppl. 7: 59–68
Dulcan M. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/ hyperactivity disorder: American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry 1997 Oct; 36 (10 Suppl.): 85S–121S
Roy-Byrne P, Scheele L, Brinkley J, et al. Adult attention-deficit hyperactivity disorder: assessment guidelines based on clinical presentation to a specialty clinic. Compr Psychiatry 1997 May–Jun 30; 38(3): 133–40
Adler LA, Chua HC. Management of ADHD in adults. J Clin Psychiatry 2002; 63 Suppl. 12: 29–35
Shaffer D. Attention deficit hyperactivity disorder in adults. Am J Psychiatry 1994 May; 151(5): 633–8
MichelsonD, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002 Nov; 159(11): 1896–901
Michelson D, Adler L, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003 Jan 15; 53(2): 112–20
Stahl SM. Neurotransmission of cognition, part 2. Selective NRIs are smart drugs: exploiting regionally selective actions on both dopamine and norepinephrine to enhance cognition. J Clin Psychiatry 2003 Feb; 64(2): 110–1
Biederman J, Spencer T. Genetics of childhood disorders: XIX. ADHD, Part 3: is ADHD a noradrenergic disorder? J Am Acad Child Adolesc Psychiatry 2000 Oct; 39(10): 1330–3
Wong DT, Penny G, Threlkeld KL, et al. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharmacol Exp Ther 1982 Jul; 222(1): 61–5
Bolden-Watson C, Richelson E. Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci 1993; 52(12): 1023–9
Cusack B, Nelson A, Richelson E. Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl) 1994 May; 114(4): 559–65
Gehlert DR, Schober DA, Gackenheimer SL. Comparison of (R)-[3H]tomoxetine and (R/S)-[3H]nisoxetine binding in rat brain. J Neurochem 1995 Jun; 64(6): 2792–800
Gehlert DR, Gackenheimer SL, Robertson DW. Localization of rat brain binding sites for [3H] tomoxetine an enantiomerically pure ligand for norepinephrine reuptake sites. Neurosci Lett 1993 Jul 23; 157(2): 203–6
Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002 Nov; 27(5): 699–711
Fuller RW, Hemrick-Leucke SK. Antagonism by tomoxetine of the depletion of norepinephrine and epinephrine in rat brain by a-methyl-m-tyrosine. Res Commun Chem Pathol Pharmacol 1983 Jul; 41(1): 169–72
Zerbe RL, Rowe H, Enas GG, et al. Clinical pharmacology of tomoxetine, a potential antidepressant. J Pharmacol Exp Ther 1985 Jan; 232(1): 139–43
Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998 May; 155(5): 693–5
Adler L, Spencer T, Sutton V, et al. Dose and time response of atomoxetine use in adult ADHD [poster]. American Academy of Child and Adolescent Psychiatry (AACAP); 2003 Oct 14–19; Miami
Wernicke FJ, Faries D, Girod D, et al. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf 2003; 26(10): 729–40
Yeo KP, Kelly R, Lowe SL, et al. Haemodynamic effects of atomoxetine are less pronounced than methylphenidate, and not additive in combination. J Clin Pharmacol 2001 Sep; 41(9): 1022
Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002 Jul 1; 67(2): 149–56
Farid NA, Bergstrom RF, Ziege EA, et al. Single-dose and steady-state pharmacokinetics of tomoxetine in normal subjects. J Clin Pharmacol 1985 May–Jun 30; 25(4): 296–301
Sauer JM, Ponsler GD, Mattiuz EL, et al. Disposition and metabolic fate of atomoxetine hydrochloride: the role of CYP2D6 in human disposition and metabolism. Drug Metab Dispos 2003 Jan; 31(1): 98–107
Eli Lilly and Company. Indianapolis. Strattera (R) (atomoxetine HCl) [online]. Available from URL: http://www.strattera.com [Accessed 2003 Jul 28]
Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/ hyperactivity disorder: a randomised, placebo-controlled study, dose-response study. Paediatrics 2001; 108(5): E83
Belle DJ, Ernest CS, Sauer JM, et al. Effect of potent CYP2D6 inhibition by paroxetine on atomoxetine pharmacokinetics. J Clin Pharmacol 2002 Nov; 42(11): 1219–27
Chalon SA, Desager JP, Desante KA, et al. Effect of hepatic impairment on the pharmacokinetics of atomoxetine and its metabolites. Clin Pharmacol Ther 2003 Mar; 73(3): 178–91
Adler LA, Spencer TJ, Reimherr FW, et al. Efficacy and safety of atomoxetine in long-term, open-label treatment of adults with ADHD [abstract no. NR645 plus poster]. American Psychiatric Association 2003 Annual Meeting; New Research Abstracts, 156th Annual Meeting; 2003 May 17–22; San Francisco
Simpson D, Perry CM. Atomoxetine. Pediatric Drugs 2003; 5(6): 407–15
Kratochvil CJ, Vaughan BS, Harrington MJ, et al. Atomoxetine: a selective noradrenaline reuptake inhibitor for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Pharmacother 2003 Jul; 4(7): 1165–74
Spencer T, Heiligenstein JH, Biederman J, et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2002 Dec; 63(12): 1140–7
Reimherr FW, Strong RE, Dawson WH, et al. Emotional dysregulation in ADHD and response to atomoxetine [abstract no. NR640]. American Psychiatric Association 2003 Annual Meeting; New Research Abstracts, 156th Annual Meeting; 2003 May 17–22; San Francisco
Data on file. Eli Lilly and Company, 2003
Rose VL. NIH issues consensus statement on attention-deficit/ hyperactivity disorder. Am Fam Physician 1999 May 1; 59(9): 2645–6
Weisler RH, Chrisman AK, Wilens TE. Adderall XR dosed once daily in adults with ADHD [abstract no. 33]. American Psychiatric Association 2003 Annual Meeting; 2003 May 17–22; San Francisco
Shire Pharmaceuticals Group. Shire receives approvable letter for adult Adderall (XR9R), from US Food and Drug Administration [media release]. 2003
National Institutes of Health Consensus Development Conference Statement. Diagnosis and treatment of attention deficit hyperactivity disorder (ADHD). NIH Consens Statement 1998 Nov; 16(2): 1–37
Popper CW. Pharmacologic alternatives to psychostimulants for the treatment of attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am 2000 Jul; 9(3): 605–46
Kratochvil CJ, Newcorn J, Gao H, et al. Atomoxetine for comorbid ADHD and affective symptoms [poster]. 50th Annual Meeting of the American Academy of Child and Adolescent Psychiatry (AACAP); 2003 Oct 14–19; Miami
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: P.J. Ambrosini, Eastern Pennsylvania Psychiatric Institute, Drexel University College of Medicine, Philadelphia, Pennsylvania, USA; J. Elia, Department of Psychiatry, University of Pennsylvania, Philadelphia, Pennsylvania, USA; J.H. Newcorn, Department of Psychiatry, Mount Sinai Medical Center, New York, New York, USA; F. Reimherr, Department of Psychiatry, University of Utah Health Sciences Center, Salt Lake City, Utah, USA; M. Weiss, Children’s and Women’s Health Centre of British Columbia, Vancouver, British Columbia, Canada.
Data Selection
Sources: Medical literature published in any language since 1980 on atomoxetine, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘atomoxetine’ and (‘attention deficit disorder with hyperactivity’ or ‘ADHD’ in title) and ‘adults’. EMBASE search terms were ‘atomoxetine’ and (‘attention deficit disorder’ or ‘ADHD’ in title) and ‘adults’. AdisBase search terms were ‘atomoxetine’ and (‘attention deficit hyperactivity disorder’ or ‘ADHD’ in title) and ‘adults’. Searches were last updated 28 November 2003.
Selection: Studies in adult patients with attention deficit hyperactivity disorder who received atomoxetine. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Atomoxetine, attention deficit hyperactivity disorder, adults, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Simpson, D., Plosker, G.L. Atomoxetine. Drugs 64, 205–222 (2004). https://doi.org/10.2165/00003495-200464020-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464020-00005